Introduction
Extravasation of chemotherapy is a rare devastating, cutaneous complication associated with administration of intravenous chemotherapy.1 Extravasation of chemotherapeutic agents can cause significant morbidity to already arduous chemotherapy regimens.1 Traditional treatment of extravasation is largely conservative in nature using compression, elevation, and topical or systemic steroids as the primary therapeutic modalities with varying efficacy.1, 2 We present a case of docetaxel extravasation successfully treated with intralesional steroids that clinically hastened the resolution of cutaneous symptoms.
Case report
A 72-year-old-African American man with biopsy-proven prostate cancer with metastases to the bone was started on androgen depravation therapy with degarelix and referred to the National Cancer Institute at the National Institutes of Health to start a protocol (NCT02649855) with docetaxel, androgen depravation therapy, and prostvac, a vaccine-based therapy. The patient received the vaccine via subcutaneous injection 24 hours before standard intravenous infusion of docetaxel (143 mg [75 mg/m2] over 60 minutes, in a total volume of 250 mL 0.9% sodium chloride).3
Twelve hours after initial infusion of docetaxel, a painful indurated plaque over the left proximal bicep developed at site of infusion. Subsequently, the plaque spread distally down the patient's arm over the next several days. The event was reported to the National Cancer Institute's quality control/assurance system. The patient did not receive any imaging or contrast dye. When the extravasation site did not improve, he sought care with dermatology at the referring institution, Walter Reed National Military Medical Center.
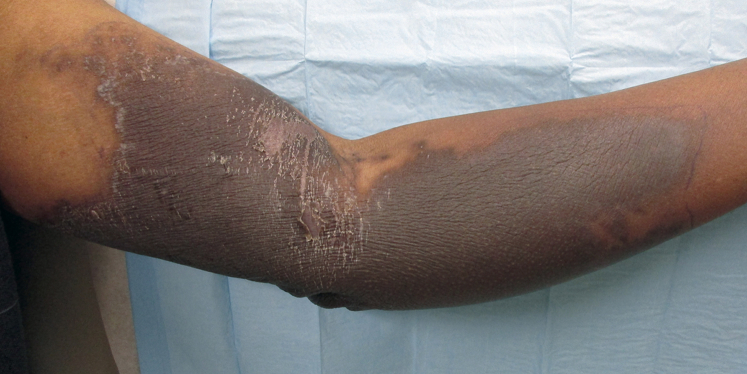
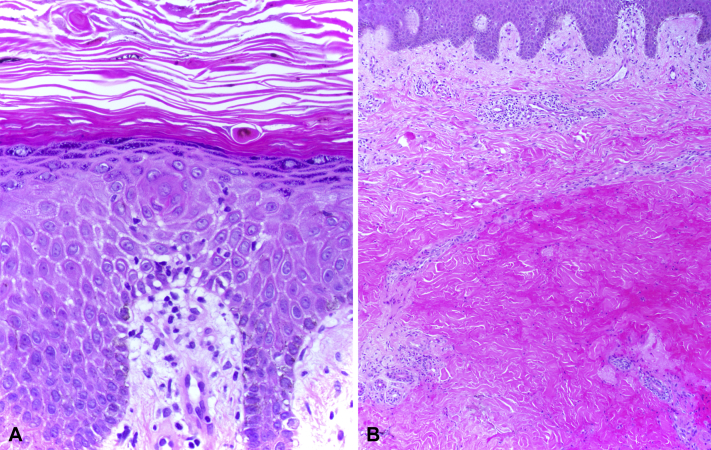
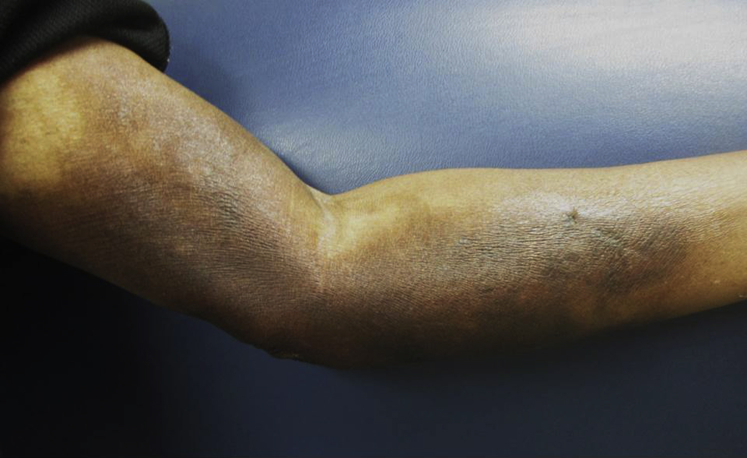
The initial evaluation 3 weeks after the event found an indurated, dusky-to-violaceous plaque on the left medial upper arm. This lesion was tender and fibrotic with a chalky-white, scaling peripheral rim (Fig 1). A punch biopsy found hyperkeratosis with a basket weave pattern, intracorneal dyskeratotic cells, hypergranulosis, focal spongiosis with lymphocytic exocytosis, and slightly pale and swollen basilar nuclei (Fig 2, A). Much of the mid dermis was occupied by a zone of brightly eosinophilic, homogenized collagen fibers and red blood cell debris (Fig 2, B). Within this zone, adnexal structures and blood vessels largely disappeared, giving the area a markedly hypocellular appearance. Intact eccrine glands and blood vessels leading up into this zone could be seen disintegrating within the zone. Immunohistochemical stains for sweat ducts and blood vessels (CEA and CD31) confirmed loss of these structures within the homogenized zone of tissue damage.
Fig 1.
Clinical extravasation 3 weeks after docetaxel infusion.
Fig 2.
A, Epidermal changes. B, Overview of dermal changes. (Original magnifications: A, ×200; B, ×100.)
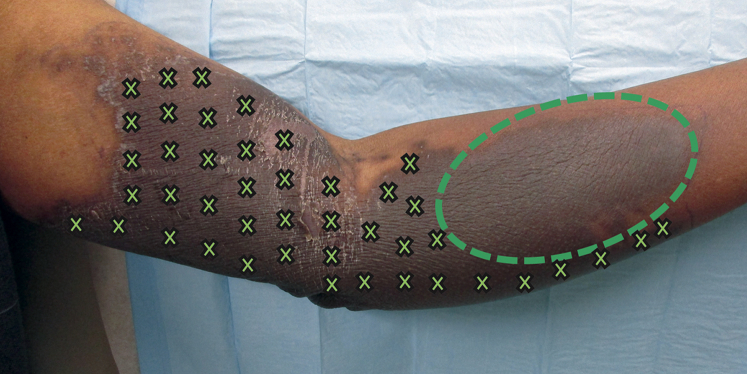
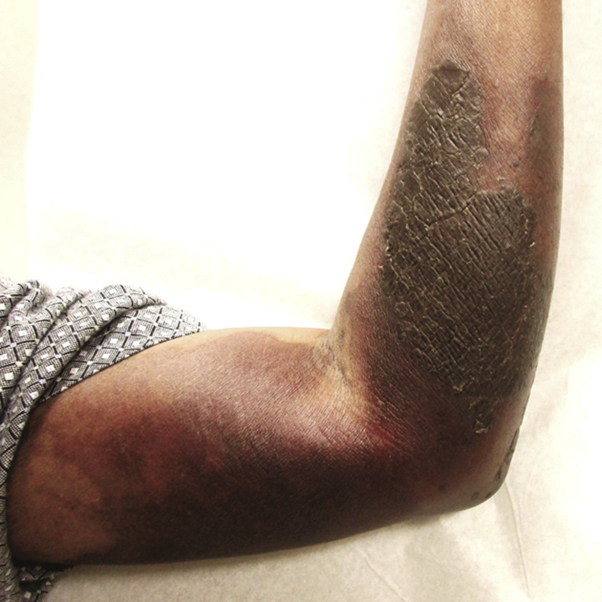
The involved area was injected intradermally in a gridlike pattern with 1.5 mL of triamcinolone 3 mg/mL suspension (0.3 mL of 10 mg/mL triamcinolone mixed with 0.7 mL of 1% lidocaine with epinephrine 1:100,000). Each site was injected with 0.05 mL of triamcinolone spaced by approximately 1 to 2 cm (Fig 3). With patient consent, the distal portion of the plaque was left untreated as a control to compare the natural resolution of extravasation versus treated areas. No topical agents were applied. Three weeks later, the treated areas showed only postinflammatory hyperpigmentation. The area treated decreased in size, and the patient reported less pain in treated areas. A roughly ovoid, brown, ashy, indurated plaque remained in the area that was not treated (Fig 4). Therefore, the remainder of the plaque was injected with triamcinolone and the patient was started on clobetasol ointment 0.05% twice daily. The patient's symptoms continued to improve, and the plaque resolved over the next 3 weeks, leaving only postinflammatory hyperpigmentation and mild parasthesias (Fig 5).
Fig 3.
Intralesional steroid approach. The x represents areas of injection. The oval represents the untreated area.
Fig 4.
Resolution of the proximal affected skin 3 weeks after treatment with distal untreated area largely unchanged.
Fig 5.
Three weeks after final treatment of distal region with intralesional steroids and treatment of entire region with topical clobetasol ointment. No induration or firmness with only postinflammatory hyperpigmentation on examination.
Discussion
Cutaneous findings
Docetaxel is a taxane class antineoplastic agent approved to treat advanced prostate cancer, breast cancer, ovarian cancer, non–small cell lung cancer, gastric cancer, and head and neck cancer.4 Docetaxel induces apoptosis of mitotically active cells by stabilizing microtubules and arresting the cell cycle in the G2/M phases.4 Cutaneous reactions associated with docetaxel include morbilliform eruption, acral erythema, fixed erythrodysesthesia plaques, nail changes (eg, onycholysis, melanonychia, onychomadesis, subungual hemorrhage, beau lines, and paronychia), alopecia, mucositis, sclerodermalike changes, recall dermatitis, drug-induced systemic lupus erythematous, and accidental extravasation.5
Extravasation
Extravasation of systemic infusional chemotherapies has an incidence of approximately 0.1% to 6%.1 Docetaxel is a chemical irritant inducing reactive inflammation and shedding of the skin without causing underlying tissue necrosis.1 Case reports have associated blistering with docetaxel extravasations suggesting some vesicantlike properties of the taxanes.6 Docetaxel extravasation results in erythema, edema, pain, and rare vesicle formation that occurs days to weeks after extravasation. This initial response is followed by desquamation that occurs approximately 3 to 6 weeks after presentation, ultimately resulting in residual hyperpigmentation.6 Extravasation seems to be self-limited with most patients experiencing few long-term side effects, as in the case of our patient.6 Rarely reported, histopathologic changes of docetaxel extravasation include epidermal dysmaturation, dyskeratotic keratocytes, vacuolar interface dermatitis, and eccrine squamous syringometaplasia.2, 7
Management and conclusions
Guidelines for management of extravasation of chemotherapy commonly suggest conservative measures to include elevation, compression, ice/heat, analgesia, topical or systemic antibiotics, and topical or systemic steroids. Other anecdotal treatments include injection of normal saline to dilute extravasated drugs, aspiration of chemotherapy through catheter at time of infusion, hyaluronidase, or topical application of dimethylsulfoxide.1, 2
There are limited studies of intralesional steroids used for treatment of extravasation. Published reports have not studied docetaxel but other vesicant-type chemotherapy extravasation associated with tissue necrosis or bullae. A single-arm clinical study with 53 patients showed that intralesional injections within 90 minutes of extravasation with hydrocortisone (10 mL of 50 mg/mL) followed by topical betamethasone prevented tissue necrosis or sloughing in vesicant extravasation.8 An argument could be made for dilutional effect early in extravasation, preventing damage to the skin. However, in our case, the time course of 3 weeks after extravasation likely precludes a dilutional effect, as the damage has already occurred. In a retrospective series of 175 cases of vesicant extravasation, up to 46% of patients receiving intralesional hydrocortisone required surgical debridement compared with 13% without steroid injections.9 However, in this study, the only indication for surgical debridement was pain at the site of extravasation, which likely artificially increased the number of patients requiring debridement. Furthermore, it is argued that vesicant chemotherapy does not show inflammation histologically, which does not support use of intralesional steroids.10 The above studies only used weak intralesional steroids like hydrocortisone and did not include taxanes. Additionally, previous reports of the histopathology of docetaxel extravasation found basilar vacuolar change, which might explain the beneficial response to corticosteroids.7 Our patient's specimen did not show interface dermatitis but did show basilar nuclei swelling, perhaps because of the patient's delayed presentation. Regardless, the treatment seemed effective and hastened resolution of the lesion and warrants further investigation of intralesional corticosteroids as an alternative treatment for taxane extravasation.
This single case is insufficient to determine if intralesional injections altered the course of the disease and are not generalizable to other chemotherapeutic classes. However, the patient's prompt symptomatic improvement and resolution of the plaque in all areas that were injected support the possibility that this treatment is efficacious. Instead, this case highlights the importance of performing prospective studies comparing intralesional steroids with other modalities in the management of taxane or irritant/exfoliative extravasation injuries.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
The views expressed in this article are those of the authors and do not reflect the official policy of the Department of Defense or US Government.
References
- 1.Kreidieh F.Y., Moukadem H.A., Saghir N.S. Overview, prevention and management of chemotherapy extravasation. World J Clin Oncol. 2016;7(1):87–97. doi: 10.5306/wjco.v7.i1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallo E., Llamas-Velasco M., Navarro R. Eccrine squamous syringometaplasia secondary to cutaneous extravasation of docetaxel: report of three cases. J Cutan Pathol. 2013;40(3):326–329. doi: 10.1111/cup.12041. [DOI] [PubMed] [Google Scholar]
- 3.Sweeney C.J., Chen Y.H., Carducci M. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737–746. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Docetaxel [prescribing information] Sanofi-aventis U.S. LLC; Bridgewater, NJ: 2010. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020449s059lbl.pdfhttp://pi.lilly.com/us/byetta-pi.pdf Available from: URL: Accessed July 17, 2016. [Google Scholar]
- 5.Reyes-Habito C.M., Roh E.K. Cutaneous reactions to chemotherapeutic drugs and targeted therapy for cancer: Part II. Targeted therapy. J Am Acad Dermatol. 2014;71(2):217. doi: 10.1016/j.jaad.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Ascherman J.A., Knowles S.L., Attkiss K. Docetaxel (taxotere) extravasation: a report of five cases with treatment recommendations. Ann Plast Surg. 2000;45(4):438–441. doi: 10.1097/00000637-200045040-00016. [DOI] [PubMed] [Google Scholar]
- 7.Ley B.D., Millán G.G., Perez J.S. Docetaxel recall phenomenon at the site of previous drug extravasation. Arch Dermatol. 2010;146(10):1190–1191. doi: 10.1001/archdermatol.2010.291. [DOI] [PubMed] [Google Scholar]
- 8.Tsavaris N.B., Karagiaouris P., Tzannou I. Conservative approach to the treatment of chemotherapy-induced extravasation. J Dermatol Surg Oncol. 1990;16(6):519–522. doi: 10.1111/j.1524-4725.1990.tb00073.x. [DOI] [PubMed] [Google Scholar]
- 9.Larson D.L. What is the appropriate management of tissue extravasation by antitumor agents? Plast Reconstr Surg. 1985;75(3):397–405. doi: 10.1097/00006534-198503000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Dorr R.T. Antidotes to vesicant chemotherapy extravasations. Blood Rev. 1990;4(1):41–60. doi: 10.1016/0268-960x(90)90015-k. [DOI] [PubMed] [Google Scholar]