Abstract
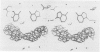
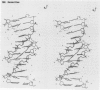
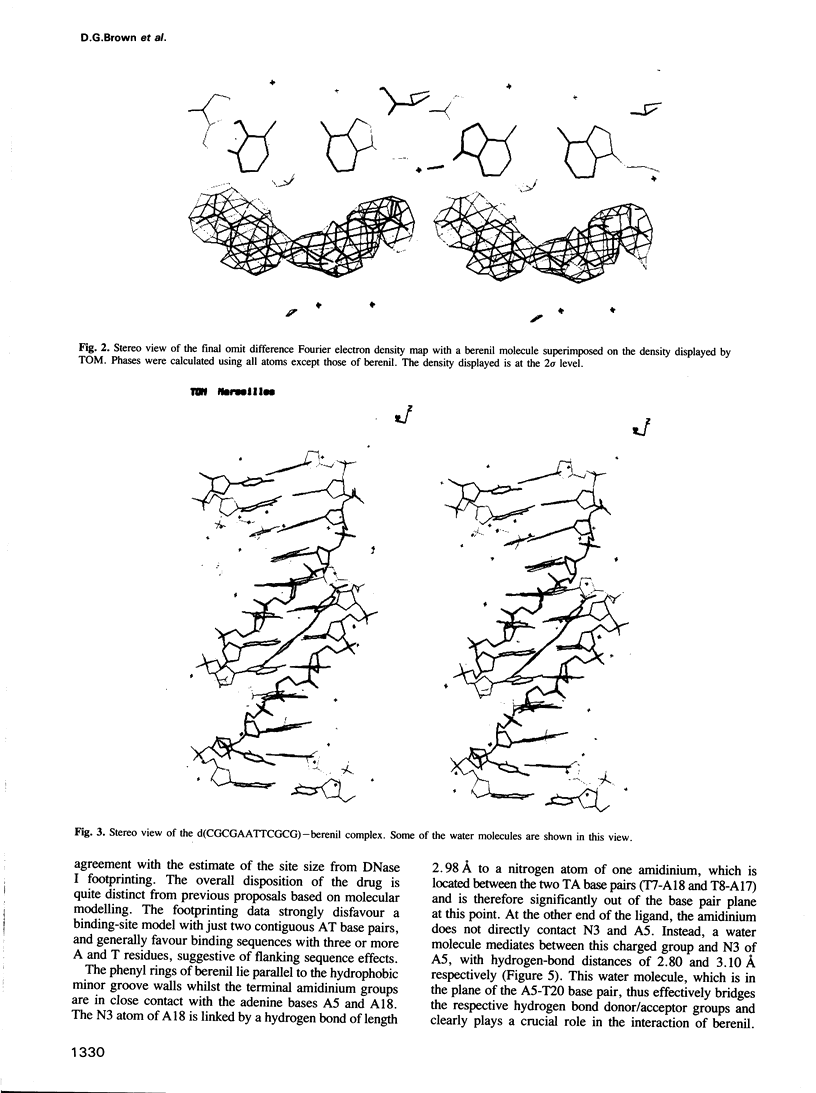
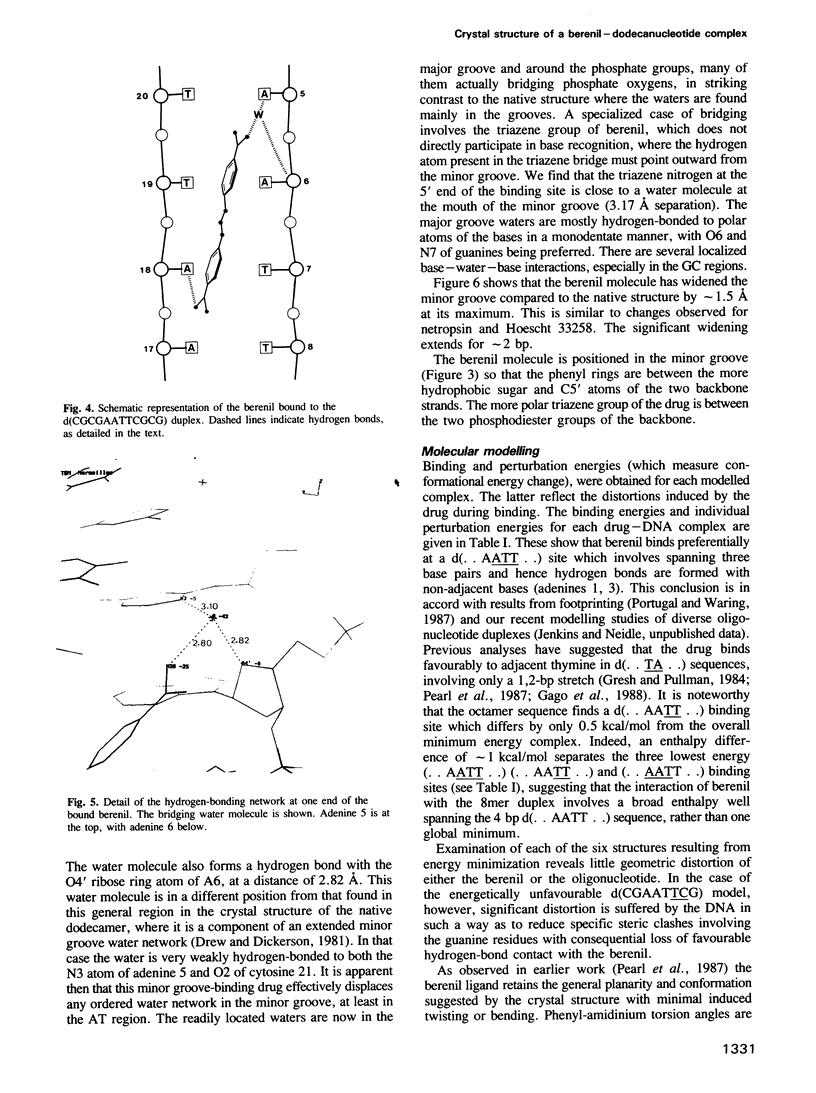
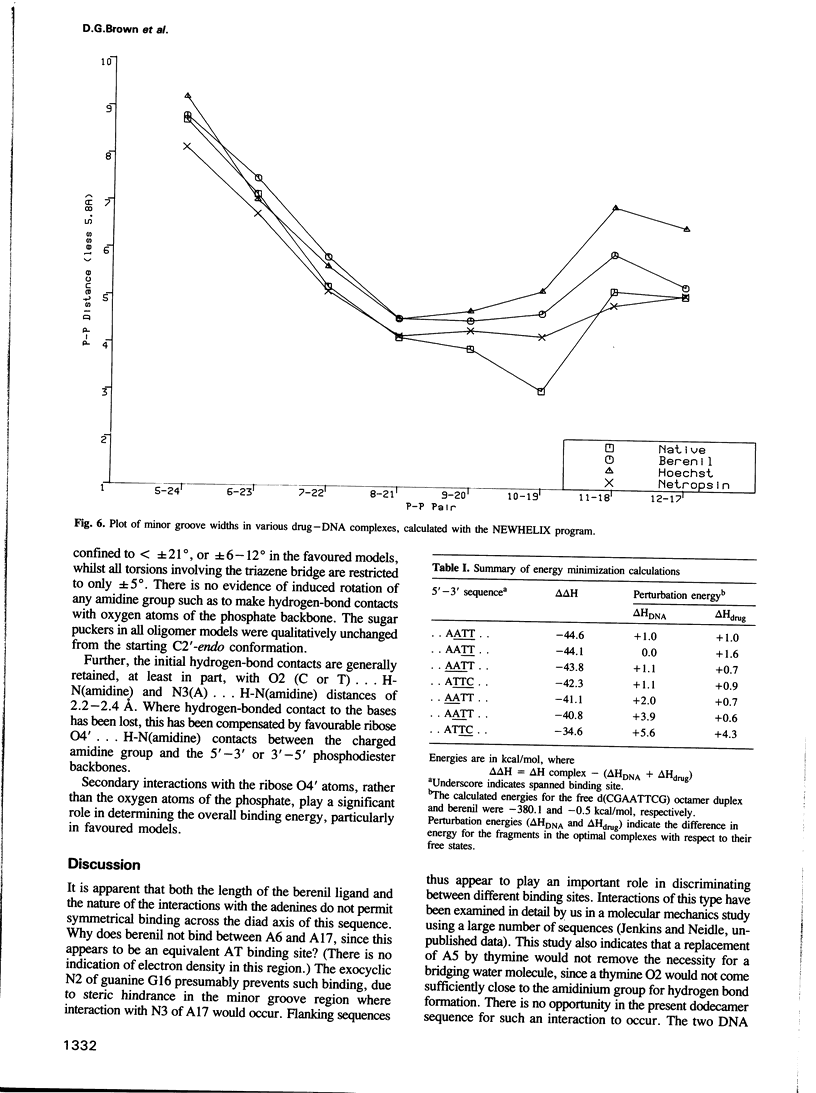
The three-dimensional structure of a complex between the dodecanucleotide d(CGCGAATTCGCG) and the anti-trypanocidal drug berenil, has been determined to a resolution of 2.5 A. The structure has been solved by molecular replacement and refined to an R factor of 0.177. A total of 49 water molecules have been located. The drug is bound at the 5'-AAT-3' region of the oligonucleotide. At one end of the drug the amidinium group is in hydrogen-bonded contact with N3 of the adenine base complementary to the thymine of the AAT. The other amidinium group does not make direct interactions with the DNA. Instead, a water molecule mediates between them. This is in hydrogen-bonded contact with an amidinium nitrogen atom, N3 of the 5' end adenine base and the ring oxygen atom of an adjacent deoxyribose. Molecular mechanics calculations have been performed on this complex, with the drug at various positions along the sequence. These show that the observed position is only 0.8 kcal/mol higher in energy than the best position. It is suggested that there is a broad energy well in the AATT region for this drug, and that water molecules as well as the neighbouring sequence, will determine precise positioning. More general aspects of minor groove binding are discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal A. K., Rodgers D. W., Drottar M., Ptashne M., Harrison S. C. Recognition of a DNA operator by the repressor of phage 434: a view at high resolution. Science. 1988 Nov 11;242(4880):899–907. doi: 10.1126/science.3187531. [DOI] [PubMed] [Google Scholar]
- Baguley B. C. Nonintercalative DNA-binding antitumour compounds. Mol Cell Biochem. 1982 Apr 2;43(3):167–181. doi: 10.1007/BF00223008. [DOI] [PubMed] [Google Scholar]
- Braithwaite A. W., Baguley B. C. Existence of an extended series of antitumor compounds which bind to deoxyribonucleic acid by nonintercalative means. Biochemistry. 1980 Mar 18;19(6):1101–1106. doi: 10.1021/bi00547a009. [DOI] [PubMed] [Google Scholar]
- Burkhoff A. M., Tullius T. D. The unusual conformation adopted by the adenine tracts in kinetoplast DNA. Cell. 1987 Mar 27;48(6):935–943. doi: 10.1016/0092-8674(87)90702-1. [DOI] [PubMed] [Google Scholar]
- Bénard J., Riou G. F. In vivo effects of intercalating and nonintercalating drugs on the tertiary structure of kinetoplast deoxyribonucleic acid. Biochemistry. 1980 Sep 2;19(18):4197–4201. doi: 10.1021/bi00559a009. [DOI] [PubMed] [Google Scholar]
- Carrondo M. A., Coll M., Aymami J., Wang A. H., van der Marel G. A., van Boom J. H., Rich A. Binding of a Hoechst dye to d(CGCGATATCGCG) and its influence on the conformation of the DNA fragment. Biochemistry. 1989 Sep 19;28(19):7849–7859. doi: 10.1021/bi00445a047. [DOI] [PubMed] [Google Scholar]
- Clercq E. D., Dann O. Diaryl amidine derivatives as oncornaviral DNA polymerase inhibitors. J Med Chem. 1980 Jul;23(7):787–795. doi: 10.1021/jm00181a016. [DOI] [PubMed] [Google Scholar]
- Coll M., Aymami J., van der Marel G. A., van Boom J. H., Rich A., Wang A. H. Molecular structure of the netropsin-d(CGCGATATCGCG) complex: DNA conformation in an alternating AT segment. Biochemistry. 1989 Jan 10;28(1):310–320. doi: 10.1021/bi00427a042. [DOI] [PubMed] [Google Scholar]
- Coll M., Frederick C. A., Wang A. H., Rich A. A bifurcated hydrogen-bonded conformation in the d(A.T) base pairs of the DNA dodecamer d(CGCAAATTTGCG) and its complex with distamycin. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8385–8389. doi: 10.1073/pnas.84.23.8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dervan P. B. Design of sequence-specific DNA-binding molecules. Science. 1986 Apr 25;232(4749):464–471. doi: 10.1126/science.2421408. [DOI] [PubMed] [Google Scholar]
- Drew H. R., Dickerson R. E. Structure of a B-DNA dodecamer. III. Geometry of hydration. J Mol Biol. 1981 Sep 25;151(3):535–556. doi: 10.1016/0022-2836(81)90009-7. [DOI] [PubMed] [Google Scholar]
- Gago F., Reynolds C. A., Richards W. G. The binding of nonintercalative drugs to alternating DNA sequences. Mol Pharmacol. 1989 Feb;35(2):232–241. [PubMed] [Google Scholar]
- Gresh N., Pullman B. A theoretical study of the nonintercalative binding of berenil and stilbamidine to double-stranded (dA-dT)n oligomers. Mol Pharmacol. 1984 May;25(3):452–458. [PubMed] [Google Scholar]
- Kopka M. L., Yoon C., Goodsell D., Pjura P., Dickerson R. E. Binding of an antitumor drug to DNA, Netropsin and C-G-C-G-A-A-T-T-BrC-G-C-G. J Mol Biol. 1985 Jun 25;183(4):553–563. doi: 10.1016/0022-2836(85)90171-8. [DOI] [PubMed] [Google Scholar]
- Lane M. J., Dabrowiak J. C., Vournakis J. N. Sequence specificity of actinomycin D and Netropsin binding to pBR322 DNA analyzed by protection from DNase I. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3260–3264. doi: 10.1073/pnas.80.11.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lown J. W. Lexitropsins: rational design of DNA sequence reading agents as novel anti-cancer agents and potential cellular probes. Anticancer Drug Des. 1988 Jun;3(1):25–40. [PubMed] [Google Scholar]
- Marini J. C., Levene S. D., Crothers D. M., Englund P. T. Bent helical structure in kinetoplast DNA. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7664–7668. doi: 10.1073/pnas.79.24.7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marky L. A., Breslauer K. J. Origins of netropsin binding affinity and specificity: correlations of thermodynamic and structural data. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4359–4363. doi: 10.1073/pnas.84.13.4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. H., Hunter W. N., d'Estaintot B. L., Kennard O. DNA-drug interactions. The crystal structure of d(CGATCG) complexed with daunomycin. J Mol Biol. 1989 Apr 20;206(4):693–705. doi: 10.1016/0022-2836(89)90577-9. [DOI] [PubMed] [Google Scholar]
- Pearl L. H., Skelly J. V., Hudson B. D., Neidle S. The crystal structure of the DNA-binding drug berenil: molecular modelling studies of berenil-DNA complexes. Nucleic Acids Res. 1987 Apr 24;15(8):3469–3478. doi: 10.1093/nar/15.8.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pjura P. E., Grzeskowiak K., Dickerson R. E. Binding of Hoechst 33258 to the minor groove of B-DNA. J Mol Biol. 1987 Sep 20;197(2):257–271. doi: 10.1016/0022-2836(87)90123-9. [DOI] [PubMed] [Google Scholar]
- Portugal J., Waring M. J. Antibiotics which can alter the rotational orientation of nucleosome core DNA. Nucleic Acids Res. 1986 Nov 25;14(22):8735–8754. doi: 10.1093/nar/14.22.8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal J., Waring M. J. Comparison of binding sites in DNA for berenil, netropsin and distamycin. A footprinting study. Eur J Biochem. 1987 Sep 1;167(2):281–289. doi: 10.1111/j.1432-1033.1987.tb13334.x. [DOI] [PubMed] [Google Scholar]
- Suck D., Lahm A., Oefner C. Structure refined to 2A of a nicked DNA octanucleotide complex with DNase I. Nature. 1988 Mar 31;332(6163):464–468. doi: 10.1038/332464a0. [DOI] [PubMed] [Google Scholar]
- Teng M. K., Usman N., Frederick C. A., Wang A. H. The molecular structure of the complex of Hoechst 33258 and the DNA dodecamer d(CGCGAATTCGCG). Nucleic Acids Res. 1988 Mar 25;16(6):2671–2690. doi: 10.1093/nar/16.6.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnell W. G., Satchwell S. C., Travers A. A. A decapeptide motif for binding to the minor groove of DNA. A proposal. FEBS Lett. 1988 May 23;232(2):263–268. doi: 10.1016/0014-5793(88)80750-6. [DOI] [PubMed] [Google Scholar]
- Van Dyke M. W., Hertzberg R. P., Dervan P. B. Map of distamycin, netropsin, and actinomycin binding sites on heterogeneous DNA: DNA cleavage-inhibition patterns with methidiumpropyl-EDTA.Fe(II). Proc Natl Acad Sci U S A. 1982 Sep;79(18):5470–5474. doi: 10.1073/pnas.79.18.5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. H., Ughetto G., Quigley G. J., Rich A. Interactions between an anthracycline antibiotic and DNA: molecular structure of daunomycin complexed to d(CpGpTpApCpG) at 1.2-A resolution. Biochemistry. 1987 Feb 24;26(4):1152–1163. doi: 10.1021/bi00378a025. [DOI] [PubMed] [Google Scholar]
- Westhof E., Dumas P., Moras D. Crystallographic refinement of yeast aspartic acid transfer RNA. J Mol Biol. 1985 Jul 5;184(1):119–145. doi: 10.1016/0022-2836(85)90048-8. [DOI] [PubMed] [Google Scholar]
- Wu H. M., Crothers D. M. The locus of sequence-directed and protein-induced DNA bending. Nature. 1984 Apr 5;308(5959):509–513. doi: 10.1038/308509a0. [DOI] [PubMed] [Google Scholar]
- Zimmer C., Wähnert U. Nonintercalating DNA-binding ligands: specificity of the interaction and their use as tools in biophysical, biochemical and biological investigations of the genetic material. Prog Biophys Mol Biol. 1986;47(1):31–112. doi: 10.1016/0079-6107(86)90005-2. [DOI] [PubMed] [Google Scholar]