Abstract
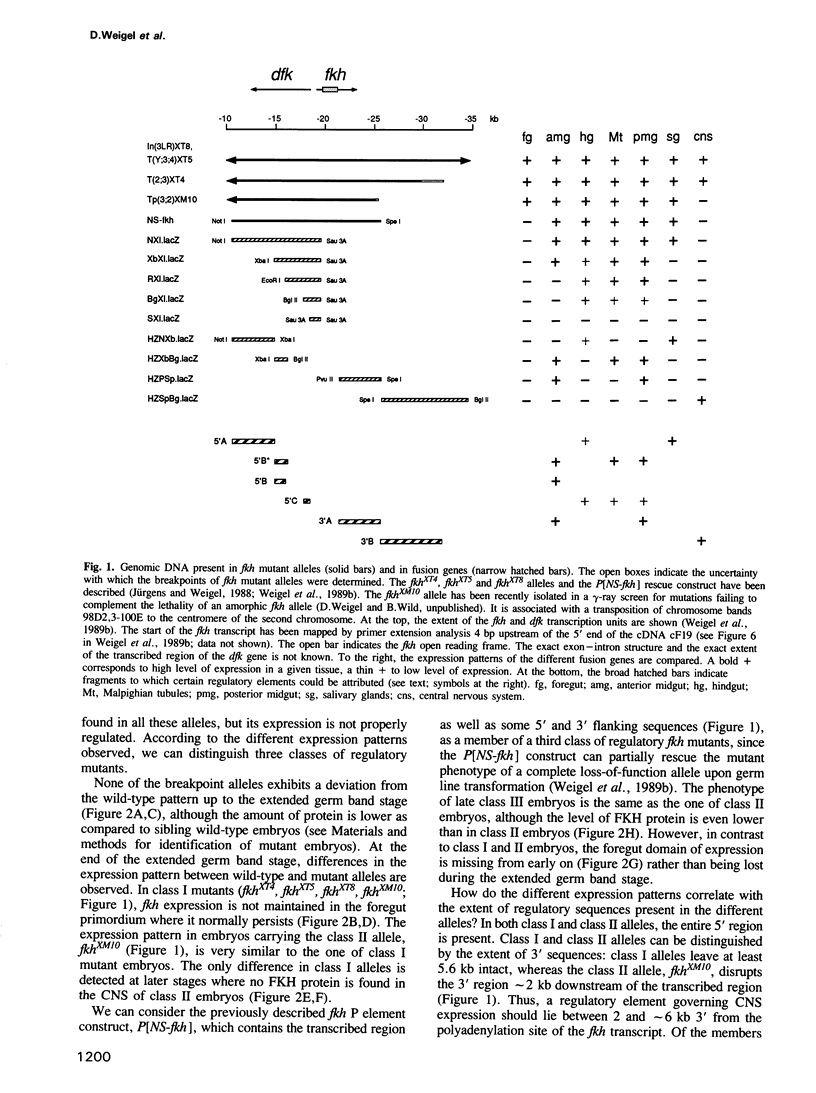
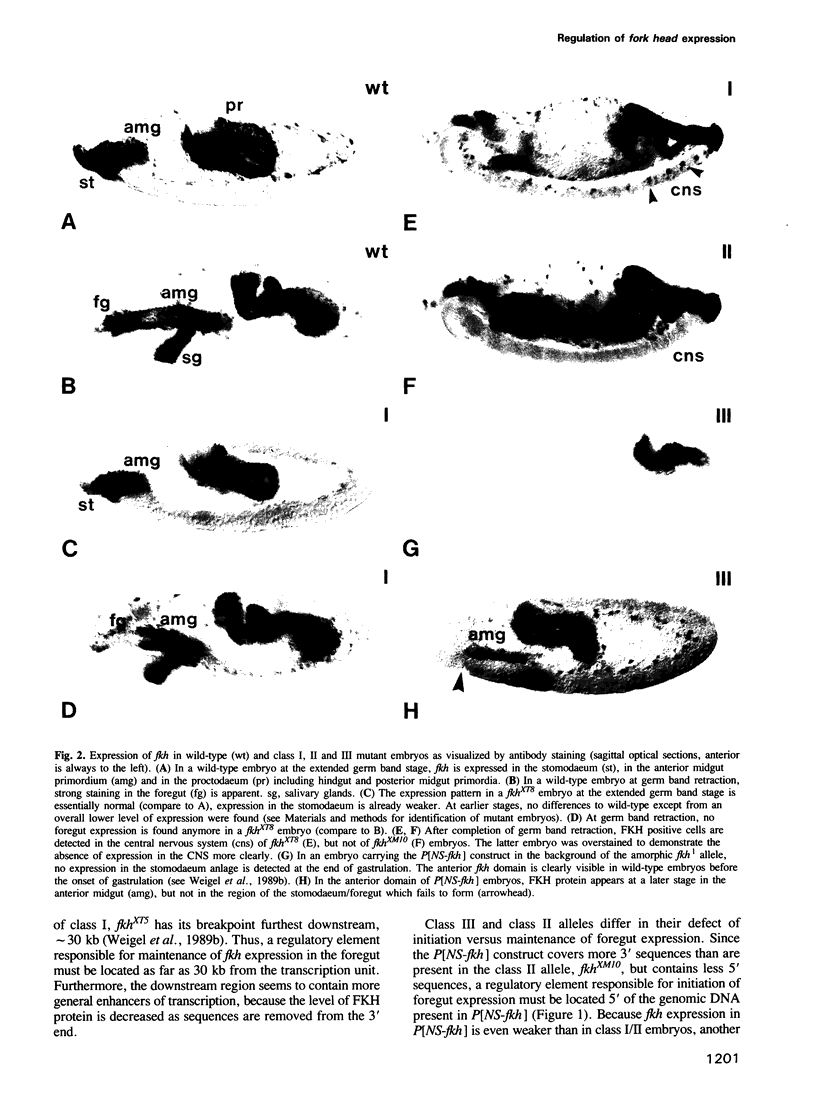
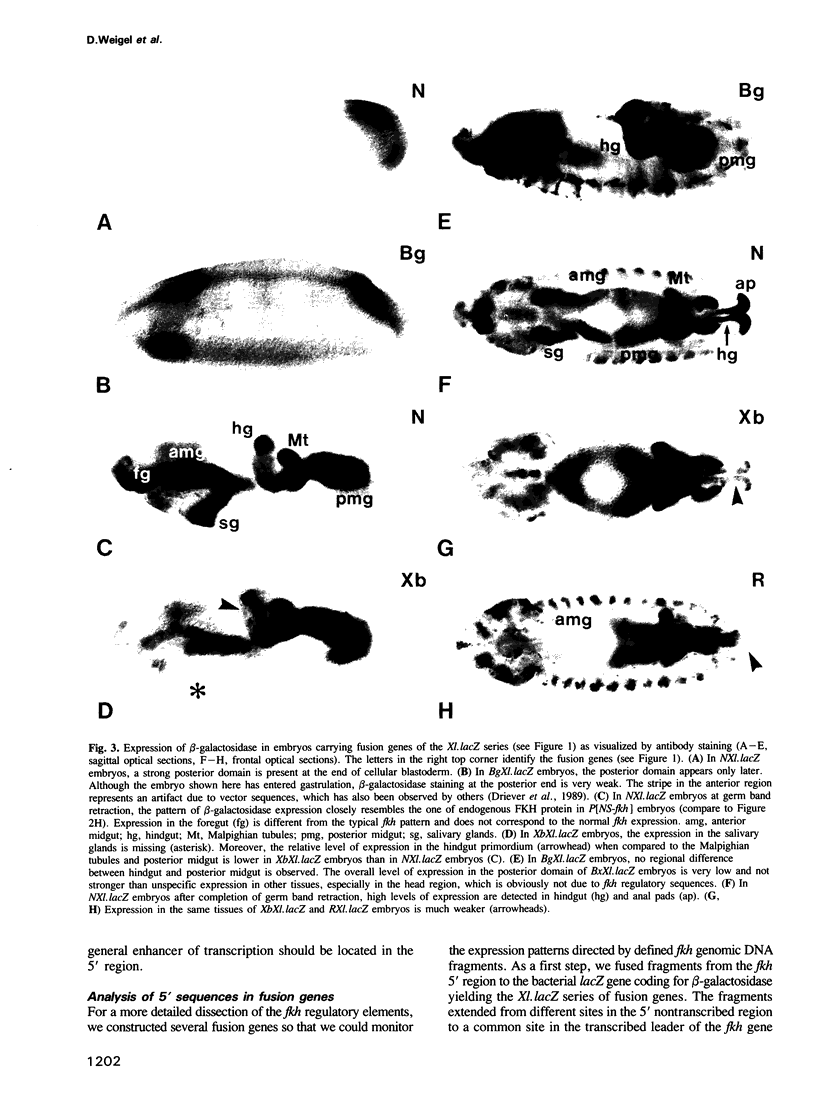
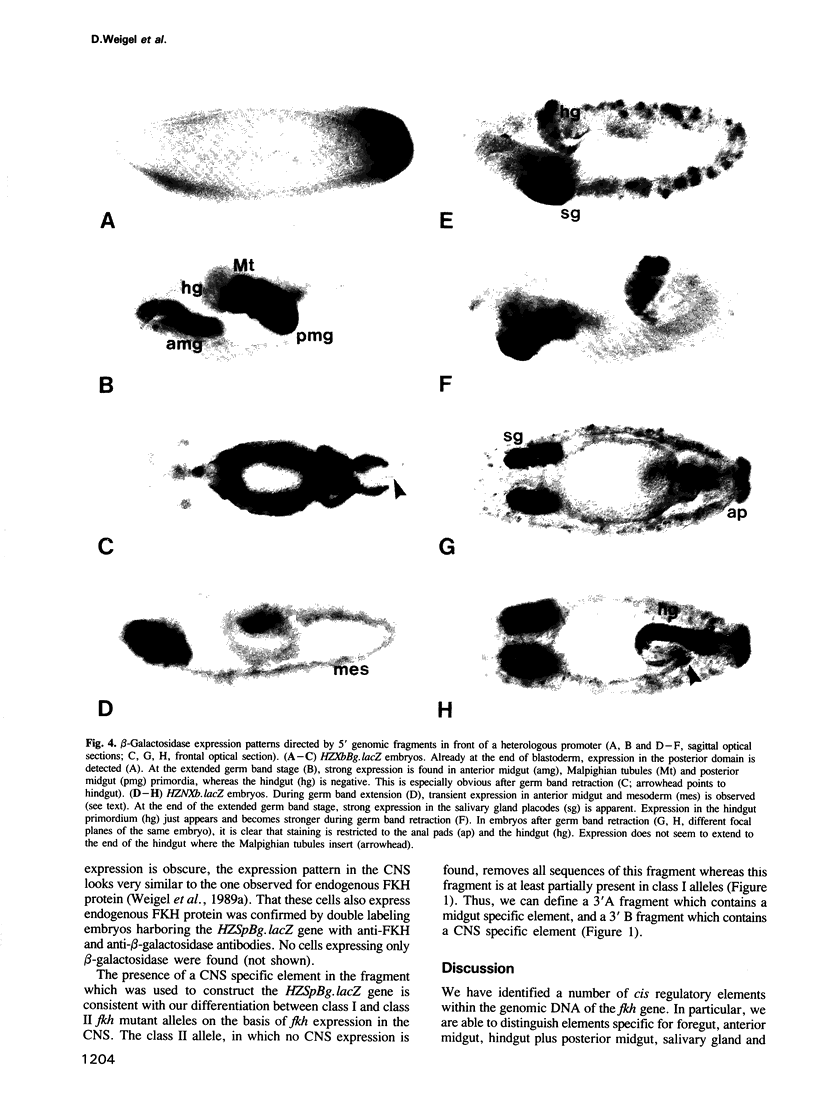
The region-specific homeotic gene fork head (fkh) is expressed and required in a variety of tissues of the developing Drosophila embryo. In order to identify the cis regulatory elements directing the complex spatio-temporal expression pattern of fkh, we have studied the subpatterns directed by defined fragments of fkh genomic DNA. These experiments enabled us to distinguish separate regulatory elements specific for the different expression domains of fkh. In addition, our analysis revealed several unexpected features such as the redundancy of regulatory elements and the overlap of regulatory elements with the transcribed regions of other genes. Moreover, the separation of normally contiguous elements effecting expression in the posterior terminal fkh domain appears to lead to novel expression domains which do not correspond to known developmental units in the embryo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akam M. The molecular basis for metameric pattern in the Drosophila embryo. Development. 1987 Sep;101(1):1–22. [PubMed] [Google Scholar]
- Bienz M., Saari G., Tremml G., Müller J., Züst B., Lawrence P. A. Differential regulation of Ultrabithorax in two germ layers of Drosophila. Cell. 1988 May 20;53(4):567–576. doi: 10.1016/0092-8674(88)90573-9. [DOI] [PubMed] [Google Scholar]
- Bienz M., Tremml G. Domain of Ultrabithorax expression in Drosophila visceral mesoderm from autoregulation and exclusion. Nature. 1988 Jun 9;333(6173):576–578. doi: 10.1038/333576a0. [DOI] [PubMed] [Google Scholar]
- Carroll S. B., DiNardo S., O'Farrell P. H., White R. A., Scott M. P. Temporal and spatial relationships between segmentation and homeotic gene expression in Drosophila embryos: distributions of the fushi tarazu, engrailed, Sex combs reduced, Antennapedia, and Ultrabithorax proteins. Genes Dev. 1988 Mar;2(3):350–360. doi: 10.1101/gad.2.3.350. [DOI] [PubMed] [Google Scholar]
- Dearolf C. R., Topol J., Parker C. S. The caudal gene product is a direct activator of fushi tarazu transcription during Drosophila embryogenesis. Nature. 1989 Sep 28;341(6240):340–343. doi: 10.1038/341340a0. [DOI] [PubMed] [Google Scholar]
- DiNardo S., Sher E., Heemskerk-Jongens J., Kassis J. A., O'Farrell P. H. Two-tiered regulation of spatially patterned engrailed gene expression during Drosophila embryogenesis. Nature. 1988 Apr 14;332(6165):604–609. doi: 10.1038/332604a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle H. J., Kraut R., Levine M. Spatial regulation of zerknüllt: a dorsal-ventral patterning gene in Drosophila. Genes Dev. 1989 Oct;3(10):1518–1533. doi: 10.1101/gad.3.10.1518. [DOI] [PubMed] [Google Scholar]
- Driever W., Nüsslein-Volhard C. The bicoid protein is a positive regulator of hunchback transcription in the early Drosophila embryo. Nature. 1989 Jan 12;337(6203):138–143. doi: 10.1038/337138a0. [DOI] [PubMed] [Google Scholar]
- Driever W., Thoma G., Nüsslein-Volhard C. Determination of spatial domains of zygotic gene expression in the Drosophila embryo by the affinity of binding sites for the bicoid morphogen. Nature. 1989 Aug 3;340(6232):363–367. doi: 10.1038/340363a0. [DOI] [PubMed] [Google Scholar]
- Frasch M., Warrior R., Tugwood J., Levine M. Molecular analysis of even-skipped mutants in Drosophila development. Genes Dev. 1988 Dec;2(12B):1824–1838. doi: 10.1101/gad.2.12b.1824. [DOI] [PubMed] [Google Scholar]
- Hafen E., Levine M., Gehring W. J. Regulation of Antennapedia transcript distribution by the bithorax complex in Drosophila. Nature. 1984 Jan 19;307(5948):287–289. doi: 10.1038/307287a0. [DOI] [PubMed] [Google Scholar]
- Harbecke R., Janning W. The segmentation gene Krüppel of Drosophila melanogaster has homeotic properties. Genes Dev. 1989 Jan;3(1):114–122. doi: 10.1101/gad.3.1.114. [DOI] [PubMed] [Google Scholar]
- Harding K., Hoey T., Warrior R., Levine M. Autoregulatory and gap gene response elements of the even-skipped promoter of Drosophila. EMBO J. 1989 Apr;8(4):1205–1212. doi: 10.1002/j.1460-2075.1989.tb03493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding K., Wedeen C., McGinnis W., Levine M. Spatially regulated expression of homeotic genes in Drosophila. Science. 1985 Sep 20;229(4719):1236–1242. doi: 10.1126/science.3898362. [DOI] [PubMed] [Google Scholar]
- Hiromi Y., Gehring W. J. Regulation and function of the Drosophila segmentation gene fushi tarazu. Cell. 1987 Sep 11;50(6):963–974. doi: 10.1016/0092-8674(87)90523-x. [DOI] [PubMed] [Google Scholar]
- Hiromi Y., Kuroiwa A., Gehring W. J. Control elements of the Drosophila segmentation gene fushi tarazu. Cell. 1985 Dec;43(3 Pt 2):603–613. doi: 10.1016/0092-8674(85)90232-6. [DOI] [PubMed] [Google Scholar]
- Ingham P. W. The molecular genetics of embryonic pattern formation in Drosophila. Nature. 1988 Sep 1;335(6185):25–34. doi: 10.1038/335025a0. [DOI] [PubMed] [Google Scholar]
- Irish V. F., Martinez-Arias A., Akam M. Spatial regulation of the Antennapedia and Ultrabithorax homeotic genes during Drosophila early development. EMBO J. 1989 May;8(5):1527–1537. doi: 10.1002/j.1460-2075.1989.tb03537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuziora M. A., McGinnis W. Autoregulation of a Drosophila homeotic selector gene. Cell. 1988 Nov 4;55(3):477–485. doi: 10.1016/0092-8674(88)90034-7. [DOI] [PubMed] [Google Scholar]
- Kuziora M. A., McGinnis W. Different transcripts of the Drosophila Abd-B gene correlate with distinct genetic sub-functions. EMBO J. 1988 Oct;7(10):3233–3244. doi: 10.1002/j.1460-2075.1988.tb03190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald P. M., Struhl G. A molecular gradient in early Drosophila embryos and its role in specifying the body pattern. Nature. 1986 Dec 11;324(6097):537–545. doi: 10.1038/324537a0. [DOI] [PubMed] [Google Scholar]
- Martizez Arias A., Baker N. E., Ingham P. W. Role of segment polarity genes in the definition and maintenance of cell states in the Drosophila embryo. Development. 1988 May;103(1):157–170. doi: 10.1242/dev.103.1.157. [DOI] [PubMed] [Google Scholar]
- Mullins M. C., Rio D. C., Rubin G. M. cis-acting DNA sequence requirements for P-element transposition. Genes Dev. 1989 May;3(5):729–738. doi: 10.1101/gad.3.5.729. [DOI] [PubMed] [Google Scholar]
- Pankratz M. J., Hoch M., Seifert E., Jäckle H. Krüppel requirement for knirps enhancement reflects overlapping gap gene activities in the Drosophila embryo. Nature. 1989 Sep 28;341(6240):337–340. doi: 10.1038/341337a0. [DOI] [PubMed] [Google Scholar]
- Strecker T. R., Merriam J. R., Lengyel J. A. Graded requirement for the zygotic terminal gene, tailless, in the brain and tail region of the Drosophila embryo. Development. 1988 Apr;102(4):721–734. doi: 10.1242/dev.102.4.721. [DOI] [PubMed] [Google Scholar]
- Struhl G., White R. A. Regulation of the Ultrabithorax gene of Drosophila by other bithorax complex genes. Cell. 1985 Dec;43(2 Pt 1):507–519. doi: 10.1016/0092-8674(85)90180-1. [DOI] [PubMed] [Google Scholar]
- Sánchez-Herrero E., Crosby M. A. The Abdominal-B gene of Drosophila melanogaster: overlapping transcripts exhibit two different spatial distributions. EMBO J. 1988 Jul;7(7):2163–2173. doi: 10.1002/j.1460-2075.1988.tb03055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel C. S., Boulet A. M., Lipshitz H. D. Vectors for Drosophila P-element-mediated transformation and tissue culture transfection. Gene. 1988 Dec 30;74(2):445–456. doi: 10.1016/0378-1119(88)90177-1. [DOI] [PubMed] [Google Scholar]
- Wedeen C., Harding K., Levine M. Spatial regulation of Antennapedia and bithorax gene expression by the Polycomb locus in Drosophila. Cell. 1986 Mar 14;44(5):739–748. doi: 10.1016/0092-8674(86)90840-8. [DOI] [PubMed] [Google Scholar]
- Weigel D., Jürgens G., Küttner F., Seifert E., Jäckle H. The homeotic gene fork head encodes a nuclear protein and is expressed in the terminal regions of the Drosophila embryo. Cell. 1989 May 19;57(4):645–658. doi: 10.1016/0092-8674(89)90133-5. [DOI] [PubMed] [Google Scholar]