Abstract
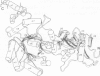
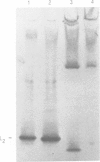
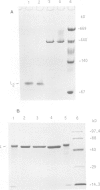
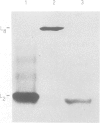
Truncations of the subunit of ribulose bisphosphate carboxylase/oxygenase (Rubisco) from Rhodospirillum rubrum were generated by site-directed mutagenesis to examine the role of the C-terminal tail section. Removal of the last and the penultimate alpha-helices in the tail section changes the quaternary structure of the protein. Electrophoretic and electron microscope analysis revealed that the truncated subunits assemble into an octamer, whereas the wild-type enzyme has a dimeric structure. The octomerization of the mutant protein is due to a hydrophobic patch exposed to the solvent by truncation of the subunit. The mutant protein thus consists of four dimers, bound end-to-end by hydrophobic interactions. Insertion of a polar amino acid in the hydrophobic patch by a L424 to N424 substitution restores the familiar dimeric structure. Truncation of the subunit is associated with a considerable decrease in catalytic activity. The mutants undergo carbamylation but bind the reaction intermediate analog, 2-carboxy arabinitol-1,5-bisphosphate, poorly. This indicates that loss of activity in the mutant is due to weakened substrate binding. These findings suggest that the mutations in the tail section of the subunit are transmitted to the active site, although the C-terminal region is far from the active site. On the basis of the crystal structure of Rubisco, we propose a model for how the truncations of the enzyme subunit induce conformational changes in one of the two phosphate binding sites.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banner D. W., Bloomer A. C., Petsko G. A., Phillips D. C., Pogson C. I., Wilson I. A., Corran P. H., Furth A. J., Milman J. D., Offord R. E. Structure of chicken muscle triose phosphate isomerase determined crystallographically at 2.5 angstrom resolution using amino acid sequence data. Nature. 1975 Jun 19;255(5510):609–614. doi: 10.1038/255609a0. [DOI] [PubMed] [Google Scholar]
- Chapman M. S., Suh S. W., Cascio D., Smith W. W., Eisenberg D. Sliding-layer conformational change limited by the quaternary structure of plant RuBisCO. Nature. 1987 Sep 24;329(6137):354–356. doi: 10.1038/329354a0. [DOI] [PubMed] [Google Scholar]
- Chapman M. S., Suh S. W., Curmi P. M., Cascio D., Smith W. W., Eisenberg D. S. Tertiary structure of plant RuBisCO: domains and their contacts. Science. 1988 Jul 1;241(4861):71–74. doi: 10.1126/science.3133767. [DOI] [PubMed] [Google Scholar]
- Estelle M., Hanks J., McIntosh L., Somerville C. Site-specific mutagenesis of ribulose-1,5-bisphosphate carboxylase/oxygenase. Evidence that carbamate formation at Lys 191 is required for catalytic activity. J Biol Chem. 1985 Aug 15;260(17):9523–9526. [PubMed] [Google Scholar]
- Hartman F. C., Soper T. S., Niyogi S. K., Mural R. J., Foote R. S., Mitra S., Lee E. H., Machanoff R., Larimer F. W. Function of Lys-166 of Rhodospirillum rubrum ribulosebisphosphate carboxylase/oxygenase as examined by site-directed mutagenesis. J Biol Chem. 1987 Mar 15;262(8):3496–3501. [PubMed] [Google Scholar]
- Kabsch W., Sander C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983 Dec;22(12):2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- Knight S., Andersson I., Brändén C. I. Reexamination of the Three-Dimensional Structure of the Small Subunit of RuBisCo from Higher Plants. Science. 1989 May 12;244(4905):702–705. doi: 10.1126/science.244.4905.702. [DOI] [PubMed] [Google Scholar]
- Larimer F. W., Lee E. H., Mural R. J., Soper T. S., Hartman F. C. Intersubunit location of the active site of ribulose-bisphosphate carboxylase/oxygenase as determined by in vivo hybridization of site-directed mutants. J Biol Chem. 1987 Nov 15;262(32):15327–15329. [PubMed] [Google Scholar]
- Lasters I., Wodak S. J., Alard P., van Cutsem E. Structural principles of parallel beta-barrels in proteins. Proc Natl Acad Sci U S A. 1988 May;85(10):3338–3342. doi: 10.1073/pnas.85.10.3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer G. H., Badger M. R., Andrews T. J. D-Ribulose-1,5-bisphosphate carboxylase-oxygenase. Improved methods for the activation and assay of catalytic activities. Anal Biochem. 1977 Mar;78(1):66–75. doi: 10.1016/0003-2697(77)90009-4. [DOI] [PubMed] [Google Scholar]
- Lorimer G. H., Hartman F. C. Evidence supporting lysine 166 of Rhodospirillum rubrum ribulosebisphosphate carboxylase as the essential base which initiates catalysis. J Biol Chem. 1988 May 15;263(14):6468–6471. [PubMed] [Google Scholar]
- Lorimer G. H. Ribulosebisphosphate carboxylase: amino acid sequence of a peptide bearing the activator carbon dioxide. Biochemistry. 1981 Mar 3;20(5):1236–1240. doi: 10.1021/bi00508a028. [DOI] [PubMed] [Google Scholar]
- Pierce J., Tolbert N. E., Barker R. Interaction of ribulosebisphosphate carboxylase/oxygenase with transition-state analogues. Biochemistry. 1980 Mar 4;19(5):934–942. doi: 10.1021/bi00546a018. [DOI] [PubMed] [Google Scholar]
- Schloss J. V., Phares E. F., Long M. V., Norton I. L., Stringer C. D., Hartman F. C. Ribulosebisphosphate carboxylase/oxygenase from Rhodospirillum rubrum. Methods Enzymol. 1982;90(Pt E):522–528. doi: 10.1016/s0076-6879(82)90179-3. [DOI] [PubMed] [Google Scholar]
- Schneider G., Lindqvist Y., Brändén C. I., Lorimer G. Three-dimensional structure of ribulose-1,5-bisphosphate carboxylase/oxygenase from Rhodospirillum rubrum at 2.9 A resolution. EMBO J. 1986 Dec 20;5(13):3409–3415. doi: 10.1002/j.1460-2075.1986.tb04662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soper T. S., Mural R. J., Larimer F. W., Lee E. H., Machanoff R., Hartman F. C. Essentiality of Lys-329 of ribulose-1,5-bisphosphate carboxylase/oxygenase from Rhodospirillum rubrum as demonstrated by site-directed mutagenesis. Protein Eng. 1988 Apr;2(1):39–44. doi: 10.1093/protein/2.1.39. [DOI] [PubMed] [Google Scholar]
- Tabita F. R., McFadden B. A. D-ribulose 1,5-diphosphate carboxylase from Rhodospirillum rubrum. II. Quaternary structure, composition, catalytic, and immunological properties. J Biol Chem. 1974 Jun 10;249(11):3459–3464. [PubMed] [Google Scholar]
- WEISSBACH A., HORECKER B. L., HURWITZ J. The enzymatic formation of phosphoglyceric acid from ribulose diphosphate and carbon dioxide. J Biol Chem. 1956 Feb;218(2):795–810. [PubMed] [Google Scholar]