Abstract
Irritable bowel syndrome (IBS) is a functional bowel disorder of unknown aetiology. There is currently no known cure, and pharmacological interventions are usually targeting symptomatic relief, where natural and herbal remedies also play a role. This study aimed to evaluate the benefit and tolerability of IQP‐CL‐101 in symptomatic IBS relief. A double‐blinded, randomised, placebo‐controlled trial was conducted over 8 weeks. A total of 99 subjects fulfilling ROME‐III criteria for IBS were randomised into two groups, given either two IQP‐CL‐101 softgels or matching placebo twice daily before main meals. The primary endpoint was the difference in change of IBS Symptom Severity Score (IBS‐SSS) after an 8‐week intake of IQP‐CL‐101 compared to placebo. After 8 weeks, subjects on IQP‐CL‐101 showed a significant reduction in IBS‐SSS (113.0 ± 64.9‐point reduction) compared to subjects on placebo (38.7 ± 64.5‐point reduction) (p < 0.001). A significant improvement could be seen as early as 4 weeks. No serious adverse events were reported throughout. IQP‐CL‐101 can be considered beneficial in the improvement of IBS symptom severity, regardless of IBS type, and therefore able to improve quality of life in patients suffering from abdominal pain and discomfort. © 2017 The Authors. Phytotherapy Research published by John Wiley & Sons Ltd.
Keywords: irritable bowel syndrome, IQP‐CL‐101, curcumin, Xanthofen
Introduction
Irritable bowel syndrome (IBS) is a functional bowel disorder with unknown aetiology. Irritable bowel syndrome patients suffer from a variety of symptoms such as abdominal pain/discomfort and bloating, constipation, diarrhoea or alternating symptoms of constipation and diarrhoea (Farthing, 2004; Ohman and Simren, 2007; Spiller and Gased, 2009). Worldwide prevalence of IBS is estimated to be around 11%, with highly variable numbers reported in different countries (Canavan et al., 2014). Both physical factors, such as low‐grade inflammation, and psychological factors have been implicated in causing and exacerbating symptoms of IBS (Barbara et al., 2002; Akiho et al., 2010).
Because IBS is a symptom‐based disorder, treatments focus on symptomatic relief; these include over‐the‐counter medications and pharmacological agents aiming to improve abdominal pain, cramping, bloating, diarrhoea and constipation. Nevertheless, the efficacy of pharmacological methods is variable, and placebo response has been shown to be high, ranging from 30% to 80% (Talley, 2003; Ford and Moayyedi, 2010). For many patients, IBS is a chronic and relapsing disease that significantly affects their health‐related quality of life. Frustrated by the inefficacy of conventional treatments, many patients have turned to complementary and alternative therapies (Hussain and Quigley, 2006).
To date, there is no known cure for IBS. However, there is increasing evidence that low‐grade inflammation may be a causative factor for IBS (Spiller and Major, 2016). A product that targets chronic low‐grade inflammation in the intestines besides providing symptomatic relief could hence be beneficial and improve treatment outcome in IBS patients.
IQP‐CL‐101 contains a proprietary mixture of curcuminoids and essential oils from different Curcuma species, besides fish oil, peppermint oil, caraway oil and vitamins B1, B9 and D3. Curcuminoids, related essential oils and fish oil have been known for their antiinflammatory effects (Maroon and Bost, 2006; Jurenka, 2009; Kunnumakkara et al., 2016), while peppermint oil and caraway oil provide antispasmodic effects (Grigoleit and Grioleit, 2005). This combination is hypothesised to improve quality of life of IBS sufferers by providing symptomatic relief of abdominal pain and discomfort while reducing low‐grade inflammation in the intestines.
Extracts from the rhizomes of Curcuma longa (syn. Curcuma domestica) are standardised to contain at least 90% curcuminoids—mainly curcumin—while essential oils are obtained by steam distillation from both C. longa and Curcuma xanthorrhiza. The proprietary mixture in a specific ratio contained in IQP‐CL‐101 was found to provide higher antiinflammatory properties in comparison to the individual constituents (InQpharm internal data).
The fish oil consists of oils from anchovy (Engraulis ringens), sardine (Sardinops sagax sagax) or mackerel (Trachurus symmetricus) or a combination thereof. It is standardised to 18% eicosapentaenoic acid and 12% docosahexaenoic acid. Fish oils are well known for their antiinflammatory properties (Maroon and Bost, 2006).
Peppermint oil is derived from the aerial parts of the peppermint plant (Mentha piperita) by steam distillation. Peppermint oil has been widely used as an herb and spice and has strong evidence for use as a natural and effective antispasmodic agent in relaxing smooth muscles (Grigoleit and Grioleit, 2005).
The major source of caraway essential oil is from the dried caraway fruit (Carum carvi) and contains 3–7% essential oil that consists mainly of carvone and limonene (ESCOP Monographs, 2003). The combination of peppermint oil and caraway oil has been demonstrated to be efficient in reducing dyspepsia, providing symptomatic relief of abdominal pains and discomfort (Freise and Kohler, 1999).
Although animal and human studies on the individual ingredients of IQP‐CL‐101 showed promising results for the treatment of IBS‐related symptoms, no study has yet been performed on the specific combination and dosage of IQP‐CL‐101. We therefore conducted this study to evaluate the efficacy of IQP‐CL‐101 in relieving IBS symptoms.
Material and Methods
Trial design
This double‐blinded, randomised, placebo‐controlled study was conducted at two study sites in Germany from January 2013 to February 2014. The study was conducted over 10 weeks including a 2‐week run‐in phase and an 8‐week treatment phase. Subjects were randomly allocated to either an IQP‐CL‐101 or placebo group in a 1:1 ratio, using a block size of four. Randomisation was carried out by an independent biostatistician using the randomisation scheme BiAS for Windows V9.2 (http://www.bias‐online.de).
The clinical investigation was approved by the ethics committee of Charité Universitätsmedizin Berlin and was performed in compliance with the Declaration of Helsinki and the Guideline for Good Clinical Practice (CPMP/ICH/135/95).
Each enteric coated IQP‐CL‐101 softgel contains 330 mg proprietary mixture of curcuminoids and essential oils from C. longa and C. xanthorrhiza, 70 mg fish oil, 15 mg peppermint oil and 8 mg caraway oil as well as 263 μg thiamine, 39 μg folic acid and 625 μg vitamin D3. The concentrations of the key ingredients were chosen based on prior research that provided evidence of efficacy. The placebo was identical to verum softgels in terms of size, shape, colour and opacity, with soybean oil replacing the active ingredients. Subjects were asked to consume two softgels of either the verum or placebo twice a day immediately before or with main meals for 8 weeks.
Eligible subjects included Caucasian men and women aged 18–70 years old, fulfilling the ROME‐III criteria for IBS diagnosis with an average worst daily abdominal pain/discomfort score of ≥4 and ≤8 on an 11‐point Likert scale during the run‐in period. Subjects had to refrain from any major lifestyle changes and had to stop using other IBS treatments for a washout period of 4 weeks and throughout the duration of the study. Women of childbearing potential were included with their agreement to use appropriate birth control.
Exclusion criteria included known sensitivities to any of the ingredients of IQP‐CL‐101; any clinically relevant abnormalities in colonoscopy within the last 2 years; measured faecal calprotectin levels of 30 mg/kg and above during the run‐in period; history of chronic liver, heart, pulmonary or renal disease; abnormal electrocardiogram; presence of faecal occult blood; nocturnal gastrointestinal (GI) symptoms; lactose intolerance; active GI infection; significant and unexplained weight loss within the past 6 months; active malignancy in the past 5 years; acute or chronic psychiatric diseases; pregnancy or nursing; use of medications that may influence GI functions within 1 month prior to randomisation; history of current abuse of drugs, alcohol or medication; inability to comply with study requirements; participation in other studies 30 days before enrolment; and excursion of safety parameters. Subjects were also excluded if their IBS symptoms had other GI causes.
Endpoints
Endpoints were clearly defined at the start of the study. The primary endpoint was the difference in the change of IBS Symptom Severity Score (IBS‐SSS) after an 8‐week intake of IQP‐CL‐101 from baseline in comparison to placebo intake for the same duration. The IBS‐SSS contains five questions measured on a 100‐point visual analogue scale: severity of abdominal pain, frequency of abdominal pain, severity of abdominal distension, dissatisfaction with bowel habits and interference with quality of life. The IBS‐SSS is a validated questionnaire for monitoring severity of IBS, which is reproducible and sensitive to change (Francis et al., 1997). The German version of the questionnaire used in the study was validated by Betz et al. (2013). Secondary endpoints included the difference in change of the IBS Global Improvement Scale (IBS‐GIS) and the IBS Quality of Life (IBS‐QOL) score between the IQP‐CL‐101 and placebo groups. The number of responders with ≥30% improvement of the mean score on the self‐reported abdominal pain/discomfort diary and a global assessment on the benefit rated by subjects and investigators on a 4‐point Likert scale were also included as secondary analysis.
Safety parameters
Safety assessments included measurements of vital signs (pulse rate and blood pressure) and clinical chemistry (full blood count and liver and renal function parameters), haematology and lipid profile at baseline and at the end of the study. Resting blood pressure was measured using standard devices. The subjects and investigators provided subjective ratings for the tolerability of the study medication at the end of the study. All adverse effects were recorded regardless of causality.
Compliance was checked through tablet counting and questioning of the subjects. Compliance was then defined as the percentage of actual deviation from the expected intake.
Statistical analyses
The sample size calculation for the primary endpoint was using assumptions based on results from two studies on IBS (Madisch et al., 2004; Merat et al., 2010). According to Madisch et al., a medium effect size of 0.625 was expected, which was confirmed by Merat et al. Based on two‐sample t‐tests, effect size, a significance level of 5% (two‐tailed), a power of 80% and a dropout rate of 10%, the calculated sample size was 50 subjects per group. The sample size was further confirmed in the course of the study through a blinded interim analysis, which did not allow for any conclusion on the study groups and therefore did not affect the global level of significance of the statistical analysis.
For the primary endpoint, the IBS‐SSS at baseline and at study end after 8 weeks was determined. The primary endpoint was analysed with the non‐parametric Mann–Whitney U‐test using the rank sums of the individual changes in IBS‐SSS. The influence of the baseline values was analysed with ANOVA.
All secondary endpoints and further endpoints were analysed with non‐parametric tests:
the Mann–Whitney U‐test for comparison of metric and ordinal variables between (sub)groups;
the Wilcoxon test for analyses of changes of metric and ordinal variables within (sub)groups; and
the chi‐square test for comparison of frequency distribution.
In case of small sample sizes or imbalance between groups, especially regarding subgroup analyses, exact tests were to be used.
For all statistical analyses, the level of significance (p < 0.05) was assumed. Analyses are based on intention‐to‐treat population (ITT), where subjects received at least one dose of the investigational product and data on benefit were available, especially if the subject discontinued before V3. All data were analysed using spss statistics software, version 19.0 (SPSS, Chicago, IL, USA).
Results
A total of 99 subjects were recruited into the study. All subjects complied with the treatment regimen during the run‐in phase and were randomised into the study. Ninety subjects were included in the ITT population, which consisted of 43 subjects from the IQP‐CL‐101 group and 47 subjects from the placebo group. Six subjects (four from the IQP‐CL‐101 group and two from the placebo group) were excluded from the ITT population because of violation of inclusion criteria during the run‐in period, and three subjects (two from the IQP‐CL‐101 group and one from the placebo group) were excluded as they had no further data other than baseline (Fig. 1). There was no statistically significant difference in compliance in the verum and placebo groups (−1.4% vs 0.4% deviation from expected intake, p = 0.317).
Figure 1.
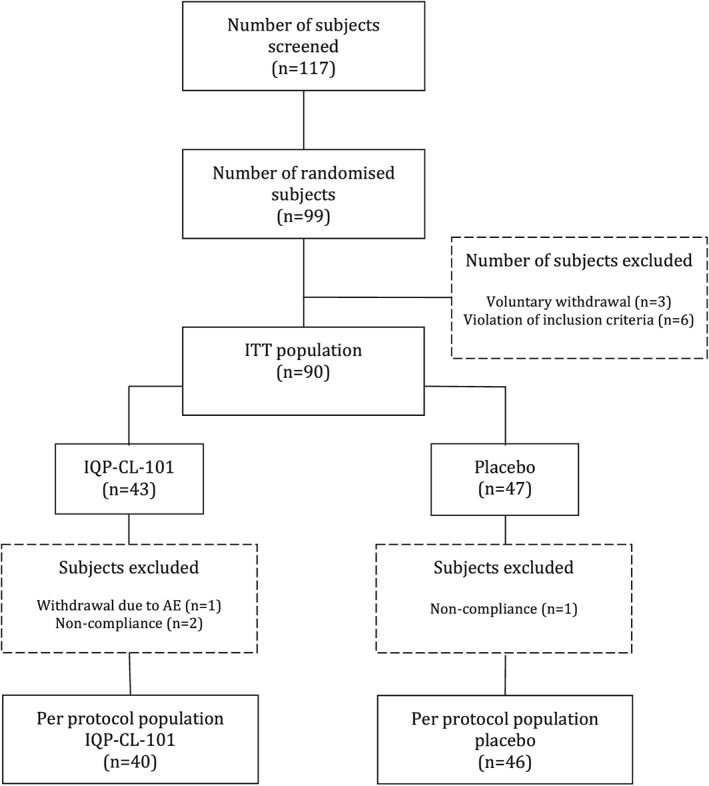
CONSORT flow of participants. ITT, intention to treat.
The baseline characteristics of the ITT population are shown in Table 1. There were no significant differences between both groups at baseline.
Table 1.
Baseline characteristics of intention‐to‐treat population
| Parameter | Intention‐to‐treat population (n = 90) | p‐value | |
|---|---|---|---|
| IQP‐CL‐101 (n = 43) | Placebo (n = 47) | ||
| Gender | |||
| Male | 12 (30%) | 15 (31.9%) | 0.679 |
| Female | 31 (70%) | 32 (68.1%) | |
| Age (years) | 44.0 (13.0) | 47.5 (14.8) | 0.192 |
| Weight (kg) | 73.3 (12.9) | 73.6 (15.2) | 0.747 |
| BMI (kg/m2) | 24.9 (4.4) | 25.3 (4.4) | 0.793 |
Numbers represent mean (SD) unless specified. BMI, body mass index.
Efficacy
At baseline, there was a statistically significant difference in IBS‐SSS between the IQP‐CL‐101 group and the placebo group (p = 0.015). The IQP‐CL‐101 group had a slightly higher score (315.3 ± 60.9) compared to the placebo group (291.6 ± 52.4) (Table 2).
Table 2.
IBS‐SSS scores during the study period
| IQP‐CL‐101 (n = 41) | Placebo (n = 42) | p‐value | |
|---|---|---|---|
| Baseline (V2) | 315.3 (60.9) | 291.6 (52.4) | 0.015 |
| Week 4 (V3) | 251.8 (74.1) | 261.7 (62.6) | 0.389 |
| Week 8 (V4) | 202.3 (71.3) | 252.9 (80.8) | 0.002 |
Numbers represent mean (SD).
A statistically significant difference in the change in IBS‐SSS was seen as early as week 4 between the IQP‐CL‐101 (63.5 ± 62.3‐point reduction) and placebo groups (29.9 ± 49.3‐point reduction) (p = 0.001) (Table 3).
Table 3.
Change in Irritable Bowel Syndrome Severity Scoring System (IBS‐SSS) during the study period
| Change in IBS‐SSS (reduction) | |||
|---|---|---|---|
| IQP‐CL‐101 (n = 41) | Placebo (n = 42) | p‐value | |
| After 4 weeks (V2–V3) | 63.5 (62.3) | 29.9 (49.3) | 0.001 |
| After 8 weeks (V2–V4) | 113.0 (64.9) | 38.7 (64.5) | <0.001 |
Numbers represent mean (SD).
After 8 weeks, subjects on IQP‐CL‐101 showed a significant reduction in IBS‐SSS (113.0 ± 64.9‐point reduction) compared to subjects on placebo (38.7 ± 64.5‐point reduction) (p < 0.001), despite having a significantly higher IBS‐SSS at baseline (Table 3).
Considering the baseline value as covariate, there was a statistically significant difference between the IQP‐CL‐101 and placebo groups regarding the changes in IBS‐SSS from week 8 to baseline (p < 0.001), and the influence of the baseline value was not statistically significant.
Post hoc analysis on IBS‐SSS responders revealed that 63.4% of subjects in the IQP‐CL‐101 group experienced at least 30% reduction in IBS‐SSS from baseline to end of study compared to only 21.4% of subjects from the placebo group. This difference in the frequency distribution of responders was statistically significant (p < 0.001) (Table 4).
Table 4.
Frequency distribution of Irritable Bowel Syndrome Severity Scoring System (IBS‐SSS) responders
| Responder to IBS‐SSS | IQP‐CL‐101 (n = 41) | Placebo (n = 42) | ||
|---|---|---|---|---|
| Number | Percentage | Number | Percentage | |
| Increased/unchanged | 2 | 4.9 | 15 | 35.7 |
| <30% decreased | 13 | 31.7 | 18 | 42.9 |
| ≥30% and <50% decreased | 18 | 43.9 | 4 | 9.5 |
| ≥50% decreased | 8 | 19.5 | 5 | 11.9 |
| p‐value | <0.001 | |||
Irritable Bowel Syndrome Global Improvement Score
At baseline, there was no statistically significant difference between the IQP‐CL‐101 and placebo groups in the frequency distribution of IBS‐GIS (p = 0.658).
The IBS‐GIS at the end of study (week 8) significantly differed in the IQP‐CL‐101 group compared to that in the placebo group (p < 0.001). Of the subjects in the IQP‐CL‐101 group, 53.5% experienced at least a moderate improvement of symptoms (rated 6 and above), compared to only 21.3% of subjects in the placebo group (Table 5).
Table 5.
Frequency distribution of Irritable Bowel Syndrome Global Improvement Scale (IBS‐GIS) at week 8
| IBS‐GIS rating | IQP‐CL‐101 (n = 43) | Placebo (n = 47) | ||
|---|---|---|---|---|
| Number | Percentage | Number | Percentage | |
| Substantially worse (1) | 0 | 0.0 | 0 | 0.0 |
| Moderately worse (2) | 1 | 2.3 | 0 | 0.0 |
| Slightly worse (3) | 0 | 0.0 | 0 | 0.0 |
| No change (4) | 4 | 9.3 | 26 | 55.3 |
| Slightly improved (5) | 15 | 34.9 | 11 | 23.4 |
| Moderately improved (6) | 11 | 25.6 | 8 | 17.0 |
| Substantially improved (7) | 12 | 27.9 | 2 | 4.3 |
| p‐value | <0.001 | |||
Irritable Bowel Syndrome Quality of Life
At baseline, there was no statistically significant difference in total score of IBS‐QOL between the IQP‐CL‐101 and placebo groups (p = 0.639).
The change in IBS‐QOL (total score) from baseline to end of study was statistically significant (p = 0.008) with 3.79 ± 2.93 points in the IQP‐CL‐101 group compared to 2.19 ± 3.25 points in the placebo group (Table 6). A positive change indicates improvement in quality of life.
Table 6.
Change in Irritable Bowel Syndrome Quality of Life during the study period
| IQP‐CL‐101 (n = 42) | Placebo (n = 47) | p‐value | |
|---|---|---|---|
| After 8 weeks | 3.79 (2.93) | 2.19 (3.25) | 0.008 |
Numbers represent mean (SD).
Pain and discomfort
Subjects with at least 30% reduction in abdominal pain
At the end of study, 64.3% of subjects in the IQP‐CL‐101 group showed a reduction in abdominal pain of at least 30% compared to 31.9% in the placebo group. This difference was statistically significant (p = 0.002) (Fig. 2).
Figure 2.
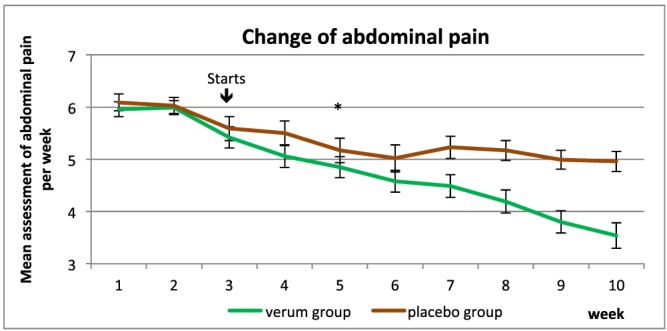
Mean abdominal pain during the study period. The asterisk signifies the first point of significance. [Colour figure can be viewed at wileyonlinelibrary.com]
Post hoc analysis revealed that the mean weekly abdominal pain was already significantly lower in the IQP‐CL‐101 group compared to the placebo group from week 5 onwards.
Subjects with at least 30% reduction in abdominal discomfort
At the end of study, 59.5% of subjects in the IQP‐CL‐101 group showed a reduction in abdominal discomfort of at least 30% compared to 29.8% in the placebo group. This difference was statistically significant (p = 0.005) (Fig. 3).
Figure 3.
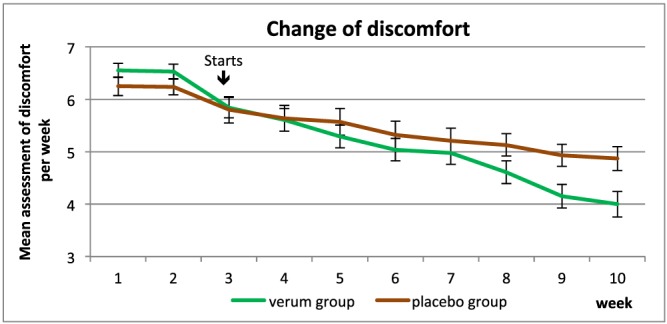
Mean abdominal discomfort during the study period. [Colour figure can be viewed at wileyonlinelibrary.com]
Post hoc analysis revealed that the mean weekly abdominal discomfort was already significantly lower in the IQP‐CL‐101 group compared to the placebo group from week 7 onwards.
Global assessment of benefit
Table 7 shows that 97.6% of subjects in the IQP‐CL‐101 group gave a rating of good or very good compared to only 12.8% of subjects in the placebo group. The difference was statistically significant (p < 0.001).
Table 7.
Global assessment of benefit by subject
| Global assessment | IQP‐CL‐101 (n = 41) | Placebo (n = 47) | ||
|---|---|---|---|---|
| Number | Percentage | Number | Percentage | |
| Very good | 17 | 41.5 | 0 | 0.0 |
| Good | 23 | 56.1 | 6 | 12.8 |
| Moderate | 1 | 2.4 | 24 | 51.1 |
| Poor | 0 | 0.0 | 17 | 36.2 |
| p‐value | <0.001 | |||
Investigators rated the benefit as good or very good for 97.6% of subjects in the IQP‐CL‐101 group and for only 17.0% of subjects in the placebo group. This difference was statistically significant (p < 0.001) (Table 8).
Table 8.
Global assessment of benefit by investigator (Intention‐to‐treat population)
| Global assessment | IQP‐CL‐101 (n = 41) | Placebo (n = 47) | ||
|---|---|---|---|---|
| Number | Percentage | Number | Percentage | |
| Very good | 17 | 41.5 | 0 | 0.0 |
| Good | 23 | 56.1 | 8 | 17.0 |
| Moderate | 1 | 2.4 | 21 | 44.7 |
| Poor | 0 | 0.0 | 18 | 38.3 |
| p‐value | <0.001 | |||
Discussion
Irritable bowel syndrome is a commonly diagnosed GI condition. It is mainly characterised by the presence of abdominal pain or discomfort, bloating and altered bowel habits, in the absence of any other diseases that may cause similar symptoms. Individuals with IBS can be grouped into three broad groups according to their bowel habits: constipation predominant, diarrhoea predominant or mixed constipation/diarrhoea (Schoenfeld, 2016).
There are no standard therapies for IBS, and IBS therapies are largely aimed at symptomatic control and relief. Treatments for diarrhoea‐predominant IBS include antidiarrhoeals, 5‐HT3 receptor antagonists and antispasmodics, while fibre supplements, laxative agents and prosecretory agents are used for constipation‐predominant IBS. Centrally acting interventions such as antidepressants are treatment options for patients with moderate to severe IBS (Chey et al., 2011). Additionally, modification of intestinal microbiota and use of herbal therapies have become widely accepted among patients with IBS owing to lack of long‐term efficacy of pharmacological agents (Hussain and Quigley, 2006).
Irritable bowel syndrome is a chronic disease that requires the sufferers to seek therapy throughout their lives. Although this study was only conducted over 8 weeks, which is a relatively short duration, the results showed that subjects on IQP‐CL‐101 achieved a statistically significant reduction in IBS‐SSS (113.0 ± 64.9‐point reduction) compared to subjects on placebo (38.7 ± 64.5‐point reduction) (p < 0.001). More importantly, these results showed that severity of IBS for patients on IQP‐CL‐101 has improved from a mean of 313.6 (SD 61.9) to 198.6 (SD 70.6) and such improvement is not due to placebo effect. As established in previous studies, IBS‐SSSs were correlated with clinical manifestation of the disease, where scores above 300 (of a maximum of 500) represent severe IBS, scores of 175–300 moderate severity and scores below 175 mild IBS (Francis et al., 1997). The efficacy of IQP‐CL‐101 to alleviate IBS symptoms is also supported by the positive changes in secondary endpoints measured, such as IBS‐GIS, IBS‐QOL, self‐reported abdominal pain and discomfort diary.
Various herbal remedies have been employed to relieve the suffering of patients with IBS, including the individual ingredients in our investigational product (Holtmann and Talley, 2015; Currò et al., 2016). The main challenges faced in using herbal and complementary therapies are often the lack of understanding of the actives and their interaction, the lack of standardisation of such actives in the extracts or preparations and especially the lack of evidence from well‐designed clinical studies (Izzo et al., 2016). A Cochrane systematic review in the use of herbal medicines in IBS showed a significant number of trials conducted, but majority of trials were of low methodological quality (Liu et al., 2006). As different diagnostic tools were used in the trials, it is also difficult to compare the efficacy of respective therapies. In a recent systematic review, Currò et al. (2016) also concluded that more confirmatory studies are required to provide more evidence on the efficacy of herbal supplements in IBS. In the present study, we provide evidence of efficacy and safety for a standardised herbal combination product. We have also shown that IQP‐CL‐101 was well tolerated regardless of the type of IBS the subjects were suffering from.
Limitations
The study reports result from an 8‐week treatment, which does not provide indication of efficacy in the longer term, particularly when IBS symptoms seem to vary over time (Chey et al., 2011). Although the chosen endpoints of IBS‐SSS were acceptable at the time the trial was conducted, a new set of guidelines for designing treatment trials for functional GI disorders had been released (Irvine et al., 2016), where the current recommended outcome measure is a composite endpoint that takes into consideration both the pain severity and bowel habits. There was also no post‐trial follow‐up on subjects, and thus, it is not known if relapse of IBS symptoms occurred. The study was also not sufficiently powered to identify if IQP‐CL‐101 shows better efficacy towards different types of IBS, that is, constipation, diarrhoea or mixed type. Future research on IQP‐CL‐101 should be conducted according to current guidelines. It would also be beneficial to identify if IQP‐CL‐101 would be more effective towards any subtype of IBS.
Conclusion
In conclusion, IQP‐CL‐101 can be considered as beneficial in the improvement of IBS symptom severity, regardless of IBS type as early as 4 weeks after the start of the treatment, and is at the same time able to improve the quality of life in patients suffering from abdominal pain and discomfort.
Funding
This study was supported by InQpharm Europe Ltd.
Conflict of Interest
Emily Teng is currently employed by InQpharm Europe Ltd. Pee‐Win Chong was an employee of InQpharm Europe Ltd at the time of the study.
Alt, F. , Chong, P.‐W. , Teng, E. , and Uebelhack, R. (2017) Evaluation of Benefit and Tolerability of IQP‐CL‐101 (Xanthofen) in the Symptomatic Improvement of Irritable Bowel Syndrome: A Double‐Blinded, Randomised, Placebo‐Controlled Clinical Trial. Phytother. Res., 31: 1056–1062. doi: 10.1002/ptr.5826.
Trial registration Clinicaltrials.gov no.: NCT01774825
References
- Akiho A, Ihara E, Nakamura K. 2010. Low‐grade inflammation plays a pivotal role in gastrointestinal dysfunction in irritable bowel syndrome. World J Gastrointest Pathophysiol 1(3): 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbara G, Giorgio E, Stanghellini V, Cremon C, Corinaldesi R. 2002. A role of inflammation in irritable bowel syndrome? Gut 51(Suppl 1): i41–i44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz C, Mannsdorfer K, Bischoff S. 2013. Validation of the IBS‐SSS. Z Gastroenterol 51(10): 1171–1176. [DOI] [PubMed] [Google Scholar]
- Canavan C, West J, Card T. 2014. The epidemiology of irritable bowel syndrome. Clin Epidemiol 6: 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chey W, Kurlander J, Eswaran S. 2011. Irritable bowel syndrome: a clinical review. JAMA 313(9): 949–958. [DOI] [PubMed] [Google Scholar]
- Currò D, Ianiro G, Pecere S, Bibbò S, Cammarota G. 2016. Probiotics, fibre and herbal medicinal products for functional and inflammatory bowel disorders. Br J Pharmacol https://doi.org/10.1111/bph.13632. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESCOP Monographs . 2003. Carvi fructus—Caraway Fruit, 2nd edn. Georg Thieme Verlag: Stuttgart, Germany. [Google Scholar]
- Farthing M. 2004. Treatment options in irritable bowel syndrome. Best Pract Res Clin Gastroenterol 18(4): 773–786. [DOI] [PubMed] [Google Scholar]
- Ford AC, Moayyedi P. 2010. Meta‐analysis: factors affecting placebo response rate in the irritable bowel syndrome. Aliment Pharmacol Ther 32(2): 144–158. [DOI] [PubMed] [Google Scholar]
- Francis C, Morris J, Whorwell P. 1997. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther 11(2): 395–402. [DOI] [PubMed] [Google Scholar]
- Freise J, Kohler S. 1999. Peppermint oil/caraway oil fixed combination in non‐ulcer dyspepsia. Equivalent efficacy of the drug combination in an enteric coated or enteric soluble formulation. Pharmazie 54(3): 210–215. [PubMed] [Google Scholar]
- Grigoleit H, Grioleit P. 2005. Pharmacology and preclinical pharmacokinetics of peppermint oil. Phytomedicine 12: 612–616. [DOI] [PubMed] [Google Scholar]
- Holtmann G, Talley NJ. 2015. Herbal medicines for the treatment of functional and inflammatory bowel disorders. Clin Gastroenterol Hepatol 13(3): 422–432. [DOI] [PubMed] [Google Scholar]
- Hussain Z, Quigley E. 2006. Systematic review: complementary and alternative medicine in the irritable bowel syndrome. Aliment Pharmacol Ther 23(4): 465–471. [DOI] [PubMed] [Google Scholar]
- Irvine EJ, Rakc J, Crowell MD, et al. 2016. Design of treatment trials for functional gastrointestinal disorders. Gastroenterology 150(6): 1469–1480.e1. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Hoon‐Kim S, Radhakrishnan R, Williamson EM. 2016. A critical approach to evaluating clinical efficacy, adverse events and drug interactions of herbal remedies. Phytother Res 30(5): 691–700. [DOI] [PubMed] [Google Scholar]
- Jurenka J. 2009. Anti‐inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Akterbatuve Neducube Rev 14(2): 141–153. [PubMed] [Google Scholar]
- Kunnumakkara AB, Bordoloi D, Padmavathi G, et al. 2016. Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. Br J Pharmacol Sept 7. https://doi.org/10.1111/bph.13621. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yang M, Liu X, Wei M, Grimsgaard S. 2006. Herbal medicines for treatment of irritable bowel syndrome. Cochrane Database Syst Rev 1: CD004116. [DOI] [PubMed] [Google Scholar]
- Madisch A, Holtmann G, Plein K, Hotz J. 2004. Treatment of irritable bowel syndrome with herbal preparations: results of a double‐blind, randomised, placebo‐controlled, multi‐centre trial. Aliment Pharmacol Ther 19(3): 271–279. [DOI] [PubMed] [Google Scholar]
- Maroon J, Bost J. 2006. Fish Oil: The Natural Anti‐inflammatory. Basic Health Publications, Inc.: California, USA. [Google Scholar]
- Merat S, Khalili S, Mostjabi P, et al. 2010. The effect of enteric‐coated, delayed‐release peppermint oil on irritable bowel syndrome. Dig Dis Sci 55(5): 1385–1390. [DOI] [PubMed] [Google Scholar]
- Ohman L, Simren M. 2007. New insights into the pathogenesis and pathophysiology of irritable bowel syndrome. Dig Liver Dis 39: 200–215. [DOI] [PubMed] [Google Scholar]
- Schoenfeld PS. 2016. Advances in IBS 2016: a review of current and emerging data. Gastroenterol Hepatol (NY) 12(8 Suppl 3): 1–11. [PMC free article] [PubMed] [Google Scholar]
- Spiller R, Gased K. 2009. Infection, inflammation, and the irritable bowel syndrome. Dig Liver Dis 41: 844–849. [DOI] [PubMed] [Google Scholar]
- Spiller R, Major G. 2016. IBS and IBD—separate entities or on a spectrum? Nat Rev Gastroenterol Hepatol 13(10): 613–621. [DOI] [PubMed] [Google Scholar]
- Talley NJ. 2003. Evaluation of drug treatment in irritable bowel syndrome. Br J Clin Pharmacol 56(4): 362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]


