Introduction
The incidence of cutaneous metastasis in patients with cancer is estimated to be 0.6% to 10.4%.1 In women, breast cancer is the most frequent primary malignancy metastasizing to the skin.2 A large study reported that cutaneous metastasis occurs in 23.9% of patients with breast carcinoma.2 Different patterns of cutaneous breast carcinoma metastasis have been described, including nodular, erysipeloid, telangiectatic, en cuirasse (sclerodermoid) carcinoma, alopecia neoplastica, and zosteriform patterns.3, 4 However, purpura is a very rare manifestation. Here, we report an atypical case of telangiectatic metastatic breast carcinoma presenting as a large purpuric patch on the abdomen around the umbilicus.
Case report
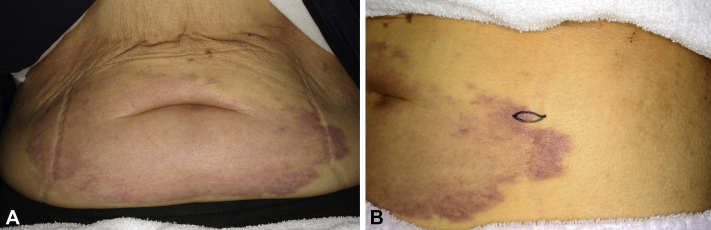
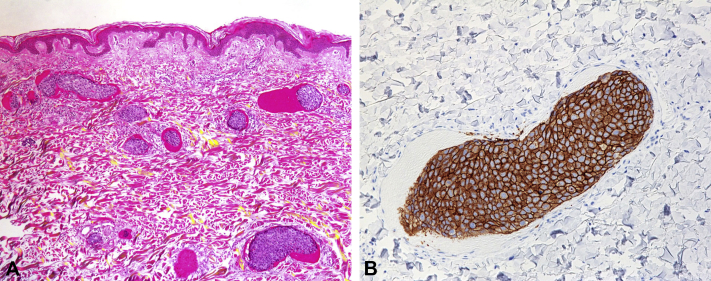
A 71-year-old woman presented to our department with a 6-month history of violet macular lesions on the abdomen around the umbilicus. Traumatic purpura was diagnosed by several other dermatologic clinics before the patient visited our department, but she received no treatment. Macular lesions gradually spread and became a large purpuric patch (25 × 10 cm in diameter) without pain or itch (Fig 1, A and B). Eleven years previously (in 2004), she had invasive breast carcinoma of the right breast. The primary breast cancer cells were positive for HER2 and negative for both estrogen and progesterone receptors (luminal HER2 type). She had been treated with radical mastectomy and chemotherapy. Unfortunately, the breast cancer metastasized to the liver in 2008 and spread to the lungs in 2013 without cutaneous metastasis. Based on this medical history and the atypical clinical features, we performed a skin biopsy from the margin of the purpuric patch. The specimen showed infiltration and occlusion of the dermal blood vessels by cancer cells, which formed multiple intravascular tumor emboli obstructing the blood vessels. Not only cancer cells, but also erythrocytes had aggregated and occluded the dermal blood vessels (Fig 2, A). No infiltration of cancer cells into the dermis was detected. Immunohistochemical staining found that the cancer cells were positive for HER2 (Fig 2, B) and negative for estrogen and progesterone receptors. These results were the same as the immunostaining pattern of the primary breast carcinoma. Considering the history, cutaneous breast carcinoma metastasis was suspected. Further studies, including laboratory tests, found elevated serum levels of cancer antigen 15-3 (36.5 U/mL; normal, <25 U/mL) and HER2 (273.5 ng/mL; normal, 0.0–15.2 ng/mL). Computed tomography found multiple lymphatic metastases and peritoneal dissemination. Chemotherapy dosing regimens were subsequently changed from T-DM1 (trastuzumab emtansine) to VNR (vinorelbine)+HER (trastuzumab) therapy. However, the purpuric lesions on the abdomen did not resolve, and the patient died 4 months after the initial diagnosis of cutaneous metastasis.
Fig 1.
A, Large purpuric patch (25 × 10 cm in diameter) on the abdomen. B, Biopsy section taken from the margin of the purpuric patch.
Fig 2.
A, The skin biopsy sample shows numerous dilated vessels in the dermis with thrombi of neoplastic cells and erythrocytes. B, Neoplastic cells show positive results for HER2. (A, Hematoxylin-eosin stain; original magnifications: A, ×40; B ×100.)
Discussion
Cutaneous breast carcinoma metastasis can be of different clinicopathologic types. The most common manifestation is nodules, which are often found on the chest wall. Carcinoma telangiectaticum is rare, with only a few cases reported in the literature.5, 6, 7, 8, 9, 10
Clinically, carcinoma telangiectaticum is characterized by yellowish to reddish or violaceous lymphangioma circumscriptum–like papulovesicles ipsilateral to the side affected by breast carcinoma.3 Our patient presented with a large purpuric patch mimicking traumatic purpura on the abdomen without pain or itch, which is a relatively rare manifestation of cutaneous breast cancer metastasis even in carcinoma telangiectaticum.
Histologically, tumor nests are found in dilated small blood vessels, mainly in the subepidermal zone in carcinoma telangiectaticum.9, 10 Blood vessels in the lower dermis may occasionally be involved. Tumor nests usually lie free in the vascular lumina, although focal connection to the vessel walls or breaching into the surrounding dermis may occur.9 Our case is mostly consistent with these histologic characteristics. Interestingly, our specimen showed not only metastatic breast cancer cells but also most erythrocytes aggregating and occluding the dermal blood vessels and forming multiple intravascular thrombi. Extravasation of erythrocytes into the dermis was not detected. We speculated that the atypical clinical manifestation of large purpuric patch mimicking traumatic purpura in our case was possibly caused by aggregation of erythrocytes in the dermis forming multiple intravascular thrombi. This clinicopathologic presentation was similar to that seen with hemangioma simplex or capillary-venous malformation.
Metastasis of breast carcinoma to distant areas of the skin is thought to be mediated by lymphatic vessels and rarely by blood vessels. Some cases of carcinoma erysipeloides, which is another type of inflammatory carcinoma, have also been reported previously. Both carcinoma erysipeloides and telangiectaticum are characterized by the intravascular spread of tumor cells in the dermis.10 In carcinoma erysipeloides, tumor cells involve the lymphatic vessels in the dermis, whereas in carcinoma telangiectaticum, the tumor cells are spread mainly within the lumen of blood capillaries.10 In 2009, Marneros et al5 reviewed 100 skin biopsy specimens from cutaneous breast cancer metastatic lesions obtained from patients seen at their dermatopathology unit between 1999 and 2008. Ninety specimens showed interstitial tumor cell infiltration, and only 10 cases showed strictly intravascular tumor cell localization. In 9 cases, tumor cells were restricted to lymph vessels, and only one case (1%) showed exclusively dermal blood vessel involvement.5 Telangiectatic metastatic breast cancer is therefore exceedingly rare. The prognosis for this type is considered poor, because microvascular metastases in other organs had already expanded when cutaneous metastasis was initially identified. In our case, multiple lymphatic metastases and peritoneal dissemination were also detected at diagnosis.
Here, we reported an atypical case of telangiectatic metastatic breast carcinoma. Unusual aspects of this case included cutaneous metastasis to the abdomen around the umbilicus, which does not appear to have been reported previously. Furthermore, the clinical presentations of large purpura such as traumatic purpura were rather unique. Finally, histopathologic examination found that not only metastatic cancer cells, but also most erythrocytes aggregated and occluded the dermal blood vessels and formed multiple intravascular thrombi. These unusual histologic characteristics resulted in an atypical purpuric appearance similar to that of traumatic purpura. High clinical suspicion for cutaneous metastasis should be maintained in the initial evaluation of purpura, particularly in any patient with a history of malignancy.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Alcaraz I., Cerroni L., Rütten A. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347–393. doi: 10.1097/DAD.0b013e31823069cf. [DOI] [PubMed] [Google Scholar]
- 2.Lookingbill D.P., Spangler N., Helm K.F. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29:228–236. doi: 10.1016/0190-9622(93)70173-q. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz R.A. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33:161–182. doi: 10.1016/0190-9622(95)90231-7. quiz 183-186. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan R.P. Specific cutaneous manifestations of internal malignancy. Adv Dermatol. 1986;1:3–42. [PubMed] [Google Scholar]
- 5.Marneros A.G., Blanco F., Husain S. Classification of cutaneous intravascular breast cancer metastases based on immunolabeling for blood and lymph vessels. J Am Acad Dermatol. 2009;60:633–638. doi: 10.1016/j.jaad.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Das P., Ahuja A., Gupta S.D. Calcifying telangiectatic cutaneous breast carcinoma metastasis. Int J Surg Pathol. 2009;17:455–456. doi: 10.1177/1066896908318580. [DOI] [PubMed] [Google Scholar]
- 7.Lin J.H., Lee J.Y., Chao S.C. Telangiectatic metastatic breast carcinoma preceded by en cuirasse metastatic breast carcinoma. Br J Dermatol. 2004;151:523–524. doi: 10.1111/j.1365-2133.2004.06140.x. [DOI] [PubMed] [Google Scholar]
- 8.Dobson C.M., Tagor V., Myint A.S. Telangiectatic metastatic breast carcinoma in face and scalp mimicking cutaneous angiosarcoma. J Am Acad Dermatol. 2003;48:635–636. doi: 10.1067/mjd.2003.256. [DOI] [PubMed] [Google Scholar]
- 9.Ingram J.T. Carcinoma erysipelatodes and carcinoma telangiectaticum. AMA Arch Derm. 1958;77:227–231. doi: 10.1001/archderm.1958.01560020081011. [DOI] [PubMed] [Google Scholar]
- 10.Weber F.P. Bilateral thoracic zosteroid spreading marginate telangiectasia—probably a variety of “carcinoma erysipelatodes” (C. Rasch)-associated with unilateral mammary carcinoma, and better termed “carcinoma telangiectaticum”. Proc R Soc Med. 1933;26:1549–1551. doi: 10.1177/003591573302601236. [DOI] [PMC free article] [PubMed] [Google Scholar]