Abstract
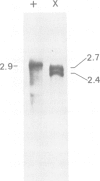
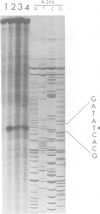
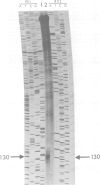
The regulatory gene areA mediating nitrogen metabolite repression in Aspergillus nidulans has been sequenced and its transcript mapped and orientated. A single ORF can encode a protein of 719 amino acids. A 52 amino acid region including a putative 'zinc finger' strongly resembles putative DNA binding regions of the major regulatory protein of erythroid cells. The derived protein sequence also contains a highly acidic region possibly involved in gene activation and 22 copies of the motif S(T)PXX, abundant in DNA binding proteins. Analysis of chromosomal rearrangements and transformation with deletion clones identified 342 N-terminal and 124 C-terminal residues as inessential and localized a C-terminal region required for nitrogen metabolite repressibility. A -1 frameshift eliminating the inessential 122 C-terminal amino acids is a surprising loss-of-function mutation. Extraordinary basicity of the replacement C terminus might explain its phenotype. Mutant sequencing also identified a polypeptide chain termination and several missense mutations, but most interesting are sequence changes associated with specificity mutations. A mutation elevating expression of some structural genes under areA control whilst reducing or not affecting expression of others is a leucine to valine change in the zinc finger loop. It reverts to a partly reciprocal phenotype by replacing the mutant valine by methionine.
Full text
PDF

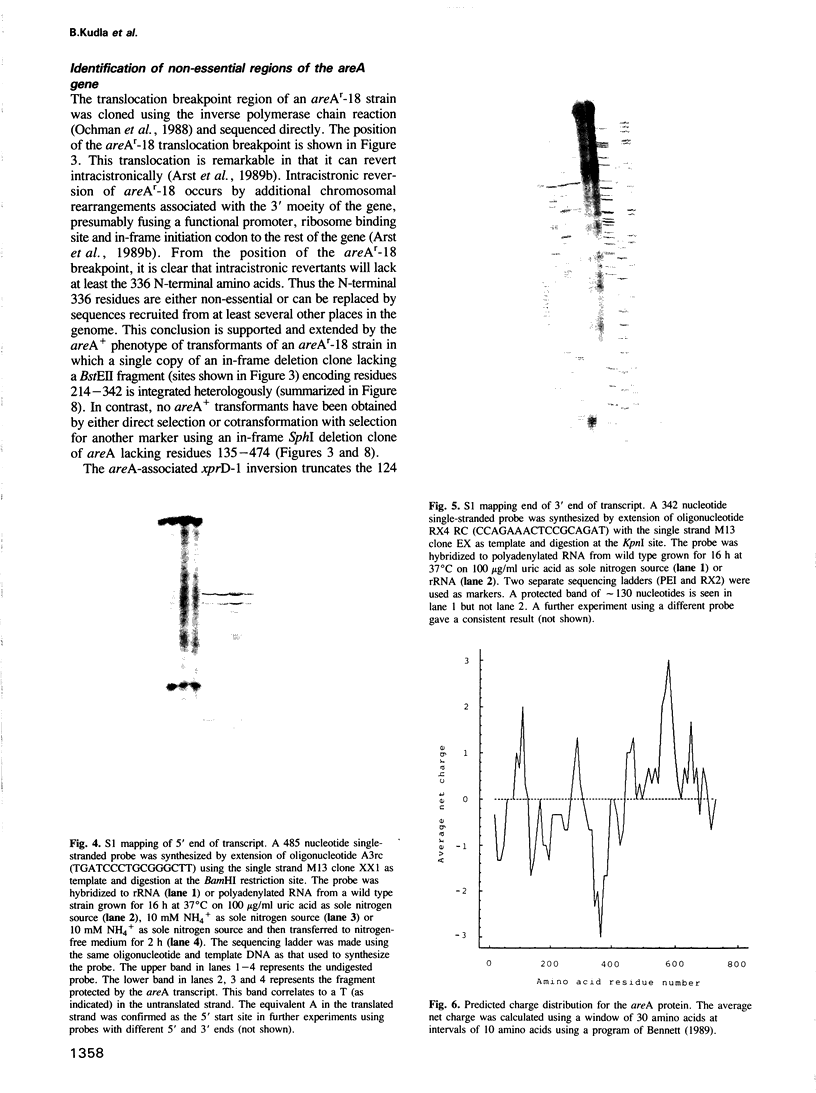
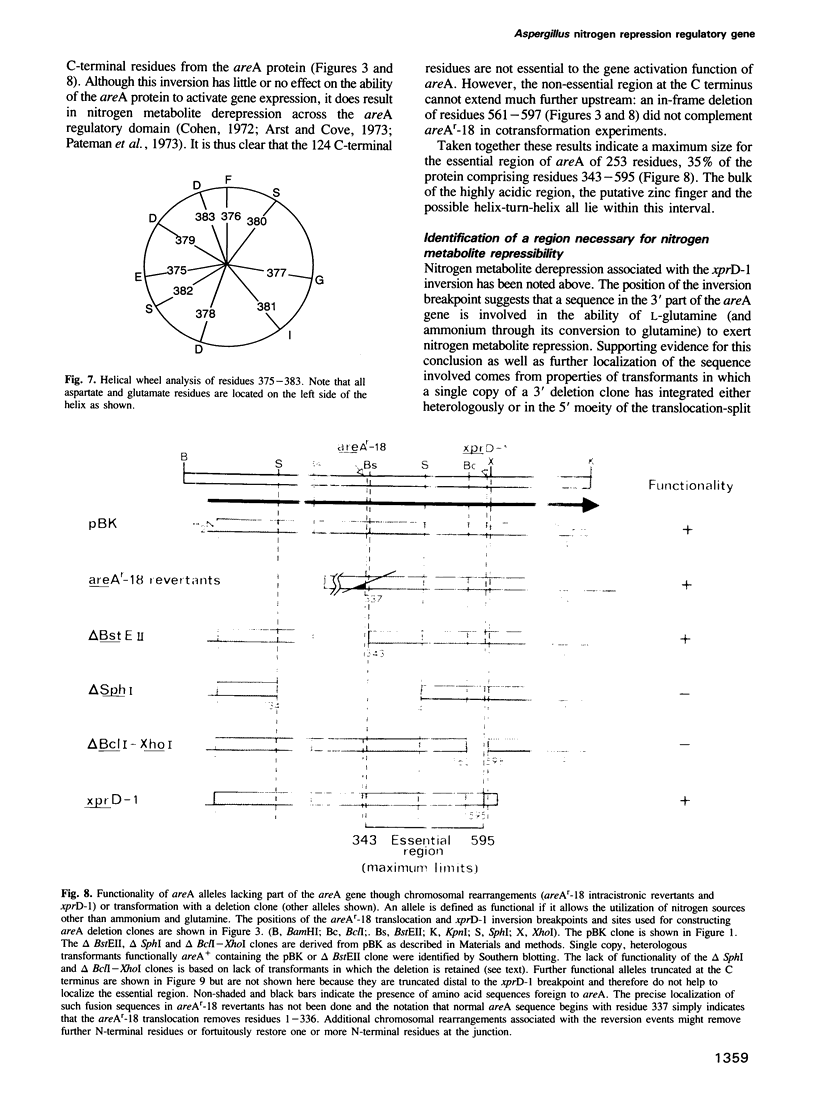

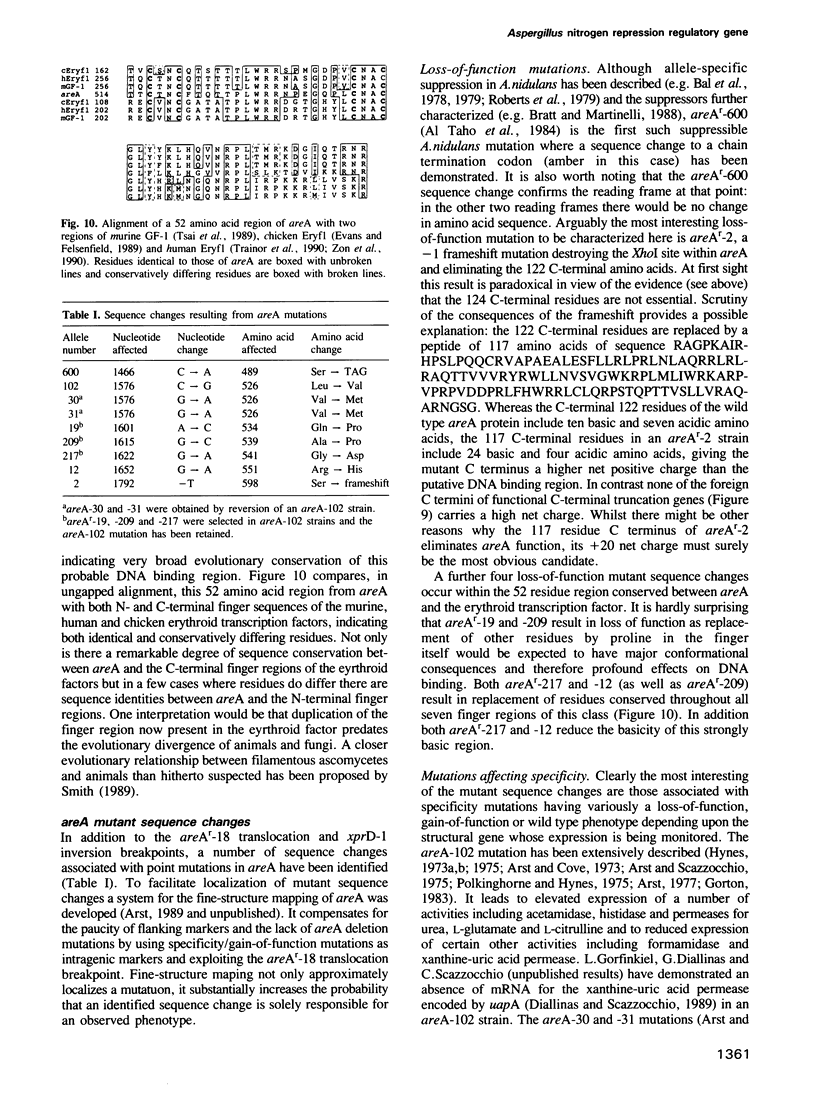



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arst H. N., Jr A near terminal pericentric inversion leads to nitrogen metabolite derepression in Aspergillus nidulans. Mol Gen Genet. 1982;188(3):490–493. doi: 10.1007/BF00330054. [DOI] [PubMed] [Google Scholar]
- Arst H. N., Jr, Cove D. J. Methylammonium resistance in Aspergillus nidulans. J Bacteriol. 1969 Jun;98(3):1284–1293. doi: 10.1128/jb.98.3.1284-1293.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arst H. N., Jr, Cove D. J. Nitrogen metabolite repression in Aspergillus nidulans. Mol Gen Genet. 1973 Nov 2;126(2):111–141. doi: 10.1007/BF00330988. [DOI] [PubMed] [Google Scholar]
- Arst H. N., Jr, Kudla B., Martinez-Rossi N., Caddick M. X., Sibley S., Davies R. W. Aspergillus and mouse share a new class of 'zinc finger' protein. Trends Genet. 1989 Sep;5(9):291–291. doi: 10.1016/0168-9525(89)90105-4. [DOI] [PubMed] [Google Scholar]
- Arst H. N., Jr, Scazzocchio C. Initiator constitutive mutation with an 'up-promoter' effect in Aspergillus nidulans. Nature. 1975 Mar 6;254(5495):31–34. doi: 10.1038/254031a0. [DOI] [PubMed] [Google Scholar]
- Arst H. N., Jr Some genetical aspects of ornithine metabolism in Aspergillus nidulans. Mol Gen Genet. 1977 Feb 28;151(1):105–110. doi: 10.1007/BF00446919. [DOI] [PubMed] [Google Scholar]
- Arst H. N., Jr, Tollervey D. W., Sealy-Lewis H. M. A possible regulatory gene for the molybdenum-containing cofactor in Aspergillus nidulans. J Gen Microbiol. 1982 May;128(5):1083–1093. doi: 10.1099/00221287-128-5-1083. [DOI] [PubMed] [Google Scholar]
- Arst H. N., Jr, Tollervey D., Caddick M. X. A translocation associated, loss-of-function mutation in the nitrogen metabolite repression regulatory gene of Aspergillus nidulans can revert intracistronically. Mol Gen Genet. 1989 Jan;215(2):364–367. doi: 10.1007/BF00339744. [DOI] [PubMed] [Google Scholar]
- Bal J., Kowalska I. E., Maciejko D. M., Wegleński P. Allele specific and locus non-specific suppressors in Aspergillus nidulans. J Gen Microbiol. 1979 Dec;115(2):457–470. doi: 10.1099/00221287-115-2-457. [DOI] [PubMed] [Google Scholar]
- Bal J., Maciejko D. M., Kajtaniak E. M., Gajewski W. Supersuppressors in Aspergillus nidulans. Mol Gen Genet. 1978 Feb 16;159(2):227–228. doi: 10.1007/BF00270899. [DOI] [PubMed] [Google Scholar]
- Ballance D. J. Sequences important for gene expression in filamentous fungi. Yeast. 1986 Dec;2(4):229–236. doi: 10.1002/yea.320020404. [DOI] [PubMed] [Google Scholar]
- Ballance D. J., Turner G. Development of a high-frequency transforming vector for Aspergillus nidulans. Gene. 1985;36(3):321–331. doi: 10.1016/0378-1119(85)90187-8. [DOI] [PubMed] [Google Scholar]
- Bratt R., Martinelli S. D. Every ribosomal suppressor mutation in Aspergillus nidulans has a unique and highly pleiotropic phenotype. Curr Genet. 1988 Jul;14(1):29–36. doi: 10.1007/BF00405850. [DOI] [PubMed] [Google Scholar]
- Burke J. F. High-sensitivity S1 mapping with single-stranded [32P]DNA probes synthesized from bacteriophage M13mp templates. Gene. 1984 Oct;30(1-3):63–68. doi: 10.1016/0378-1119(84)90105-7. [DOI] [PubMed] [Google Scholar]
- Caddick M. X., Arst H. N., Jr, Taylor L. H., Johnson R. I., Brownlee A. G. Cloning of the regulatory gene areA mediating nitrogen metabolite repression in Aspergillus nidulans. EMBO J. 1986 May;5(5):1087–1090. doi: 10.1002/j.1460-2075.1986.tb04326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzone F. J., Britten R. J., Davidson E. H. Mapping of gene transcripts by nuclease protection assays and cDNA primer extension. Methods Enzymol. 1987;152:611–632. doi: 10.1016/0076-6879(87)52069-9. [DOI] [PubMed] [Google Scholar]
- Clements J. M., Roberts C. F. Molecular cloning of the 3-phosphoglycerate kinase (PGK) gene from Aspergillus nidulans. Curr Genet. 1985;9(4):293–298. doi: 10.1007/BF00419958. [DOI] [PubMed] [Google Scholar]
- Corton J. C., Johnston S. A. Altering DNA-binding specificity of GAL4 requires sequences adjacent to the zinc finger. Nature. 1989 Aug 31;340(6236):724–727. doi: 10.1038/340724a0. [DOI] [PubMed] [Google Scholar]
- Cove D. J. The induction and repression of nitrate reductase in the fungus Aspergillus nidulans. Biochim Biophys Acta. 1966 Jan 11;113(1):51–56. doi: 10.1016/s0926-6593(66)80120-0. [DOI] [PubMed] [Google Scholar]
- Darlington A. J., Scazzocchio C. Use of analogues and the substrate-sensitivity of mutants in analysis of purine uptake and breakdown in Aspergillus nidulans. J Bacteriol. 1967 Mar;93(3):937–940. doi: 10.1128/jb.93.3.937-940.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diallinas G., Scazzocchio C. A gene coding for the uric acid-xanthine permease of Aspergillus nidulans: inactivational cloning, characterization, and sequence of a cis-acting mutation. Genetics. 1989 Jun;122(2):341–350. doi: 10.1093/genetics/122.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T., Felsenfeld G. The erythroid-specific transcription factor Eryf1: a new finger protein. Cell. 1989 Sep 8;58(5):877–885. doi: 10.1016/0092-8674(89)90940-9. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Giniger E., Ptashne M. Transcription in yeast activated by a putative amphipathic alpha helix linked to a DNA binding unit. Nature. 1987 Dec 17;330(6149):670–672. doi: 10.1038/330670a0. [DOI] [PubMed] [Google Scholar]
- Hope I. A., Mahadevan S., Struhl K. Structural and functional characterization of the short acidic transcriptional activation region of yeast GCN4 protein. Nature. 1988 Jun 16;333(6174):635–640. doi: 10.1038/333635a0. [DOI] [PubMed] [Google Scholar]
- Hynes M. J. Alterations in the control of glutamate uptake in mutants of Aspergillus nidulans. Biochem Biophys Res Commun. 1973 Sep 18;54(2):685–689. doi: 10.1016/0006-291x(73)91477-0. [DOI] [PubMed] [Google Scholar]
- Hynes M. J. Pleiotropic mutants affecting the control of nitrogen metabolism in Aspergillus nidulans. Mol Gen Genet. 1973 Sep 5;125(2):99–107. doi: 10.1007/BF00268862. [DOI] [PubMed] [Google Scholar]
- Hynes M. J. Studies on the role of the areA gene in the regulation of nitrogen catabolism in Aspergillus nidulans. Aust J Biol Sci. 1975 Jun;28(3):301–313. doi: 10.1071/bi9750301. [DOI] [PubMed] [Google Scholar]
- Johnstone I. L., Hughes S. G., Clutterbuck A. J. Cloning an Aspergillus nidulans developmental gene by transformation. EMBO J. 1985 May;4(5):1307–1311. doi: 10.1002/j.1460-2075.1985.tb03777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. S., Guarente L. Mutations that alter transcriptional activation but not DNA binding in the zinc finger of yeast activator HAPI. Nature. 1989 Nov 9;342(6246):200–203. doi: 10.1038/342200a0. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Ochman H., Gerber A. S., Hartl D. L. Genetic applications of an inverse polymerase chain reaction. Genetics. 1988 Nov;120(3):621–623. doi: 10.1093/genetics/120.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PONTECORVO G., ROPER J. A., HEMMONS L. M., MACDONALD K. D., BUFTON A. W. J. The genetics of Aspergillus nidulans. Adv Genet. 1953;5:141–238. doi: 10.1016/s0065-2660(08)60408-3. [DOI] [PubMed] [Google Scholar]
- Pateman J. A., Kinghorn J. R., Dunn E., Forbes E. Ammonium regulation in Aspergillus nidulans. J Bacteriol. 1973 Jun;114(3):943–950. doi: 10.1128/jb.114.3.943-950.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polkinghorne M., Hynes M. J. Mutants affecting histidine utilization in Aspergillus nidulans. Genet Res. 1975 Apr;25(2):119–135. doi: 10.1017/s0016672300015524. [DOI] [PubMed] [Google Scholar]
- Prentki P., Karch F., Iida S., Meyer J. The plasmid cloning vector pBR325 contains a 482 base-pair-long inverted duplication. Gene. 1981 Sep;14(4):289–299. doi: 10.1016/0378-1119(81)90161-x. [DOI] [PubMed] [Google Scholar]
- Rand K. N., Arst H. N., Jr A mutation in Aspergillus nidulans which affects the regulation of nitrite reductase and is tightly linked to its structural gene. Mol Gen Genet. 1977 Sep 21;155(1):67–75. doi: 10.1007/BF00268562. [DOI] [PubMed] [Google Scholar]
- Roberts T., Martinelli S., Scazzocchio C. Allele specific, gene unspecific suppressors in Aspergillus nidulans. Mol Gen Genet. 1979;177(1):57–64. doi: 10.1007/BF00267253. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer P. M., Arst H. N., Jr, Estberg L., Fernando L., Ly T., Sitter M. An asparaginase of Aspergillus nidulans is subject to oxygen repression in addition to nitrogen metabolite repression. Mol Gen Genet. 1988 May;212(2):337–341. doi: 10.1007/BF00334704. [DOI] [PubMed] [Google Scholar]
- Smith T. L. Disparate evolution of yeasts and filamentous fungi indicated by phylogenetic analysis of glyceraldehyde-3-phosphate dehydrogenase genes. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7063–7066. doi: 10.1073/pnas.86.18.7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M. SPXX, a frequent sequence motif in gene regulatory proteins. J Mol Biol. 1989 May 5;207(1):61–84. doi: 10.1016/0022-2836(89)90441-5. [DOI] [PubMed] [Google Scholar]
- Trainor C. D., Evans T., Felsenfeld G., Boguski M. S. Structure and evolution of a human erythroid transcription factor. Nature. 1990 Jan 4;343(6253):92–96. doi: 10.1038/343092a0. [DOI] [PubMed] [Google Scholar]
- Tsai S. F., Martin D. I., Zon L. I., D'Andrea A. D., Wong G. G., Orkin S. H. Cloning of cDNA for the major DNA-binding protein of the erythroid lineage through expression in mammalian cells. Nature. 1989 Jun 8;339(6224):446–451. doi: 10.1038/339446a0. [DOI] [PubMed] [Google Scholar]
- Tuite M. F., Bossier P., Fitch I. T. A highly conserved sequence in yeast heat shock gene promoters. Nucleic Acids Res. 1988 Dec 23;16(24):11845–11845. doi: 10.1093/nar/16.24.11845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdière J., Gaisne M., Guiard B., Defranoux N., Slonimski P. P. CYP1 (HAP1) regulator of oxygen-dependent gene expression in yeast. II. Missense mutation suggests alternative Zn fingers as discriminating agents of gene control. J Mol Biol. 1988 Nov 20;204(2):277–282. doi: 10.1016/0022-2836(88)90575-x. [DOI] [PubMed] [Google Scholar]
- Wiame J. M., Grenson M., Arst H. N., Jr Nitrogen catabolite repression in yeasts and filamentous fungi. Adv Microb Physiol. 1985;26:1–88. doi: 10.1016/s0065-2911(08)60394-x. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zon L. I., Tsai S. F., Burgess S., Matsudaira P., Bruns G. A., Orkin S. H. The major human erythroid DNA-binding protein (GF-1): primary sequence and localization of the gene to the X chromosome. Proc Natl Acad Sci U S A. 1990 Jan;87(2):668–672. doi: 10.1073/pnas.87.2.668. [DOI] [PMC free article] [PubMed] [Google Scholar]