Abstract
Background
Systemic sclerosis (SSc) is an autoimmune disease involving the skin and several internal organs. Most therapies available for this disease are symptomatic. Given the difficulty in treating SSc, we conducted this study to investigate the effect of combined plasmapheresis (PE) and allogeneic mesenchymal stem cells transplantation (MSCT) therapy on SSc.
Methods
Fourteen patients underwent three repeated PE treatments with subsequent pulse cyclophosphamide on days 1, 3 and 5. Patients received a single MSCT (1 × 106 cells/kg of body weight) on day 8. During follow up, evaluations performed included complete physical examination, serologic testing, and organ function.
Results
The mean modified Rodnan skin score (MRSS) improved from 20.1 ± 3.1 to 13.8 ± 10.2 (P < 0.001) at 12 months of follow up. Three patients had interstitial lung disease, all had improvement of lung function and improved computed tomography (CT) images after 12 months of combined therapy. This combined treatment also significantly decreased the anti-Scl70 autoantibody titer and serum transforming growth factor-β and vascular endothelial growth factor levels during follow up.
Conclusion
The results indicate that PE combined with MSCT is a feasible treatment associated with possible clinical benefit for SSc patients.
Trial registration
ClinicalTrials.gov, NCT00962923. Registered on 19 August 2009.
Keywords: Systemic sclerosis (SSc), Plasmapheresis (PE), Mesenchymal stem cells (MSCs)
Background
Systemic sclerosis (SSc) is a rare chronic autoimmune disease characterized by increased synthesis and deposition of extra-cellular matrix in skin and various internal organs. Skin involvement, important in diagnosis and classification of SSc, is an almost universal feature of SSc [1]. Depending on the extent of skin fibrosis, SSc can be classified as two main subtypes, the limited cutaneous form (lcSSc) and the diffuse cutaneous form (dcSSc). The extensive degree of skin involvement coincides with future severe internal organ manifestations, poor prognosis and mortality, at least in the early phase of dcSSc [2]. Although skin disease does not directly threaten life, the thick skin can lead to psychological stress and thus worsens the quality of life.
Many inflammatory cytokines and growth factors are associated with the onset and progression of fibrosis, such as transforming growth factor (TGF)-β, endothelin-1, interleukin (IL)-17, IL-23 and tumor necrosis factor (TNF)-α [3–6]. In general, skin disease in SSc patients is treated with immunosuppressive agents such as methotrexate (MTX) or mycophenolate mofetil (MMF) [7, 8], but are not acceptable to all Chinese patients in terms of side effects and cost. There are also various novel therapies focusing on skin disease, including anti-inflammatory immunosuppressive agents such as imatinib or rituximab, and extracorporeal shock waves [9–12]. However, assessment of their effects remains inconclusive. A few studies on plasmapheresis (PE) for treatment of SSc have demonstrated improvement in the modified Rodnan Skin Score (MRSS), decreased level of cytokines, soluble adhesion molecules and immunolaboratory markers after treatment [13–15]. However, patients often received three repeated PE treatments every 2–3 months in these studies as a high frequency of PE would increase the risk of infection due to allogeneic blood transfusion. Thus, newer therapies are needed with enhanced efficacy and less toxicity in the treatment of skin disease in SSc.
Mesenchymal stem cells (MSCs) are a subset of multipotent adult somatic stem cells that have the ability to undergo self-renewal, proliferation and pluripotent differentiation. They can be obtained from different sources such as bone marrow, umbilical cord, and adipose tissue in the human body [16–19]. Besides their multi-lineage differentiation potential [20–22], MSCs also harbor immunosuppressive activities owing to their paracrine effects and interaction with different immune cells [23–26], and limited immunogenicity with low human leukocyte antigen (HLA) I and no HLA II expression [27]. These properties of MSCs have offered a new strategy in the treatment of numerous autoimmune inflammatory diseases and demonstrated promising results in safety and efficacy. To date, MSC transplantation (MSCT) has been proved in our center to be effective in the treatment of refractory systemic lupus erythematosus, rheumatoid arthritis and inflammatory bowel disease [28–31].
Recently studies have shown deficiency of MSCs in SSc patients. Compared with healthy controls, bone marrow MSCs (BM-MSCs) isolated from SSc patients display a more mature and myofibroblast-like phenotype, which re-programs these cells toward pro-angiogenic behavior [32]. MSCs from SSc patients also exhibit abnormal functional activities, such as increased expression of TGF-β and vascular endothelial growth factor (VEGF), and impairment of endothelial cell differentiation, which may play critical roles during the development of fibrosis in SSc [33, 34]. Based on these findings, allogeneic MSCT appears a promising therapy for SSc. Indeed, one animal experiment has shown that MSCT results in lower expression of fibrotic markers in both skin and lung, and decreased levels of anti-topoisomerase (anti-scl70) autoantibodies, suggesting systemic effect of MSCs [35]. Our previous pilot study in five SSc patients also showed that MSCT results in decreased anti-nuclear antibody (ANA) titer, and improvement in the MRSS and Health Assessment Questionnaire (HAQ) [36]. Thus, we aimed to evaluate the effectiveness and safety of combination therapy with PE and MSCT for treatment of SSc on the basis of their different mechanisms in treating SSc patients.
Methods
Patient eligibility
Fourteen patients were recruited after approval by the Ethics Committee of Drum Tower Hospital. All patients provided signed, written, informed consent. Inclusion criteria were age 18–70 years, diagnosed as dcSSc according to 1980 American College of Rheumatology and/or the LeRoy and Medsger criteria. Exclusion criteria were: (1) pregnancy and lactation period; (2) heart failure and ventricular arrhythmia; (3) human immunodeficiency virus or hepatitis C virus seropositivity; (4) serum hepatitis B virus DNA of more than 10,000 copies/ml in patients with positive hepatitis B surface antigen; (5) presence of active untreated infectious disease; (6) presence of hepatic, portal or splenic vein thrombosis on ultrasonography; (7) presence of severe comorbid diseases (e.g., severe respiratory or cardiac disease), or presence of any type of malignancy.
MSC culturing
The MSCs were obtained from the umbilical cord (UC). The UC-derived MSCs were prepared by the Stem Cell Center of Jiangsu Province (Jiangsu Beike Bio-Technology, Taizhou, Jiangsu). Fresh UCs was obtained from informed healthy mothers in a local maternity hospital after normal deliveries. The UCs were rinsed twice in PBS consisting of penicillin and streptomycin to remove the cord blood. Then the washed cords were cut into 1-mm2 pieces and floated in low-glucose DMEM containing FBS (Stemcell, Vancouver, Canada). The pieces of cord were subsequently incubated at 37 °C in a humidified atmosphere consisting of 5% CO2 in air. Non-adherent cells were removed by washing. The medium was replaced every 3 days after the initial plating. When well-developed colonies of fibroblast-like cells appeared after 10 days, the cultures were trypsinized and passaged into a new flask for further expansion. Flow cytometric analysis confirmed the cells expressed CD106, CD105, CD90, CD71, CD44, CD29, but not CD34, CD14, CD3 or CD45. The capacity of MSCs to differentiate along adipogenic and osteogenic lineages was evaluated as previously described [25]. MSCs at passage 3 with a purity of more than 95% were used.
PE and MSCT
Patients received three repeated PE treatments every two days on days 1, 3 and 5, with the removal and reinfusion of 800–1000 ml plasma at each time. Patients received an intravenous cyclophosphamide (CTX) regimen to inhibit B cell proliferation triggered by rapidly decreased autoantibodies and circulating immune complex. The total amount of CTX (1.0 g/m2 body surface area) was divided to use on the following day after each PE (0.4–0.6 g each time). On day 8, patients received single MSC infusion (1 × 106 cells/kg of body weight). Patients were discharged after at least 48 hours of observation post MSCT.
Follow up and outcome measurements
After MSCT, each patient returned for follow up at 1, 3, 6 and 12 months. At each follow-up visit, complete physical examination, serologic testing and organ function were performed. Skin thickening was measured using the MRSS score, which was performed by at least one experienced attending physician. Serum levels of anti-scl70 IgG and the changes in TGF-β, VEGF, interferon (IFN)-γ, IL-4 and IL-10 were measured by ELISA (R&D, USA).
Statistical analysis
GraphPad Prism 5.0 software was used for statistical analyses. Results were expressed as median (range). The paired or unpaired t test was used for statistical comparison of variables before and after treatment by GraphPad Prism 5.0 software. A level of P < 0.05 was considered statistically significant.
Results
Demographic findings
Fourteen patients with SSc underwent allogeneic MSCT; all of those were classified as having the diffuse cutaneous subsets of the disease. Their average age was 37.4 years (range 19–67). The average disease duration was 27 months (range 6–84). Table 1 displays patients’ demographics and drug regimens received at the time of MSCT. The mean follow-up period was 15.6 ± 4.3 months (range 7–21). Twelve patients were followed up for more than 12 months.
Table 1.
Clinical and demographic characteristics of the patients enrolled in the study
| Case | Age (years) | Sex | Duration (mo) | MRSS baseline | Organ involvement | Previous treatments | Maintain treatments | Infectious AE |
|---|---|---|---|---|---|---|---|---|
| 1 | 26 | M | 17 | 26 | P 15 mg/d + MTX 10 mg/w | P 5 mg/d + MTX 10 mg/w | Minor respiratory tract infection | |
| 2 | 21 | F | 24 | 23 | P10mg/d + penicillamine 0.375/d | No treatment | ||
| 3 | 25 | M | 6 | 19 | no treatment | No treatment | ||
| 4 | 35 | F | 30 | 17 | P 5 mg/d + penicillamine 0.375/d | No treatment | ||
| 5 | 28 | F | 84 | 21 | ILD acral ulcers | P 20 mg/d + CTX 0.4/2 w | P 10 mg/d + CTX 0.6/2 mo | Minor respiratory tract infection |
| 6 | 43 | F | 60 | 20 | Penicillamine 0.375/d | No treatment | ||
| 7 | 19 | F | 36 | 19 | P 10 mg/d + AZA100 mg/d | P 5 mg/d + AZA50 mg/d | Minor respiratory tract infection | |
| 8 | 56 | M | 40 | 15 | ILD | P 15 mg/d CTX 0.4/2 w | P 5 mg/d CTX 0.4/mo | Minor respiratory tract infection |
| 9 | 46 | F | 7 | 21 | P 10 mg/d + GTW | GTW | ||
| 10 | 30 | F | 42 | 18 | P10mg/d + MMF 0.75 BID | No treatment | Minor respiratory tract infection | |
| 11 | 38 | F | 12 | 20 | ILD dysphagia | P 20 mg/d + CTX 0.1 QD + GTW | P10mg + CTX 0.4/2 w + GTW | |
| 12 | 67 | F | 6 | 25 | P 10 mg/d + AZA 100 mg/d | P 5 mg/d + AZA 50 mg/d | Diarrhea | |
| 13 | 53 | F | 6 | 17 | P 5 mg/d + MMF 0.75 BID | P 5 mg/d | ||
| 14 | 36 | F | 6 | 21 | No treatment | No treatment |
Duration was from the first symptom of disease to the time receiving plasmapheresis (PE) + mesenchymal stem cell transplantation (MSCT). Skin MRSS modified Rodnan skin score, AE adverse events, ILD interstitial lung disease, P prednisone, CTX cyclophasphomide, MTX methotrexate, AZA azathioprine, GTW glycosides of Tripterygium wilfordi, MMF mycophenolate mofetil, BID twice daily
Modified Rodnan skin score
There was a significant improvement in the MRSS over the course of 1 year following the treatment. At 12 months of treatment, the mean MRSS improved from 20.1 ± 3.1 to 13.8 ± 10.2 (P < 0.0001) and the mean difference in MRSS was −6.2 points (95% CI −3.1 to −9.3). This change was not seen after 1 month of treatment, but was evident at 3 months, with a mean improvement of −3.3 points (−0.3 to −6.3; P < 0.5) and at 6 months with a mean improvement of −4.7 points (−1.7 to −7.7; P < 0.001) (Fig. 1).
Fig. 1.
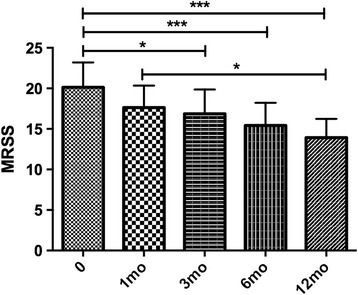
Evaluation of the modified Rodnan skin score (MRSS). At baseline the MRSS was 20.1 ± 3.1 (n = 14). After 1 month, the MRSS was 17.6 ± 2.7. After 3 months, the MRSS was 16.9 ± 3.0 (n = 14). After 6 months, the MRSS was 15.4 ± 2.8 (n = 14). After 12 months of treatment the mean MRSS was 13.9 ± 2.3 (n = 12). *P < 0.05,***P < 0.001
Non-skin fibrosis-related manifestations
Three of fourteen patients had interstitial lung disease (ILD); all patients had improvement in lung function after 12 months of combined therapy, with increased CO diffusing capacities (DLco) and forced vital capacity (FVC) (Fig. 2). Improved computed tomography (CT) images were also observed in these patients (Fig. 3). One of fourteen patients had an acral ulcer. Pain from the skin ulcer improved 1 month after combined therapy and the lesion size was reduced and healed 3 months after the treatment and did not recur till the last follow up. One of fourteen patients had dysphagia, which responded to the combined treatment during the whole follow up.
Fig. 2.
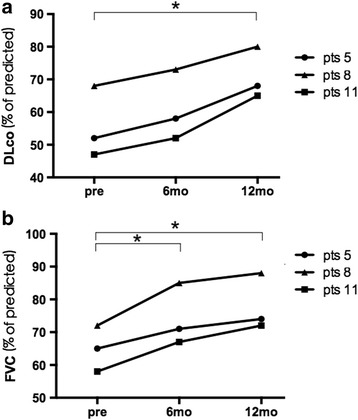
Evaluation of variables associated with interstitial lung disease (ILD) in three patents with systemic sclerosis before mesenchymal stem cell transplantation (MSCT), and at 6 months and 12 months after MSCT. a Diffusing capacity of the lung for carbon monoxide (DLco). b Forced vital capacity (FVC). *P < 0.05. Pts patients
Fig. 3.
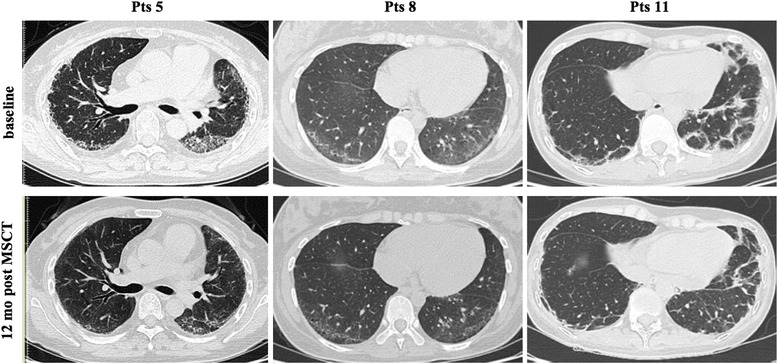
Pulmonary high-resolution computed tomography in patients with systemic sclerosis. Upper panel before mesenchymal stem cell transplantation (MSCT); lower panel 12 months after MSCT. Pts patients
Serology changes
This combined therapy significantly decreased serum anti-Scl70 autoantibody titer, TGF-β and VEGF levels (Fig. 4) during the follow up. There were no changes in the levels of IFN-γ, IL-4 or IL-10. The anti-Scl70 autoantibody titers decreased from 125.98 ± 91.13 RU/ml at baseline to 98.77 ± 88.46 RU/ml (P = 0.66, n = 7) at 1-month follow up; the titers were reduced to 66.91 ± 74.69 RU/ml (3-month follow up, P < 0.05, n = 7), 50.98 ± 71.39 RU/ml (6-month follow up, P < 0.05, n = 7) and 61.32 ± 52.68 RU/ml (12-month follow up, P < 0.01, n = 7), respectively. The serum TGF-β levels were decreased from 148.94 ± 79.85 ng/ml at baseline to 52.47 ± 21.98 ng/ml (1-month follow up, P < 0.05, n = 9), 45.94 ± 22.33 ng/ml (3-month follow up, P < 0.01, n = 9), 57.25 ± 40.56 ng/ml (6-month follow up, P < 0.05, n = 9) and 71.64 ± 58.20 ng/ml (12-month follow up, P = 0.0547, n = 9), respectively. The serum VEGF levels were decreased from 275.71 ± 108.15 pg/ml at baseline to 101.54 ± 69.88 pg/ml (1-month follow up, P < 0.01, n = 9), 75.84 ± 42.58 pg/ml (3-month follow up, P < 0.01, n = 9), 104.64 ± 56.6 pg/ml (6-month follow up, P < 0.01, n = 9) and 145.89 ± 88.20 pg/ml (12-month follow up, P = 0.1125, n = 9), respectively.
Fig. 4.
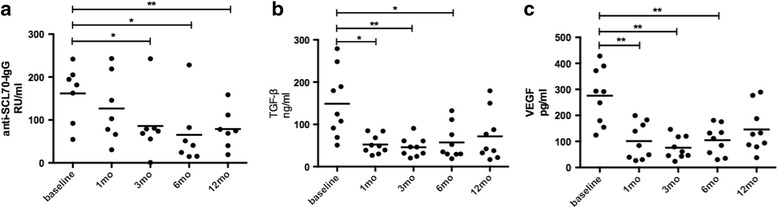
Serum anti-SCL70 IgG (a), transforming growth factor (TGF)-β (b) and vascular endothelial growth factor (VEGF) (c) levels in patients with systemic sclerosis were decreased after combined plasmapheresis and allogeneic mesenchymal stem cell transplantation therapy.*P < 0.05,**P < 0.01
Adverse events
No serious adverse events were observed during or immediately after PE and MSCT in any of the 14 patients. None of these patients developed graft versus host disease (GvHD) during follow up. Adverse events noted were upper respiratory tract infections reported by five patients and diarrhea reported by one patient during follow-up visits (Table 1). No serious infections occurred.
Discussion
Skin involvement is the hallmark of SSc; improvement in skin thickening may be useful as a surrogate for improvement in survival in clinical trials [37]. Therefore, the treating skin symptoms have been the focus of investigation in many clinical trials. Recent studies have assessed different options for the treatment of skin thickness; however, most of these therapies did not show significant efficacy [38]. Herein, we proposed allogeneic MSCT combined with PE as a potential therapy for the diffuse cutaneous form of SSc. In this study, of the total of 14 patients, 11 only had diffuse sclerosis and thickening of the skin without internal organ involvement, including two newly diagnosed patients who were not receiving any treatment before and after PE + MSCT; the other 9 patients received small doses of glucocorticoid in combination with immunosuppresive agents such as MTX or MMF, etc., which 3 patients gradually stopped taking after the combined therapy, and the other 6 cases also had reduced dosage of glucocorticoids and immunosuppressants. The results indicate this combined therapy has a possible benefit in improving MRSS and reducing inflammatory markers including anti-Scl70 autoantibody titer, TGF-β and VEGF levels.
Fibrosis is the final step and is the basis of most prominent clinical manifestations in SSc patients, including skin thickness and tightness [39]. Two fundamental biological processes contribute to the development of skin fibrosis, including vasculopathy with perivascular inflammation and coagulation activation, and fibroblast activation with the excess accumulation of extracellular matrix components. Multiple factors and signaling pathways are involved in the development or persistence of skin involvement in SSc, such as TGF-β, IL-4, IL-6, platelet-derived growth factor (PDGF), IL-1, IL-13, IL-17, IL-5, monocyte chemoattractant protein (MCP)-1, VEGF and connective tissue growth factor (CTGF) [6]. Recently, some experimental studies have revealed MSC deficiency in SSc. MSCs in SSc have a different phenotype from healthy controls [32]. BM-MSCs isolated from SSc patients have upregulation of α-smooth muscle actin (SMA) and smooth muscle (SM)22α genes and reduced proliferative activity, displaying a more mature and myofibroblast-like phenotype. Cipriani P et al. have observed that BM-MSCs from SSc patients have increased senescence biomarkers, through increased activation of the IL-6 pathway [40]. Furthermore, MSCs have been proved to not only have the properties of reduced inflammatory and fibrotic processes, but also have the ability to differentiate into endothelial cells [41]. These findings supported the hypothesis that MSCs from SSc patients are structurally and functionally defective, and provide basis for allogeneic MSCT as a potential therapy for SSc patients. Our results showed that MSCT combined with PE downregulated serum levels of TGF-β, which is a major cytokine involved in early angiogenesis and latent collagen production leading to fibrosis [42]. In addition, the combined therapy was shown to reduce the levels of VEGF, which was elevated in SSc patients and could stimulate angiogenesis [6]. We also noted lower levels of anti-Scl70 antibodies after the combined treatment, suggesting reduced B cell activation.
Some studies have suggested that MSCs can exhibit a protective effect on skin tightness and thickness [36, 43]. PE is also reported to be able to improve the Rodnan skin score in small case series of SSc patients [13]. These two therapies were taken into account for combination in view of their seemingly complementary characteristics. PE could quickly remove serum pro-inflammatory substances such as inflammatory cytokines, antibodies, immunoglobulins and complements, which play major roles in the immune responses against normal skin and fibrosis. MSC could secrete anti-inflammatory cytokines, anti-fibrotic factors and trophic molecules, and differentiate into epithelial cells, which will all promote regeneration of skin. Moreover, the self-renewal capacity of MSCs would make the therapeutic effects last a long time. However, it is difficult to tell which treatment is important in the resulting clinical and laboratory test improvements. Future randomized clinical trials with larger sample sizes and long-term follow up are required to further verify the results of this study.
In addition to improving the skin lesions, the data also suggest that the combined therapy can improve internal function in these patients. ILD is a common visceral lesion in SSc patients. For more than 15 years, CTX has commonly been used in the treatment of SSc-ILD. CTX is a cytotoxic immunosuppressive agent that suppresses lymphokine production and modulates lymphocyte function. In a landmark study, the Scleroderma Lung Study Research Group noted that CTX achieved a modest but significant beneficial effect on lung function and patients’ quality of life. After 12 months of therapy, FVC increased in 49.3% of the patients who received the CTX treatment. However, unfortunately, it also provoked a serious adverse event [44]. In our study, there were three patients with different degrees of ILD, all of whom had received glucocorticoids and CTX for at least 3 ~ 6 months before the combined therapy, but without improvement in the pulmonary symptoms. However, lung function and CT images in these three patients all improved significantly, after 12 months of combined therapy, FVC increased from 65.0 ± 7.0% to 81.7 ± 8.5%. We have gradually reduced the dosage of glucocorticoids and CTX to maintaining treatment. Compared with glucocorticoids and immunosuppressive agents, this combined therapy has shown a low side reaction and good safety; only a few patients had mild upper respiratory tract infections and diarrhea.
Our study has limitations. First, we were aware of the limitation of the small sample size of this study population, thus this is an exploratory analysis. The adequacy of sample size is important to clinical trials but depends on the availability of patients. A lower prevalence of SSc explained the sample size in this trial to some extent. Second, the uncontrolled study design (automatically also not blinded) may result in a substantial overestimation of therapeutic effect.
Conclusions
This study indicates that MSCT combined with PE is a feasible treatment associated with possible clinical benefit in SSc patients. The true value and safety will require much more robust data from a controlled trial and longer-term follow up in many more SSc patients.
Acknowledgements
We thank all the patients participating in this study.
Funding
This work was supported by the National Natural Science Foundation of China (number 81671608, 81202350, 81571586 and 81302559), Jiangsu Six Talent Peaks Project (2015-WSN-074), Jiangsu 333 High Level Talents Project, Jiangsu Government Scholarship for Overseas Studies, Jiangsu Health International Exchange Program sponsorship, Nanjing Young Medical Talents Project, Nanjing Health Bureau Key Project (ZKX15018) and Jiangsu Provincial Special Program of Medical Science (BE2015602).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Abbreviations
- ANA
Anti-nuclear antibody
- BM-MSCs
Bone marrow mesenchymal stem cells
- CT
Computed tomography
- CTGF
Connective tissue growth factor
- CTX
Cyclophosphamide
- dcSSc
Diffuse cutaneous systemic sclerosis
- DLco
Diffusing capacity of the lung for carbon monoxide
- DMEM
Dulbecco’s modified Eagle’s medium
- ELISA
Enzyme-linked immunosorbent assay
- FBS
Fetal bovine serum
- FVC
Forced vital capacity
- GvHD
Graft versus host disease
- HAQ
Health Assessment Questionnaire
- HLA
Human leukocyte antigen
- IL
Interleukin
- ILD
Interstitial lung disease
- IFN
Interferon
- lcSSc
Limited cutaneous systemic sclerosis
- MCP
Monocyte chemoattractant protein
- MMF
Mycophenolate mofetil
- MRSS
Modified Rodnan skin score
- MSCs
Mesenchymal stem cells
- MSCT
Mesenchymal stem cell transplantation
- MTX
Methotrexate
- PBS
Phosphate-buffered saline
- PDGF
Platelet-derived growth factor
- PE
Plasmapheresis
- scl70
Topoisomerase
- SM
Smooth muscle
- SMA
Smooth muscle actin
- SSc
Systemic sclerosis
- TGF
Transforming growth factor
- TNF
Tumor necrosis factor
- UC
Umbilical cord
- VEGF
Vascular endothelial growth factor
Authors’ contributions
HZ, JL and LS contributed to study design, data acquisition, data interpretation and drafting the manuscript. XT and DW analyzed the data and revised the manuscript. XF, FW, BH and HW recruited patients and revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Affiliated Drum Tower Hospital of Nanjing University Medical School in December 2008. All patients provided signed, written, informed consent.
Consent for publication
Written informed consent was obtained from the patients for publication of their anonymized data in this manuscript. No identifiable patient data are contained in this manuscript.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Huayong Zhang, Email: huayong.zhang@nju.edu.cn.
Jun Liang, Email: 13505193169@163.com.
Xiaojun Tang, Email: xjtang09@163.com.
Dandan Wang, Email: dandanwang2007@163.com.
Xuebing Feng, Email: xb.feng@163.com.
Fan Wang, Email: 270043834@qq.com.
Bingzhu Hua, Email: pinker68@163.com.
Hong Wang, Email: xyz6345@163.com.
Lingyun Sun, Phone: +86-25-68182422, Email: lingyunsun@nju.edu.cn.
References
- 1.Czirjak L, Foeldvari I, Muller-Ladner U. Skin involvement in systemic sclerosis. Rheumatology (Oxford) 2008;47(Suppl 5):v44–5. doi: 10.1093/rheumatology/ken309. [DOI] [PubMed] [Google Scholar]
- 2.Domsic RT, Rodriguez-Reyna T, Lucas M, Fertig N, Medsger TA., Jr Skin thickness progression rate: a predictor of mortality and early internal organ involvement in diffuse scleroderma. Ann Rheum Dis. 2011;70:104–9. doi: 10.1136/ard.2009.127621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cipriani P, Di Benedetto P, Ruscitti P, Verzella D, Fischietti M, Zazzeroni F, et al. Macitentan inhibits the transforming growth factor-beta profibrotic action, blocking the signaling mediated by the ETR/TbetaRI complex in systemic sclerosis dermal fibroblasts. Arthritis Res Ther. 2015;17:247. doi: 10.1186/s13075-015-0754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnett FC, Gourh P, Shete S, Ahn CW, Honey RE, Agarwal SK, et al. Major histocompatibility complex (MHC) class II alleles, haplotypes and epitopes which confer susceptibility or protection in systemic sclerosis: analyses in 1300 Caucasian, African-American and Hispanic cases and 1000 controls. Ann Rheum Dis. 2010;69:822–7. doi: 10.1136/ard.2009.111906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Czeslick EG, Simm A, Grond S, Silber RE, Sablotzki A. Inhibition of intracellular tumour necrosis factor (TNF)-alpha and interleukin (IL)-6 production in human monocytes by iloprost. Eur J Clin Invest. 2003;33:1013–7. doi: 10.1046/j.1365-2362.2003.01241.x. [DOI] [PubMed] [Google Scholar]
- 6.Pattanaik D, Brown M, Postlethwaite BC, Postlethwaite AE. Pathogenesis of systemic sclerosis. Front Immunol. 2015;6:272. doi: 10.3389/fimmu.2015.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson SR, Feldman BM, Pope JE, Tomlinson GA. Shifting our thinking about uncommon disease trials: the case of methotrexate in scleroderma. J Rheumatol. 2009;36:323–9. doi: 10.3899/jrheum.071169. [DOI] [PubMed] [Google Scholar]
- 8.Walker KM, Pope J, participating members of the Scleroderma Clinical Trials C, Canadian Scleroderma Research G Treatment of systemic sclerosis complications: what to use when first-line treatment fails − a consensus of systemic sclerosis experts. Semin Arthritis Rheum. 2012;42:42–55. doi: 10.1016/j.semarthrit.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Quillinan NP, McIntosh D, Vernes J, Haq S, Denton CP. Treatment of diffuse systemic sclerosis with hyperimmune caprine serum (AIMSPRO): a phase II double-blind placebo-controlled trial. Ann Rheum Dis. 2014;73:56–61. doi: 10.1136/annrheumdis-2013-203674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bosello SL, De Luca G, Rucco M, Berardi G, Falcione M, Danza FM, et al. Long-term efficacy of B cell depletion therapy on lung and skin involvement in diffuse systemic sclerosis. Semin Arthritis Rheum. 2015;44:428–36. doi: 10.1016/j.semarthrit.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Khanna D, Saggar R, Mayes MD, Abtin F, Clements PJ, Maranian P, et al. A one-year, phase I/IIa, open-label pilot trial of imatinib mesylate in the treatment of systemic sclerosis-associated active interstitial lung disease. Arthritis Rheum. 2011;63:3540–6. doi: 10.1002/art.30548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tinazzi E, Amelio E, Marangoni E, Guerra C, Puccetti A, Codella OM, et al. Effects of shock wave therapy in the skin of patients with progressive systemic sclerosis: a pilot study. Rheumatol Int. 2011;31:651–6. doi: 10.1007/s00296-009-1339-z. [DOI] [PubMed] [Google Scholar]
- 13.Szucs G, Szamosi S, Aleksza M, Veres K, Soltesz P. Plasmapheresis therapy in systemic sclerosis. Orv Hetil. 2003;144:2213–7. [PubMed] [Google Scholar]
- 14.Dau PC, Callahan JP. Immune modulation during treatment of systemic sclerosis with plasmapheresis and immunosuppressive drugs. Clin Immunol Immunopathol. 1994;70:159–65. doi: 10.1006/clin.1994.1024. [DOI] [PubMed] [Google Scholar]
- 15.Szekanecz Z, Aleksza M, Antal-Szalmas P, Soltesz P, Veres K, Szanto S, et al. Combined plasmapheresis and high-dose intravenous immunoglobulin treatment in systemic sclerosis for 12 months: follow-up of immunopathological and clinical effects. Clin Rheumatol. 2009;28:347–50. doi: 10.1007/s10067-008-1062-2. [DOI] [PubMed] [Google Scholar]
- 16.Guillot PV, Gotherstrom C, Chan J, Kurata H, Fisk NM. Human first-trimester fetal MSC express pluripotency markers and grow faster and have longer telomeres than adult MSC. Stem Cells. 2007;25:646–54. doi: 10.1634/stemcells.2006-0208. [DOI] [PubMed] [Google Scholar]
- 17.in ’t Anker PS, Noort WA, Scherjon SA, Kleijburg-van der Keur C, Kruisselbrink AB, van Bezooijen RL, et al. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica. 2003;88:845–52. [PubMed] [Google Scholar]
- 18.Chen PM, Yen ML, Liu KJ, Sytwu HK, Yen BL. Immunomodulatory properties of human adult and fetal multipotent mesenchymal stem cells. J Biomed Sci. 2011;18:49. doi: 10.1186/1423-0127-18-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trivanovic D, Kocic J, Mojsilovic S, Krstic A, Ilic V, Djordjevic IO, et al. Mesenchymal stem cells isolated from peripheral blood and umbilical cord Wharton’s jelly. Srp Arh Celok Lek. 2013;141:178–86. doi: 10.2298/SARH1304178T. [DOI] [PubMed] [Google Scholar]
- 20.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–9. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 21.Paul D, Samuel SM, Maulik N. Mesenchymal stem cell: present challenges and prospective cellular cardiomyoplasty approaches for myocardial regeneration. Antioxid Redox Signal. 2009;11:1841–55. doi: 10.1089/ars.2009.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ju S, Teng GJ, Lu H, Jin J, Zhang Y, Zhang A, et al. In vivo differentiation of magnetically labeled mesenchymal stem cells into hepatocytes for cell therapy to repair damaged liver. Invest Radiol. 2010;45:625–33. doi: 10.1097/RLI.0b013e3181ed55f4. [DOI] [PubMed] [Google Scholar]
- 23.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 24.Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24:74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 25.Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389–97. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 26.Ooi YY, Dheen ST, Tay SS. Paracrine effects of mesenchymal stem cells-conditioned medium on microglial cytokines expression and nitric oxide production. Neuroimmunomodulation. 2015;22:233–42. doi: 10.1159/000365483. [DOI] [PubMed] [Google Scholar]
- 27.Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12:383–96. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- 28.Liang J, Zhang H, Hua B, Wang H, Lu L, Shi S, et al. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: a pilot clinical study. Ann Rheum Dis. 2010;69:1423–9. doi: 10.1136/ard.2009.123463. [DOI] [PubMed] [Google Scholar]
- 29.Liang J, Li X, Zhang H, Wang D, Feng X, Wang H, et al. Allogeneic mesenchymal stem cells transplantation in patients with refractory RA. Clin Rheumatol. 2012;31:157–61. doi: 10.1007/s10067-011-1816-0. [DOI] [PubMed] [Google Scholar]
- 30.Xu J, Wang D, Liu D, Fan Z, Zhang H, Liu O, et al. Allogeneic mesenchymal stem cell treatment alleviates experimental and clinical Sjogren syndrome. Blood. 2012;120:3142–51. doi: 10.1182/blood-2011-11-391144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang J, Zhang H, Wang D, Feng X, Wang H, Hua B, et al. Allogeneic mesenchymal stem cell transplantation in seven patients with refractory inflammatory bowel disease. Gut. 2012;61:468–9. doi: 10.1136/gutjnl-2011-300083. [DOI] [PubMed] [Google Scholar]
- 32.Cipriani P, Marrelli A, Benedetto PD, Liakouli V, Carubbi F, Ruscitti P, et al. Scleroderma Mesenchymal Stem Cells display a different phenotype from healthy controls; implications for regenerative medicine. Angiogenesis. 2013;16:595–607. doi: 10.1007/s10456-013-9338-9. [DOI] [PubMed] [Google Scholar]
- 33.Vanneaux V, Farge-Bancel D, Lecourt S, Baraut J, Cras A, Jean-Louis F, et al. Expression of transforming growth factor beta receptor II in mesenchymal stem cells from systemic sclerosis patients. BMJ Open. 2013;3(1). doi: 10.1136/bmjopen-2012-001890. [DOI] [PMC free article] [PubMed]
- 34.Guiducci S, Manetti M, Romano E, Mazzanti B, Ceccarelli C, Dal Pozzo S, et al. Bone marrow-derived mesenchymal stem cells from early diffuse systemic sclerosis exhibit a paracrine machinery and stimulate angiogenesis in vitro. Ann Rheum Dis. 2011;70:2011–21. doi: 10.1136/ard.2011.150607. [DOI] [PubMed] [Google Scholar]
- 35.Maria AT, Toupet K, Bony C, Pirot N, Vozenin MC, Petit B, et al. Antifibrotic, antioxidant, and immunomodulatory effects of mesenchymal stem cells in HOCl-induced systemic sclerosis. Arthritis Rheumatol. 2016;68:1013–25. doi: 10.1002/art.39477. [DOI] [PubMed] [Google Scholar]
- 36.Akiyama K, Chen C, Wang D, Xu X, Qu C, Yamaza T, et al. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 2012;10:544–55. doi: 10.1016/j.stem.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steen VD, Medsger TA., Jr Improvement in skin thickening in systemic sclerosis associated with improved survival. Arthritis Rheum. 2001;44:2828–35. doi: 10.1002/1529-0131(200112)44:12<2828::AID-ART470>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 38.Khanna D, Denton CP. Evidence-based management of rapidly progressing systemic sclerosis. Best Pract Res Clin Rheumatol. 2010;24:387–400. doi: 10.1016/j.berh.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carrai V, Miniati I, Guiducci S, Capaccioli G, Alterini R, Saccardi R, et al. Evidence for reduced angiogenesis in bone marrow in SSc: immunohistochemistry and multiparametric computerized imaging analysis. Rheumatology (Oxford) 2012;51:1042–8. doi: 10.1093/rheumatology/ker447. [DOI] [PubMed] [Google Scholar]
- 40.Cipriani P, Di Benedetto P, Liakouli V, Del Papa B, Di Padova M, Di Ianni M, et al. Mesenchymal stem cells (MSCs) from scleroderma patients (SSc) preserve their immunomodulatory properties although senescent and normally induce T regulatory cells (Tregs) with a functional phenotype: implications for cellular-based therapy. Clin Exp Immunol. 2013;173:195–206. doi: 10.1111/cei.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doan CC, Le TL, Hoang NS, Doan NT, Le VD, Do MS. Differentiation of umbilical cord lining membrane-derived mesenchymal stem cells into endothelial-like cells. Iran Biomed J. 2014;18:67–75. doi: 10.6091/ibj.1261.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1:169–78. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 43.Scuderi N, Ceccarelli S, Onesti MG, Fioramonti P, Guidi C, Romano F, et al. Human adipose-derived stromal cells for cell-based therapies in the treatment of systemic sclerosis. Cell Transplant. 2013;22:779–95. doi: 10.3727/096368912X639017. [DOI] [PubMed] [Google Scholar]
- 44.Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354:2655–66. doi: 10.1056/NEJMoa055120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.


