Abstract
Purpose
To describe nepafenac use in the Netherlands and Denmark with reference to its approved indications. For context, we also describe the use of ketorolac and diclofenac.
Methods
We identified users in the PHARMO Database Network (the Netherlands, 2008–2013) and the Danish national health registers (Denmark, 1994–2014). We described prevalence of cataract surgery and duration of use in patients with cataract surgery with and without diabetes.
Results
In the Netherlands, 9530 nepafenac users (mean age, 71 years; 60% women) contributed 12 691 therapy episodes, of which 21% had a recently recorded cataract surgery. Of 2266 episodes in adult non‐diabetic patients with cataract surgery, 60% had one bottle dispensed (treatment duration ≤21 days). Of 441 episodes in adult diabetic patients with cataract surgery, 90% had up to two bottles dispensed (≤60 days).
Denmark had 60 403 nepafenac users (mean age, 72 years; 58% women) and 73 648 episodes (41% had recorded cataract surgery). Of 26 649 nepafenac episodes in adult non‐diabetic patients with cataract surgery, 92% had one bottle dispensed. Of 3801 episodes in adult diabetic patients with cataract surgery, 99.8% had up to two bottles dispensed.
Use patterns of nepafenac, ketorolac and diclofenac were roughly similar in the Netherlands, but not in Denmark.
Conclusion
Less than half of therapy episodes were related to cataract surgery; around 90% of episodes with surgery were within the approved duration. Underrecording of ophthalmic conditions and procedures was a challenge in this study.
Keywords: cataract, cataract surgery, diclofenac, ketorolac, nepafenac, off‐label, ophthalmic NSAIDs
Introduction
Nepafenac is an ophthalmic non‐steroidal anti‐inflammatory drug (NSAID) sold under the trade name Nevanac®. Nevanac 1 mg/ml has been available in Europe since 2007 for the prevention and treatment of postoperative pain and inflammation after cataract surgery, in the first 2 weeks after surgery in adults. Treatment can be extended to the first 3 weeks of the postoperative period as directed by the clinician. In 2011, the European Commission approved a new indication for Nevanac 1 mg/ml: reduction in the risk of postoperative macular oedema associated with cataract surgery in patients with diabetes, for use up to 60 days after surgery. Following this, an application for a line extension was submitted to register nepafenac 3 mg/ml with the indication ‘prevention and treatment of postoperative pain and inflammation associated with cataract surgery’, which was granted in 2013. In July 2016 (after the end of the study period), a new indication was approved for nepafenac 3 mg/ml: reduction in the risk of postoperative macular oedema associated with cataract surgery in patients with diabetes.
Other ophthalmic NSAIDs are approved for broader indications; for example, ophthalmic ketorolac is approved for prevention and relief of eye inflammation after eye surgery in adults in the UK (Allergan Pharmaceuticals Ireland 2014), and diclofenac is approved for mydriasis during surgery, to control pain and inflammation after eye surgery or injury and to control symptoms of seasonal allergic conjunctivitis (Excelvision S.A.S. 2015).
As part of the risk management plan for nepafenac agreed between the European Medicines Agency and Alcon Inc., we performed a postapproval safety study (PASS). We evaluated the dispensing of nepafenac with regard to the approved indications, focusing on age, use related to cataract surgery and duration of use in patients with cataract surgery with and without diabetes. To provide context to the findings, we compared the dispensing of nepafenac with that of other ophthalmic NSAIDs (ketorolac and diclofenac).
Materials and Methods
This was a descriptive observational drug utilization study of users of nepafenac, ketorolac and diclofenac. The study was conducted in the PHARMO Database Network (PHARMO) of the PHARMO Institute for Drug Outcomes Research in the Netherlands and in the network of national health registers in Denmark. The study period was 01 September 2008 through 31 October 2013 in the Netherlands and 01 January 1994 through 31 December 2014 in Denmark.
Data sources
PHARMO Database Network is a population‐based network of electronic healthcare databases that combines data from different primary and secondary healthcare settings in the Netherlands. These different data sources, including data from general practices, inpatient and outpatient pharmacies, clinical laboratories, hospitals and more, are linked on a patient level through a validated algorithm. The longitudinal nature enables a follow‐up of more than 4 million (25%) residents of a well‐defined population in the Netherlands for an average of 10 years (Herings 1993).
The entire Danish population is provided free tax‐supported medical care by the National Health Service (Thygesen & Ersboll 2014). For administration and maintenance of this healthcare system, numerous administrative and health registries have been established. The three main data sources for this study were the Danish Prescription Registry, the Danish National Patient Registry and the Central Person Registry. The Danish Prescription Registry contains data on all prescription drugs dispensed to Danish residents since 1995 (Kildemoes et al. 2011). The data include the type of drug, date of dispensing and quantity dispensed. Drugs are categorized according to the Anatomical Therapeutic Chemical Index (Kildemoes et al. 2011). The Danish National Patient Register contains nationwide data on all non‐psychiatric hospital admissions since 1977 and all outpatient specialist contacts in hospital setting since 1995 (Schmidt et al. 2015). Discharge/contact diagnoses are coded using ICD‐8 (1977–1993) and ICD‐10 (1994‐present). The Central Person Registry contains records of deaths and migrations, which allowed us to follow patients appropriately (Schmidt et al. 2014). Data sources were linked by the civil registry number, a unique identifier assigned to all Danish residents since 1968 (Schmidt et al. 2014).
Study population
Patients became eligible for cohort entry after 6 months of enrolment in the data source. In Denmark, the cohort comprised only new users: patients entered the cohort with the first dispensing for the drug after 6 months free of dispensing for that drug. In the Netherlands, patients entered the cohort regardless of previous ophthalmic NSAID use. Follow‐up continued until the earliest of disenrolment from the database, emigration, death or end of the study period. Multiple episodes of use of one or more of the study drugs by individual patients were included if they occurred during follow‐up.
Analysis
Drug use was ascertained from community pharmacy dispensing records (Kildemoes et al. 2011). Therapy episodes were created by linking together consecutive dispensings without treatment gaps.
The indication is not recorded in either data source but was derived from diagnoses and procedures dated within 30 days before and 30 days after the prescription dispensing date.
In the Netherlands, cataract surgeries were identified from procedures in hospital discharge records in the Hospitalisation Database in PHARMO. This database captures only admissions longer than 24 hrs and admissions less than 24 hrs for which a bed is required. As the ophthalmic procedures in this study were performed mainly during outpatient visits, the number of ophthalmic procedures is likely to be underestimated. In Denmark, cataract surgeries were identified from diagnostic and procedure codes in hospital discharge records (Schmidt et al. 2015) and from the DUSAS registry, which records procedures conducted by primary care specialists. During the conduct of this study, evidence of underrecording of cataract surgery in the Danish registries appeared (Solborg Bjerrum et al. 2013, 2015).
Sensitivity analyses implemented in both populations to address underrecording of cataract surgery included expanding the list of ophthalmic conditions for which nepafenac is not approved but for which physicians may have been providing prescriptions. Additionally, in PHARMO, we identified the presence of correspondence between general practitioners and ophthalmologists and expanded the interval in which the indication was sought to within 60 days before or after the start of the therapy episode, or 6 months before. In Denmark, we conducted subgroup analyses in patients whose surgeries were likely to be captured better in our data sources: the sicker patients (Charlson comorbidity score ≥ 3) and the elderly (age ≥ 80 years) (Solborg Bjerrum et al. 2013, 2015).
Because we did not have information on when patients stopped therapy, but we did have information on the number of dispensed bottles, we used the number of dispensed bottles as a proxy for duration of therapy for nepafenac. We assumed that one bottle of nepafenac (3 ml or 5 ml) represented treatment for up to 21 days and two bottles represented treatment for up to 60 days. Because the macular oedema indication for nepafenac was approved in late 2011, we looked at the number of bottles used in two periods, before June 2011 and after January 2012 (before approval of the second indication, the approved indication would require no more than one bottle per therapy episode). We also present information on duration of use in days based on physician instructions (sparsely available) or (more frequently) the maximum possible duration based on the approved dosing and the size of the bottle (e.g. one 3‐ml bottle of nepafenac 3 mg/ml would last up to 60 days at 1 drop/day). As we do not have direct evidence that all dispensed medication was used, this is likely an overestimation of the treatment duration. Furthermore, the package leaflet recommends discarding any remaining content 4 weeks after opening the bottle to minimize the risk of infection (Alcon 2015).
Patient baseline characteristics and medication use were determined in the 6 months prior to cohort entry for nepafenac, ketorolac and diclofenac. We also determined drug use over time and concomitant use of other ophthalmic medications and selected systemic medications. Drug use in the Netherlands in 2013, quantified from 1 January to 31 October, was extrapolated to 31 December for graphic display.
The study and its protocol were registered in the EU PAS Register on 27 November 2013, prior to the start of data collection ( http://www.encepp.eu/encepp/viewResource.htm?id=13363).
Results
The Netherlands
Participants and drug dispensing
The study cohort comprised 9530 nepafenac users with 1.3 therapy episodes per person, 5351 ketorolac users (1.4 episodes per person) and 4536 diclofenac users (1.3 episodes per person). While the number of episodes was similar for the three drugs in 2009, only dispensing of nepafenac continued increasing thereafter, with a slight decrease in the last year of the study period (Fig. 1). Of nepafenac users, 60% were women, 17% were diabetic and 39% were using antithrombotic agents (Table 1). The demographics, health characteristics and dispensing of ophthalmic and systemic drugs to users of ketorolac and diclofenac were very similar to those of nepafenac users (Table 1).
Figure 1.
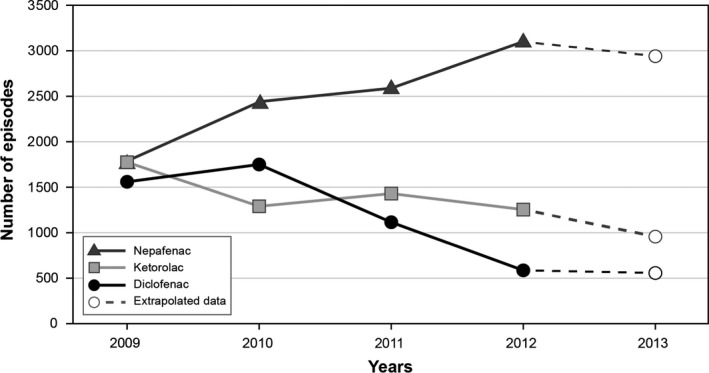
Episodes of Use of Nepafenac, Ketorolac and Diclofenac, the Netherlands. The figure starts in 2009, which is the first year with complete data. Dashed lines correspond to extrapolated data for 2013 (observed data up to 31 October 2013).
Table 1.
Patient characteristics at baseline, the Netherlands (2008–2013) and Denmark (1994–2014)
| Variables | the Netherlands | Denmark | ||||
|---|---|---|---|---|---|---|
| Users of nepafenac (n = 9530) n (%) | Users of ketorolac (n = 5351) n (%) | Users of diclofenac (n = 4536) n (%) | Users of nepafenac (n = 60 403) n (%) | Users of ketorolac (n = 54 185) n (%) | Users of diclofenac (n = 131 440) n (%) | |
| Age (years) | ||||||
| ≤ 18 | 27 (0.3) | 24 (0.4) | 35 (1) | 197 (0.3) | 2038 (4) | 6231 (5) |
| > 18 | 9503 (>99) | 5327 (>99) | 4501 (99) | 60 206 (>99) | 52 147 (97) | 125 209 (95) |
| Mean (SD) | 71 (11) | 70 (13) | 70 (14) | 72 (12) | 63 (20) | 52 (21) |
| Female sex | 5758 (60) | 3227 (60) | 2714 (60) | 35 075 (58) | 33 017 (61) | 62 880 (48) |
| Systemic conditions | ||||||
| Diabetes mellitusa | 1608 (17) | 786 (15) | 769 (17) | 7027 (12) | 3668 (7) | 6212 (5) |
| Autoimmune disorders | 104 (1) | 60 (1) | 50 (1) | 303 (0.5) | 183 (0.3) | 307 (0.2) |
| Bleeding disorders | 4 (<0.1) | 1 (<0.1) | 3 (0.1) | 6 (<0.1) | 6 (<0.1) | 26 (<0.1) |
| Use of systemic medications | ||||||
| Antithrombotic agents | 3738 (39) | 1838 (34) | 1638 (36) | 13 916 (23) | 7576 (14) | 10 874 (8) |
| NSAIDs | 1463 (15) | 811 (15) | 690 (15) | 3947 (7) | 4205 (8) | 10 613 (8) |
| Steroids | 814 (9) | 393 (7) | 304 (7) | 2104 (4) | 2106 (4) | 3428 (3) |
| Charlson comorbidity scoreb | ||||||
| 0 | – | – | – | 27 823 (46) | 32 960 (61) | 94 399 (72) |
| 1–2 | – | – | – | 20 639 (34) | 14 990 (28) | 27 041 (21) |
| ≥3 | – | – | – | 11 941 (20) | 6235 (12) | 10 000 (8) |
NSAIDs = non‐steroidal anti‐inflammatory drugs; SD = standard deviation.
Medical conditions and drug use were ascertained in the 6 months prior to the index date.
Based on dispensing records and/or diagnostic codes.
The comorbidity analysis was included in Denmark after information regarding underrecording of cataract surgery performed in private hospitals became available, and that older and sicker patients were more likely to have surgery in public hospitals, where cataract surgery is recorded more reliably.
Age
The average age of users of nepafenac was 71 years at cohort entry. Of 12 691 nepafenac therapy episodes, 34 episodes (0.3%) were in persons aged 18 years or younger (Table 2). Similarly, few episodes of ketorolac or diclofenac dispensing were in children, less than 1% for both ketorolac and diclofenac.
Table 2.
Medical conditions associated with therapy episodes of Ophthalmic nepafenac, ketorolac and diclofenac in adults, the Netherlands (2008–2013) and Denmark (1994–2014)
| Variables | the Netherlands | Denmark | ||||
|---|---|---|---|---|---|---|
| Nepafenac episodes (N = 12 691) n (%) | Ketorolac episodes (N = 7540) n (%) | Diclofenac episodes (N = 5950) n (%) | Nepafenac episodes (N = 73 648) n (%) | Ketorolac episodes (N = 102 334) n (%) | Diclofenac episodes (N = 184 361) n (%) | |
| Patients aged > 18 years | ||||||
| Number of episodes | 12 657 (>99) | 7508 (>99) | 5915 (>99) | 73 411 (>99) | 99 484 (97) | 177 754 (96) |
| Ophthalmic procedures | ||||||
| Cataract surgery | 2707 (21) | 1437 (19) | 916 (15) | 30 450 (41) | 10 951 (11) | 17 885 (10) |
| Refractive procedures | 1 (<0.1) | 1 (<0.1) | 0 (0) | 5 (<0.1) | 180 (0.2) | 2306 (1) |
| Ophthalmic conditions | ||||||
| Cataract | 4503 (36) | 2606 (35) | 2023 (34) | – | – | – |
| Dry eyes/Sjögren syndrome | 8 (0.1) | 5 (0.1) | 8 (0.1) | 24 (<0.1) | 76 (0.1) | 106 (0.1) |
| Uveitis/iritis | 13 (0.1) | 4 (0.1) | 5 (0.1) | 98 (0.1) | 106 (0.1) | 457 (0.3) |
| Ophthalmic manifestations of allergy | 3 (<0.1) | 4 (0.1) | 14 (0.2) | 0 (0) | 0 (0) | 0 (0) |
| Ocular pain | 7 (0.1) | 4 (0.1) | 8 (0.1) | – | – | – |
| Macular oedema | 6 (<0.1) | 2 (<0.1) | 1 (<0.1) | 14 (<0.1) | 33 (<0.1) | 46 (<0.1) |
| Vitreous‐related disorders | 17 (0.1) | 0 (0) | 5 (0.1) | 75 (0.1) | 99 (0.1) | 258 (0.1) |
| Infectious conjunctivitis | 21 (0.2) | 10 (0.1) | 26 (0.4) | – | – | – |
| Blepharitis/stye/chalazion | 21 (0.2) | 8 (0.1) | 5 (0.1) | – | – | – |
| Eye infection/inflammation | 11 (0.1) | 8 (0.1) | 15 (0.3) | – | – | – |
| Ophthalmic correspondence | 4763 (38) | 2227 (30) | 2172 (37) | – | – | – |
| Episodes in patients aged > 18 years with Charlson score ≥ 3 | – | – | – | 14 997 | 12 570 | 16 888 |
| Cataract surgery | – | – | – | 6649 (44) | 2233 (18) | 3292 (20) |
| Episodes in patients aged ≥ 80 years | – | – | – | 19 243 | 22 723 | 23 467 |
| Cataract surgery | – | – | – | 8552 (44) | 3277 (14) | 5186 (22) |
Data were ascertained within the 30 days before and 30 days after the start date of the therapy episode. In this table, results from the Netherlands only or from Denmark only represent database‐specific sensitivity analyses.
Cataract surgery, cataract diagnosis, other diagnoses
Of the 12 657 nepafenac episodes in adults, 21% were within the 30 days before and 30 days after cataract surgery (24% within 60 days before and 60 days after the start of the therapy episode and 12% in the 6 months before the start of the episode) (Table 2). Of the 79% without a record of cataract surgery within the 30 days before and 30 days after the start of the therapy episode, 19% had a cataract diagnosis in the same window. Furthermore, 57% had an ophthalmic procedure, ophthalmic condition or ophthalmic correspondence in that window. This was 50% for ketorolac and 54% for diclofenac. Less than 1% of the episodes in adults were associated with each of refractive procedures or ophthalmic conditions other than cataracts. Percentages for ketorolac and diclofenac were similar (Table 2).
Number of bottles
In all but two nepafenac‐use episodes, the formulation used was 1 mg/ml, sold in 5‐ml bottles (Table 3). Nepafenac therapy episodes involved one bottle in 60% of the 2266 episodes in adult patients with surgery and without diabetes (proxy for the authorized duration ≤ 21 days). Up to two bottles were involved in 90% of the 441 episodes in adult patients with cataract surgery and diabetes (two bottles were a proxy for the authorized duration ≤ 60 days). Of 2013 therapy episodes with recorded cataract surgery in adults starting between 01 September 2008 and 30 June 2011 (when only the first indication had been approved), 56% were for one bottle (Table S1). Later, between 01 January 2012 and 31 October 2013 (when both indications had been approved), 78% of 626 therapy episodes in adults with recorded cataract surgery were for the authorized duration (one bottle in patients with cataract surgery and no diabetes, or up to two bottles in patients with recorded cataract surgery and diabetes). With some variation, the distributions of duration of use of ketorolac and diclofenac were not very different from that of nepafenac (Table 3). Ketorolac use in patients not undergoing cataract surgery tended to be longer than use of nepafenac or diclofenac.
Table 3.
Number of bottles per therapy episode for ophthalmic nepafenac, ketorolac and diclofenac in adults, the Netherlands (2008–2013) and Denmark (1994–2014)
| the Netherlands | Denmark | |||||||
|---|---|---|---|---|---|---|---|---|
| Recorded cataract surgery | No cataract surgery | Recorded cataract surgery | No cataract surgery | |||||
| Diabetes n (%) | No diabetes n (%) | Diabetes n (%) | No diabetes n (%) | Diabetes n (%) | No diabetes n (%) | Diabetes n (%) | No diabetes n (%) | |
| Nepafenac | ||||||||
| Number of episodes | 441 | 2266 | 1735 | 8215 | 3801 | 26 649 | 4872 | 38 089 |
| Number of bottles (3 ml)a | ||||||||
| 1 | – | – | – | – | 139 (98) | 1023 (98) | 222 (98) | 1589 (95) |
| 2 | – | – | – | – | 3 (2) | 16 (2) | 5 (2) | 78 (5) |
| >2 | – | – | – | – | 0 (0) | (n < 3) | 0 (0) | (n < 3) |
| Number of bottles (5 ml) | ||||||||
| 1 | 260 (59) | 1360 (60) | 1212 (70) | 6086 (74) | 3319 (91) | 23 416 (91) | 4173 (90) | 32 534 (89) |
| 2 | 135 (31) | 669 (30) | 288 (17) | 1271 (15) | 328 (9) | 2088 (8) | 429 (9) | 3601 (10) |
| >2 | 46 (10) | 237 (10) | 235 (14) | 857 (10) | 9 (0.2) | 91 (0.4) | 35 (0.8) | 225 (0.6) |
| Drug supply duration | ||||||||
| ≤21 days | 1 (0.2) | 44 (2) | 84 (5) | 543 (7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| >21–60 days | 252 (57) | 1363 (60) | 1240 (71) | 5924 (72) | 1967 (52) | 13 856 (52) | 2733 (56) | 23 106 (60) |
| >60 days | 188 (43) | 859 (38) | 411 (24) | 1748 (21) | 1834 (48) | 12 793 (48) | 2139 (44) | 14 983 (39) |
| Ketorolac | ||||||||
| Number of episodes | 222 | 1215 | 968 | 5103 | 1385 | 9566 | 5479 | 83 054 |
| Duration | ||||||||
| ≤21 days | 2 (1) | 6 (0.5) | 30 (3) | 203 (4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| >21–60 days | 162 (73) | 888 (73) | 681 (70) | 3653 (72) | 648 (47) | 4845 (51) | 4077 (74) | 65 989 (77) |
| >60 days | 58 (26) | 321 (26) | 257 (27) | 1247 (24) | 737 (53) | 4721 (49) | 1402 (26) | 17 065 (20) |
| Diclofenac | ||||||||
| Number of episodes | 182 | 734 | 889 | 4110 | 1707 | 16 178 | 8326 | 151 543 |
| Duration | ||||||||
| ≤21 days | 0 (0) | 2 (0.3) | 20 (2) | 168 (4) | 192 (11) | 2271 (14) | 3126 (37) | 79 663 (50) |
| >21–60 days | 109 (60) | 525 (72) | 328 (37) | 1584 (39) | 992 (58) | 9318 (56) | 4129 (49) | 62 187 (39) |
| >60 days | 73 (40) | 207 (28) | 541 (61) | 2358 (57) | 523 (31) | 4589 (28) | 1071 (13) | 9693 (6) |
In the Netherlands, 3‐ml bottles were used in only two episodes. For 86 episodes in Denmark, the size of the bottle was not recorded as 3 or 5 ml.
Concomitant medications
The frequency of use of concomitant medications varied somewhat across drugs, with concomitant ophthalmic steroid use around 80% or higher in patients with recorded cataract surgery and lower in patients not undergoing cataract surgery (Table 4). In patients with ketorolac prescriptions, concomitant dispensing of ophthalmic NSAIDs was high, around 65%.
Table 4.
Concomitant medication use by recorded cataract surgery and diabetes for ophthalmic nepafenac, ketorolac and diclofenac therapy episodes, the Netherlands (2008–2013) and Denmark (1994–2014)
| Variables | the Netherlands | Denmark | ||||||
|---|---|---|---|---|---|---|---|---|
| Recorded cataract surgery | No cataract surgery | Recorded cataract surgery | No cataract surgery | |||||
| Diabetes n (%) | No diabetes n (%) | Diabetes n (%) | No diabetes n (%) | Diabetes n (%) | No diabetes n (%) | Diabetes n (%) | No diabetes n (%) | |
| Nepafenac | ||||||||
| Number of episodes | 441 | 2268 | 1735 | 8247 | 3801 | 26 659 | 4874 | 38 314 |
| Ophthalmic medications | ||||||||
| Other NSAIDsa | 44 (10) | 196 (9) | 24 (1) | 133 (2) | 33 (0.9) | 194 (0.7) | 50 (1) | 513 (1) |
| Steroids | 395 (90) | 2025 (89) | 1032 (59) | 4837 (59) | 2729 (72) | 19 221 (72) | 3282 (67) | 25 517 (67) |
| Prostaglandin analogues | 9 (2) | 50 (2) | 30 (2) | 223 (3) | 162 (4) | 1258 (5) | 203 (4) | 1801 (5) |
| Other ophthalmic medications | 113 (26) | 598 (26) | 774 (45) | 3597 (44) | 439 (12) | 3448 (13) | 778 (16) | 7048 (18) |
| Systemic medications | ||||||||
| Antithrombotic agents | 155 (35) | 532 (23) | 592 (34) | 1590 (19) | 1591 (42) | 6007 (23) | 1980 (41) | 7447 (19) |
| NSAIDs | 24 (5) | 148 (7) | 94 (5) | 520 (6) | 277 (7) | 1774 (7) | 405 (8) | 2517 (7) |
| Steroids | 21 (5) | 93 (4) | 70 (4) | 337 (4) | 128 (3) | 967 (4) | 159 (3) | 1389 (4) |
| Ketorolac | ||||||||
| Number of episodes | 222 | 1216 | 968 | 5134 | 1385 | 9573 | 5487 | 85 889 |
| Ophthalmic medications | ||||||||
| Other NSAIDsa | 148 (67) | 793 (65) | 25 (3) | 114 (2) | 21 (2) | 149 (2) | 53 (1) | 663 (0.8) |
| Steroids | 209 (94) | 1175 (97) | 193 (20) | 935 (18) | 1324 (96) | 8936 (93) | 1347 (25) | 15 938 (19) |
| Prostaglandin analogues | 10 (5) | 31 (3) | 22 (2) | 112 (2) | 39 (3) | 386 (4) | 230 (4) | 1949 (2) |
| Other ophthalmic medications | 174 (78) | 870 (72) | 454 (47) | 2637 (51) | 123 (9) | 1151 (12) | 1280 (23) | 23 151 (27) |
| Systemic medications | ||||||||
| Antithrombotic agents | 68 (31) | 239 (20) | 362 (37) | 928 (18) | 536 (39) | 2030 (21) | 1957 (36) | 10 864 (13) |
| NSAIDs | 13 (6) | 79 (6) | 57 (6) | 310 (6) | 96 (7) | 641 (7) | 682 (12) | 8384 (10) |
| Steroids | 12 (5) | 47 (4) | 37 (4) | 170 (3) | 51 (4) | 343 (4) | 233 (4) | 3452 (4) |
| Diclofenac | ||||||||
| Number of episodes | 182 | 734 | 890 | 4144 | 1707 | 16 210 | 8351 | 158 093 |
| Ophthalmic medications | ||||||||
| Other NSAIDsa | 1 (1) | 12 (2) | 37 (4) | 142 (3) | 20 (1) | 155 (1) | 67 (0.8) | 840 (0.5) |
| Steroids | 152 (84) | 576 (78) | 527 (59) | 2202 (53) | 899 (53) | 8452 (52) | 2097 (25) | 37 402 (24) |
| Prostaglandin analogues | 11 (6) | 21 (3) | 31 (3) | 165 (4) | 50 (3) | 690 (4) | 377 (5) | 3177 (2) |
| Other ophthalmic medications | 22 (12) | 81 (11) | 301 (34) | 1565 (38) | 286 (17) | 2940 (18) | 3676 (44) | 82 373 (52) |
| Systemic medications | ||||||||
| Antithrombotic agents | 65 (36) | 168 (23) | 304 (34) | 943 (23) | 596 (35) | 2936 (18) | 2505 (30) | 12 201 (8) |
| NSAIDs | 8 (4) | 32 (4) | 58 (7) | 358 (9) | 157 (9) | 1323 (8) | 1034 (12) | 14 289 (9) |
| Steroids | 12 (7) | 25 (3) | 36 (4) | 153 (4) | 76 (4) | 754 (5) | 327 (4) | 4510 (3) |
NSAIDs = non‐steroidal anti‐inflammatory drugs.
The category other NSAIDs reflects all other NSAIDs except the one being reported.
Denmark
Participants and drug dispensing
The study cohort comprised 60 403 users of nepafenac (1.2 episodes per person), 54 185 users of ketorolac (1.9 episodes per person) and 131 440 users of diclofenac (1.4 episodes per person). Dispensing of nepafenac increased during the study period, while dispensing of ketorolac and diclofenac was approximately stable, with a mild increase towards the end of the study period (Fig. 2). Of nepafenac users, 58% were women; 12% had diabetes and 23% used antithrombotic agents (Table 1). The demographics, health characteristics and dispensing of ophthalmic and systemic drugs in users of ketorolac and diclofenac were similar to those in nepafenac users, although users of ketorolac and diclofenac seemed to be somewhat younger and had lower Charlson comorbidity scores (Table 1).
Figure 2.
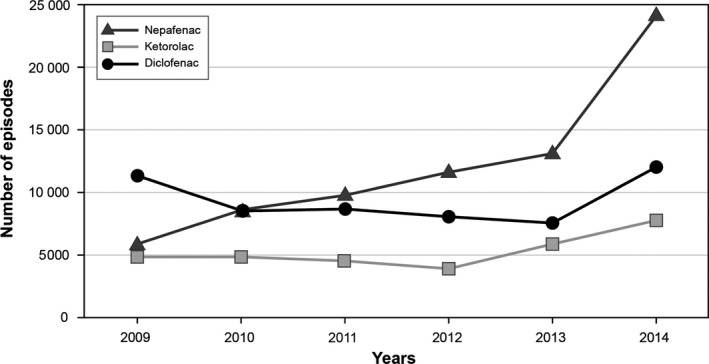
Episodes of Use of Nepafenac, Ketorolac and Diclofenac, Denmark
Age
The mean age of nepafenac users at cohort entry was 72 years. Of 73 648 nepafenac therapy episodes, 237 episodes (0.3%) were in persons aged 18 years or younger (Table 1). Few episodes of ketorolac or diclofenac dispensing occurred in children, 3% for ketorolac and 4% for diclofenac in Denmark.
Cataract surgery, cataract diagnosis, other diagnoses
Of the 73 411 nepafenac‐use episodes in adults, 41% had cataract surgery (Table 2). Less than 1% each had refractive procedures or ophthalmic conditions within 30 days before and 30 days after the start date of the therapy episode (Table 2). For both ketorolac and diclofenac, dispensing associated with cataract surgery in adults was lower: 11% of ketorolac episodes and 10% of diclofenac episodes. Using an expanded list of ophthalmic conditions that might have triggered the dispensing of ophthalmic NSAIDs, 12% of nepafenac‐, 12% of ketorolac‐ and 43% of diclofenac‐use episodes in patients with no cataract surgery were associated with these conditions (Table 5). Among patients more likely to have better capture of cataract surgery, those aged 80 years or older and those with high comorbidity (Charlson comorbidity index score of 3 or more), 44% of nepafenac episodes were associated with cataract surgery (Table 2).
Table 5.
Ophthalmologic conditions related to potential off‐label use of nepafenac, ketorolac and diclofenac, Denmark, 1994–2014
| Nepafenac episodes (n = 73 648) | Ketorolac episodes (n = 102 334) | Diclofenac episodes (n = 184 361) | |
|---|---|---|---|
| No cataract surgery | 43 188 (59) | 91 376 (89) | 166 444 (90) |
| Cataract | 2701 (6) | 908 (1) | 2550 (2) |
| Keratitis | 143 (0.3) | 305 (0.3) | 2890 (2) |
| Conjunctivitis | 2470 (6) | 9284 (10) | 64 454 (39) |
| Foreign body | 63 (0.1) | 166 (0.2) | 4059 (2) |
| Ocular trauma | 113 (0.3) | 191 (0.2) | 7693 (5) |
| Hordeolum | (n < 3) | 7 (<0.1) | 37 (<0.1) |
| Chalazion | 3 (<0.1) | (n < 3) | 10 (<0.1) |
| Blepharitis | 8 (<0.1) | 12 (<0.1) | 42 (<0.1) |
| Welder's eye | 24 (0.1) | 32 (<0.1) | 515 (0.3) |
| Traumatic corneal lesion | 90 (0.2) | 147 (0.2) | 7147 (4) |
| Acute allergic conjunctivitis | 7 (<0.1) | 36 (<0.1) | 99 (0.1) |
| Cystoid macular oedema | 62 (0.1) | 97 (0.1) | 123 (0.1) |
| Any of the above | 5235 (12) | 10 573 (12) | 70 814 (43) |
These are results from a sensitivity analysis implemented in Denmark. Results from similar analyses in the Netherlands are presented in Table 3.
Number of bottles
Of 73 411 nepafenac‐use episodes in adults, 4% involved 3‐ml bottles and 96% of episodes involved 5‐ml bottles (Table 3). Approximately 92% of the 26 649 nepafenac episodes in patients with cataract surgery and without diabetes involved one bottle; 99.8% of the 3801 episodes in patients with cataract surgery and diabetes involved up to two bottles. Of 7684 therapy episodes with recorded cataract surgery in adults when only the first indication had been approved, 81% were for one bottle (Table S1). Later, when both indications had been approved, 96% of 19 980 therapy episodes in adults with recorded cataract surgery were for the approved durations. The duration of ketorolac use in patients with cataract surgery was similar to that of nepafenac. In patients not undergoing cataract surgery, there was a tendency towards shorter treatments with ketorolac. Use of diclofenac in all patient groups tended to be shorter than use of nepafenac.
Concomitant use
Patterns of concomitant drug use varied somewhat for the three drugs (Table 4). Concomitant use of ophthalmic steroids was higher for patients with cataract surgery, but the difference was small in nepafenac users.
Discussion
This was a collaborative drug utilization study in two European countries to describe the use of nepafenac, with 9530 patients with one or more nepafenac dispensings in the Netherlands and 60 403 in Denmark. We observed increased nepafenac dispensing during the study period in both populations. Context is provided by similar analyses for ophthalmic preparations of ketorolac and diclofenac. Patterns of dispensing of the three drugs were roughly similar in the Netherlands, but not in Denmark.
There are three main aspects related to the indication for nepafenac: age (nepafenac is indicated in adults), presence of cataract surgery (nepafenac is indicated for use hrs before and several weeks after cataract surgery) and duration of treatment (up to 21 days in patients undergoing cataract surgery and up to 60 days after cataract surgery in patients with diabetes). Regarding age, 99.7% of nepafenac therapy episodes in the Netherlands and Denmark occurred in patients older than 18 years. Of the therapy episodes in adults, 21% in the Netherlands and 41% in Denmark had codes for cataract surgery. We believe this is an underestimate due to incomplete capture of cataract surgeries in the data sources in both countries. In the Netherlands, 60% of adult patients with recorded cataract surgery and no diabetes used one bottle per episode, and 90% of adult patients with recorded cataract surgery and diabetes used up to two bottles. In Denmark, duration of use was within the authorized limits for 92% of adult patients with recorded cataract surgery and no diabetes and for 99.8% of those with cataract surgery and diabetes. Using maximum days’ supply would likely overestimate unapproved product use (i.e. off‐label use); however, using the number of bottles is potentially underestimating unapproved product use.
Since the initial development of nepafenac for ophthalmic use, there have been concerns that it may be prescribed for use beyond the approved indications. Indeed, through the years, reports that describe or recommend the use of nepafenac for a number of ophthalmic conditions and procedures have been published (Wilson et al. 2015): maintenance of mydriasis during ophthalmic surgery (Cervantes‐Coste et al. 2009; Zanetti et al. 2012; Sarkar et al. 2015; Rodrigues et al. 2016) and treatment for diabetic macular oedema (Callanan & Williams 2008), cystoid macular oedema (Warren & Fox 2008), pain after photorefractive keratectomy (Caldwell & Reilly 2008; Faktorovich & Melwani 2014; ), recalcitrant macular degeneration (Chen et al. 2010; Libondi & Jonas 2010), macular oedema after epiretinal surgery (Schoenberger et al. 2011), pain and inflammation after vitreoretinal surgery (Naithani et al. 2012), acute central chorioretinopathy (Alkin et al. 2013), postoperative inflammation after small‐gauge vitrectomy (Nagpal et al. 2014), ocular discomfort after vitreous injections (Ulrich 2014) and postoperative pain associated with pterygium surgery (Ozcimen et al. 2015). However, we did not find published studies describing the frequency of nepafenac use or dispensing for the approved indication or other conditions in routine health care, as we did in this study.
The main limitation of this study is the incompleteness of capture of cataract surgeries and other ophthalmic conditions in the data sources. In the Netherlands, the Hospitalisation Database in PHARMO would miss ambulatory procedures. As many of the ophthalmic procedures in this study are performed during outpatient visits, the number of ophthalmic procedures is likely to be underestimated. In Denmark, cataract surgeries conducted in public hospitals are recorded in the Danish National Patient Registry (Schmidt et al. 2015). In 2002, to shorten the wait for this surgery, cataract surgeries started being offered by private hospitals or clinics at government expense (Solborg Bjerrum et al. 2013). Reporting these surgeries to the Danish National Patient Registry has been voluntary since 2002 and mandatory since 2004 or 2006 (Solborg Bjerrum et al. 2013, 2015). However, during the course of this study, evidence surfaced that some surgeries occurring in the private setting were not systematically recorded in the Danish National Patient Registry. In a study of infectious endophthalmitis, a dreaded but rare complication of cataract surgery, the authors found that only 59% of those cases that by manual chart review were found to be related to cataract surgery had a record of surgery in the registry. It could be estimated that 98% of surgeries performed in hospitals were recorded, while this proportion was 38% for private clinics (Solborg Bjerrum et al. 2013). Older patients and patients with a high level of comorbidity were more likely to have their surgery performed in a hospital. Thus, sensitivity analyses were designed and implemented in both data sources to address this underrecording. In the Netherlands, we observed that 57% of nepafenac episodes had an ophthalmic procedure, condition or correspondence within 1 month before or after the start of the episode. In Denmark, we found a larger percentage of therapy episodes with recorded cataracts in sicker and older patients than in the study population, providing evidence of underrecording of cataract surgery in younger and healthier patients (with less underrecording for nepafenac than for ketorolac or diclofenac) or more use of the drugs unrelated to cataract surgery in younger and healthier patients. In the Netherlands, a national cataract registry exists. This registry, maintained by the ophthalmic society Nederlands Oogheelkundig Gezelschap, does not include detailed information on medication use and would therefore not be suitable for our study. We considered linking the PHARMO Database Network and the cataract registry. However, in 2011, only 50% of cataract surgeries were entered into this registry; linkage to this cataract registry would not have solved the limitation.
Another limitation is that we did not have information on whether prescriptions were written to cover treatment for one or two eyes. However, based on the observation that 86% of patients in the Netherlands had surgery in only one eye and that cataract surgeries in fellow eyes are separated by a few weeks in Denmark (Solborg Bjerrum et al. 2015), we assumed that therapy episodes were for a single eye.
In conclusion, based on this evaluation of 12 691 nepafenac therapy episodes in the Netherlands and 73 648 in Denmark, we can confirm that practically all dispensing of nepafenac is in adults. In both populations, less than half of the therapy episodes occurred in patients with recorded cataract surgery; however, substantial underrecording of cataract surgery occurs in Denmark and is likely in the Netherlands. For this reason, our observed figures should be seen as the upper limit of the true number. Based on the dispensed number of bottles, the estimated duration of treatment is appropriate for the approved indication in 60% of adult patients without diabetes and 90% of adult patients with diabetes in the Netherlands, and in 92% and 99.8%, respectively, in Denmark. The underrecording of ophthalmic conditions in automated healthcare databases used in pharmacoepidemiology is higher than initially expected and challenges research in this important indication.
Supporting information
Table S1. Number of Bottles per Therapy Episode for Ophthalmic Nepafenac in Adults With Recorded Cataract Surgery, in the Early Study Period (Until June 2011) and Late Study Period (From January 2012), the Netherlands and Denmark.
This collaborative study was conducted by RTI Health Solutions, Southern Denmark University and PHARMO Institute for Drug Outcomes Research under an Alcon Laboratories (UK) Ltd contract, which included independent publication rights. In alignment with Good Pharmacoepidemiology and Good Pharmacovigilance Practice, Alcon has had opportunity to review the manuscript and provide critical feedback. A. Margulis, A. Arana and S. Perez‐Gutthann are employees of RTI Health Solutions, a business unit of RTI International, which has been compensated for this research. RTI International is an independent, non‐profit research organization that conducts work for government, public and private organizations including pharmaceutical companies. E. Houben and J. A. Overbeek are employees of the PHARMO Institute for Drug Outcomes Research; this independent research institute performs financially supported studies for government and related healthcare authorities and several pharmaceutical companies. Jesper Hallas and Anton Pottegård: None declared. These results have been presented at the International Conference on Pharmacoepidemiology & Therapeutic Risk Management, 25–28 August 2016, in Dublin, Ireland. The research team thanks the Alcon epidemiology and pharmacovigilance team, Wendy Ye, Dorian Villegas, Francisca Moreno, Irene Rebollo and Fred Schneiweiss, for the support and insights during the planning and reporting of the study. The research team thanks RTI staff Adele Monroe for editing, Jason Mathes for graphic art, Carla Franzoni for project management, as well as Morten Olesen for skilled data management in Denmark.
References
- Alcon (2015): Summary of product characteristics. Nevanac 1 mg/ml eye drops, suspension (nepafenac). Surry, UK: Alcon Laboratories (UK) Ltd; Available at http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000818/human_med_000928.jsp&mid=WC0b01ac058001d124. (Accessed 22 Aug 2016). [Google Scholar]
- Alkin Z, Osmanbasoglu OA, Ozkaya A, Karatas G & Yazici AT (2013): Topical nepafenac in treatment of acute central serous chorioretinopathy. Med Hypothesis Discov Innov Ophthalmol 2: 96–101. [PMC free article] [PubMed] [Google Scholar]
- Allergan Pharmaceuticals Ireland (2014): Package leaflet: information for the user. Acular® 0.5% eye drops. Westport, Ireland: Allergan Pharmaceuticals Ireland; Acular® topical ophthalmic solution (ketorolac trometamol). Available at http://www.mhra.gov.uk/home/groups/spcpil/documents/spcpil/con1423805271106.pdf. (Accessed 13 Jul 2016). [Google Scholar]
- Caldwell M & Reilly C (2008): Effects of topical nepafenac on corneal epithelial healing time and postoperative pain after PRK: a bilateral, prospective, randomized, masked trial. J Refract Surg 24: 377–382. [DOI] [PubMed] [Google Scholar]
- Callanan D & Williams P (2008): Topical nepafenac in the treatment of diabetic macular edema. Clin Ophthalmol 2: 689–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes‐Coste G, Sanchez‐Castro YG, Orozco‐Carroll M, Mendoza‐Schuster E & Velasco‐Barona C (2009): Inhibition of surgically induced miosis and prevention of postoperative macular edema with nepafenac. Clin Ophthalmol 3: 219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Benz MS, Fish RH, Brown DM, Wong TP, Kim RY & Major JC (2010): Use of nepafenac (Nevanac) in combination with intravitreal anti‐VEGF agents in the treatment of recalcitrant exudative macular degeneration requiring monthly injections. Clin Ophthalmol 4: 1249–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Excelvision S.A.S . (2015): Voltarol® Ophtha Multidose. 0.1% eye drops (diclofenac sodium). Patient information leaflet. Annonay, France: Excelvision; Available at: http://www.mhra.gov.uk/home/groups/spcpil/documents/spcpil/con1491541383012.pdf. (Accessed 12 Apr 2017). [Google Scholar]
- Faktorovich EG & Melwani K (2014): Efficacy and safety of pain relief medications after photorefractive keratectomy: review of prospective randomized trials. J Cataract Refract Surg 40: 1716–1730. [DOI] [PubMed] [Google Scholar]
- Herings RMC (1993): PHARMO: a record linkage system for postmarketing surveillance of prescription drugs in the Netherlands. Utrecht: Utrecht University, Faculty of Farmacie. [Google Scholar]
- Kildemoes HW, Sorensen HT & Hallas J (2011): The Danish National Prescription Registry. Scand J Public Health 39: 38–41. [DOI] [PubMed] [Google Scholar]
- Libondi T & Jonas JB (2010): Topical nepafenac for treatment of exudative age‐related macular degeneration. Acta Ophthalmol 88: e32–e33. [DOI] [PubMed] [Google Scholar]
- Nagpal M, Lambat S, Mehrotra N, Paranjpe G, Yadav H & Bhardwaj S (2014): Topical nepafenac 0.1% alone versus prednisolone acetate 1% as postoperative anti‐inflammatory agents in small gauge vitrectomy. Indian J Ophthalmol 62: 606–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naithani P, Puranik S, Vashisht N, Khanduja S, Kumar S & Garg S (2012): Role of topical nepafenac in prevention and treatment of macular edema after vitreoretinal surgery. Retina 32: 250–255. [DOI] [PubMed] [Google Scholar]
- Ozcimen M, Sakarya Y, Goktas S, Sakarya R, Yener HI, Bukus A & Demir LS (2015): Effect of nepafenac eye drops on pain associated with pterygium surgery. Eye Contact Lens 41: 187–189. [DOI] [PubMed] [Google Scholar]
- Rodrigues EB, Farah ME, Mantovani Bottos J & Bom Aggio F (2016): Nonsteroidal anti‐inflammatory drugs in the treatment of retinal diseases. Dev Ophthalmol 55: 212–220. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Mondal KK, Roy SS, Gayen S, Ghosh A & De RR (2015): Comparison of preoperative nepafenac (0.1%) and flurbiprofen (0.03%) eye drops in maintaining mydriasis during small incision cataract surgery in patients with senile cataract: a randomized, double‐blind study. Indian J Pharmacol 47: 491–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Pedersen L & Sorensen HT (2014): The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol 29: 541–549. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L & Sorensen HT (2015): The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol 7: 449–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenberger SD, Miller DM, Petersen MR, Foster RE, Riemann CD & Sisk RA (2011): Nepafenac for epiretinal membrane surgery. Ophthalmology 118: 1482 e1481–1483. [DOI] [PubMed] [Google Scholar]
- Solborg Bjerrum S, Kiilgaard JF, Mikkelsen KL & la Cour M (2013): Outsourced cataract surgery and postoperative endophthalmitis. Acta Ophthalmol 91: 701–708. [DOI] [PubMed] [Google Scholar]
- Solborg Bjerrum S, Mikkelsen KL & la Cour M (2015): Epidemiology of 411,140 cataract operations performed in public hospitals and private hospitals/clinics in Denmark between 2004 and 2012. Acta Ophthalmol 93: 16–23. [DOI] [PubMed] [Google Scholar]
- Thygesen LC & Ersboll AK (2014): When the entire population is the sample: strengths and limitations in register‐based epidemiology. Eur J Epidemiol 29: 551–558. [DOI] [PubMed] [Google Scholar]
- Ulrich JN (2014): Topical nepafenac after intravitreal injection: a prospective double‐masked randomized controlled trial. Retina 34: 509–511. [DOI] [PubMed] [Google Scholar]
- Warren KA & Fox JE (2008): Topical nepafenac as an alternate treatment for cystoid macular edema in steroid responsive patients. Retina 28: 1427–1434. [DOI] [PubMed] [Google Scholar]
- Wilson DJ, Schutte SM & Abel SR (2015): Comparing the efficacy of ophthalmic NSAIDs in common indications: a literature review to support cost‐effective prescribing. Ann Pharmacother 49: 727–734. [DOI] [PubMed] [Google Scholar]
- Zanetti FR, Fulco EA, Chaves FR, da Costa Pinto AP, Arieta CE & Lira RP (2012): Effect of preoperative use of topical prednisolone acetate, ketorolac tromethamine, nepafenac and placebo, on the maintenance of intraoperative mydriasis during cataract surgery: a randomized trial. Indian J Ophthalmol 60: 277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Number of Bottles per Therapy Episode for Ophthalmic Nepafenac in Adults With Recorded Cataract Surgery, in the Early Study Period (Until June 2011) and Late Study Period (From January 2012), the Netherlands and Denmark.


