Abstract
Although neuroglobin confers neuroprotection against Alzheimer's disease (AD) pathology, its expression becomes downregulated in late‐stage AD. Here, we provide evidence that indicates that this decrease is associated with the AD‐linked angiopathy. While wild‐type mice of different ages show upregulated cerebral neuroglobin expression upon whole‐body hypoxia, APP23 mice exhibit decreased cerebral transcription of neuroglobin. Interestingly, transcription of cytoglobin, whose involvement in amyloid pathology still needs to be elucidated, follows a similar pattern. To further unravel the underlying mechanism, we examined the expression levels of the RE‐1‐silencing transcription factor (REST/NRSF) after identifying a recognition site for it in the regulatory region of both globins. Neuroglobin‐cytoglobin‐REST/NRSF expression correlations are detected mainly in the cortex. This raises the possibility of REST/NRSF being an upstream regulator of these globins.
Keywords: APP23, cytoglobin, hypoxia, neuroglobin, REST/NRSF
Abbreviations
Aβ, β‐amyloid
AD, Alzheimer's disease
APP, amyloid precursor protein
CAA, cerebral amyloid angiopathy
Cygb, cytoglobin
Ets‐1, V‐ets avian erythroblastosis virus E26 oncogene homolog 1
Hif1, hypoxia‐inducible factor 1
Ngb, neuroglobin
REST, RE1‐silencing transcription factor
ROS, reactive oxygen species
SEM, standard error of the mean
Sp1, specificity protein 1
VEGF, vascular endothelial growth factor
Alzheimer's disease (AD) is a prevailing neurodegenerative disorder with a clinical representation of neuropsychiatric disturbances and a progressive cognitive deterioration 1. On the histopathological level, AD brains are typified by the emergence of insoluble neurofibrillary tangles and extracellular plaques 2. The main building blocks of these aggregates are hyperphosphorylated tau protein and amyloid precursor protein (APP)‐derived β‐amyloid (Aβ), respectively. However, a clear correlation between the level of senile plaque burden and the dementia state is lacking 2. This led to the study of soluble Aβ species which are now thought to be one of the toxic entities that drive neuronal dysregulation, culminating in widespread neurodegeneration 3. Aβ possesses a metal‐binding capacity, entailing the generation of reactive oxygen species (ROS) through Fenton and Fenton‐like chemistry 4. Knowing the Aβ burden to progressively accumulate, it may be no surprise that antioxidant mechanisms no longer meet with such requirements, initiating a runaway process through ROS‐related mitochondrial damage 4, 5.
Cytoglobin (Cygb) 6 and neuroglobin (Ngb) 7 are heme proteins of the globin family with a ROS‐scavenging potential 8. Where the involvement of Cygb in neuroprotection is largely unexplored, Ngb has been receiving much attention given its neuron‐specific localization 9. Upon oxygen and glucose deprivation, Ngb transcription is upregulated in SH‐SY5Y cells, which is associated with enhanced cell survival 8. Moreover, elevated Ngb levels not only attenuate cortical hypoxic–ischemic injury in vivo 10, but are implicated in AD as well. In vitro studies showed an increased resistance of Ngb‐overexpressing primary neurons 11 and PC12 cells 12 against Aβ25‐35‐induced neurotoxicity. Ngb also sequesters heme from Aβ‐heme complexes, entailing a possible role in reducing the oxidation of neurotransmitters like serotonin 13. Ngb interferes with Aβ production in vivo, reducing Aβ1‐40 and Aβ1‐42 levels in a double‐cross mouse model that overexpresses Ngb in an AD‐linked background with both the Swedish (K670N/M671L) and Indiana (V717F) mutation 11. In a clinical setting, however, Ngb has only been found to be upregulated in early and moderately advanced AD, while being reduced to baseline levels when the pathology progresses to more severe stages 14, 15.
Interestingly, many risk factors for dementia are linked to acute ischemic events and the impediment of cerebral perfusion, for example, smoking, hypertension, and diabetes mellitus 16, 17, 18. Brain infarcts have, in particular, been identified as important modifiers of the severity of AD clinical symptoms 19. Moreover, the prevalence of dementia increases from 57% to 88% 19. In addition, neuroimaging data revealed ~ 80% more dysregulation in the cerebral vascular system of late onset AD cases as compared to other pathogenic events as, for example, Aβ deposition and metabolic dysfunction 20. AD also shares features involving white matter changes, clinical symptoms, and pathophysiological markers with vascular dementia 21. With cerebral hypoperfusion generally foregoing neurodegeneration, some authors even suggested to classify AD as a vascular pathology 16. The detrimental effect lays in reduced brain Aβ clearance and its ensuing deposition in leptomeningeal and cerebral vessel walls as cerebral amyloid angiopathy (CAA) 22, which is observed in up to 90% of AD patients 23. Of note, sustained elevated ROS levels are a key mediator of CAA and microhemorrhages as well 24. Hence, given the additional strong link between globins and oxygen availability 25, we questioned whether hypoxia would impact Ngb expression in young APP23 mice to a similar extend as would vast Aβ pathology. Moreover, while functions of Cygb entail neuroprotection, for example, detoxification of ROS 8, 26, 27, its involvement in AD pathology remained to be elucidated. We, therefore, opted to study the alterations of Ngb and Cygb levels in APP23 mice in parallel.
Aging APP23 mice represent a valid phenocopy of human AD, with a strong Aβ pathology in the neocortex and hippocampal region, and CA1 neuronal loss reaching up to 25% in mice with a high Aβ aggregation load 28, 29. CAA initiates in 8‐month‐old APP23 mice as focal deposits in leptomeningeal vessels 23. The transgene—human APP with the Swedish double mutation (K670N/M671L)—is expressed sevenfold above endogenous APP levels and driven by the neuron‐specific murine Thy‐1.2 promoter 28.
The present study shows for the first time Cygb expression upregulation in normoxic cortices of 12‐month‐old APP23 mice. We further detected Ngb and Cygb transcription to be downregulated in Aβ‐overexpressing brains when the APP23 mice were subjected to an additional hypoxic insult. To further unravel the underlying mechanism, we studied RE‐1‐silencing transcription factor (REST/NRSF) expression levels, for which we identified a recognition site in the regulatory region of both globins. Ngb‐Cygb‐REST/NRSF expression correlations were detected mainly in the cortex. These findings provide evidence for the loss of globin‐linked neuroprotection to be linked to AD angiopathogenis, while raising the possibility of REST/NRSF being an upstream regulator together with hypoxia‐inducible factor 1 (Hif1).
Experimental procedure
Transgenic mouse model
Male transgenic APP23 mice were studied along with wild‐type control littermates at the age of 3, 6, and 12 months. The colony of experimental animals was maintained on a C57BL/6J background for at least 20 generations. All procedures were approved by the animal ethics committee of the University of Antwerp (reference number 2012‐26) and were compliant to the European Communities Council Directive on the protection of animals used for scientific purposes (2010/63/EU).
Hypoxia induction and reoxygenation
Experimental groups of wild‐type or APP23 mice were either (i) housed for 48 h at a normoxic oxygen concentration of 21%, (ii) housed for 48 h in a hypoxic chamber with 7% oxygen (premixed gases: 7% O2, 93% N2) or (iii), after the exposure to hypoxic air, reoxygenated for 6 h under normoxic conditions, as previously described 30. Mice of group 2 were euthanized by cervical dislocation immediately upon removal from the hypoxic chamber.
RNA extraction and real‐time quantitative PCR
The frontal cortex and hippocampus of each mouse was collected, snap frozen in liquid nitrogen and further processed for total RNA. RNA was extracted, using TRIzol Reagent in conjunction with the PureLink RNA Mini Kit (Life Technologies, Gent, Belgium) according to the manufacturer's instructions. First‐strand cDNA synthesis was performed on 1 μg of total RNA (NanoDrop; Thermo Scientific, Gent, Belgium) with the GoScript Reverse Transcription System (Promega, Leiden, the Netherlands) and random primers (0.5 μg per reaction). Real‐time quantitative PCR was performed on a StepOnePlus system (Life Technologies), following a 2‐min incubation at 95 °C before proceeding to 40 cycles of 95 °C for 15 s and 60 °C for 1 min. Each real‐time PCR reaction comprised Power SYBR Green PCR Master Mix (Applied Biosystems, Halle, Belgium), 7.5 ng cDNA (RNA equivalents), and 150 nm of each primer: GAPDH (F‐AGGTCGGTGTGAACGGATTTG, R‐GGGGTCGTTGATGGCAACA), β‐Actin (F‐GGCTGTATTCCCCTCCATCG, R‐CCAGTTGGTAACAATGCCATGT), B2M (F‐GTATACTCACGCCACCCACC, R‐TGGGGGTGAATTCAGTGTGAG), Pgk1 (F‐CTCCGCTTTCATGTAGAGGAAG, R‐GACATCTCCTAGTTTGGACAGTG), Ngb (F‐TACAATGGCCGCCAGTTCT, R‐TGGTCACTGCAGCATCAATCA), Cygb (F‐CCGCAGCCTACAAGGAAGTGG, R‐GGGAGCTGGGAGGGGTCTT), Hif1 (F‐CGGCTCATAACCCATCAACT, R‐CAACGTGGAAGGTGCTTCA), VEGF (F‐GCCTCCCTCAGGGTTTCGG,R‐CGATGATGGCGTGGTGGTG), or REST (F‐CATGGCCTTAACCAACGACAT, R‐CGACCAGGTAATCGCAGCAG). The four used housekeeping genes (GAPDH, β‐actin, B2M, and Pgk1) were selected from a list of 10 reference targets, using geNorm in qBase+ (Version 3.0; Biogazelle, Gent, Belgium), and achieved a value V < 0.15. Inter‐run calibration factors for each target were included to remove the run‐to‐run difference.
Immunohistochemistry
Coronal paraffin sections of brains were immunostained after microwave‐citrate (pH6) antigen retrieval for 10 min. Endogenous peroxidase activity within the tissue was blocked by incubation in 1% hydrogen peroxide in methanol for 30 min. After incubation in 1/25 normal goat serum and 1% bovine serum albumin in tris‐buffered saline to block nonspecific antibody binding, slides were stained overnight at 4 °C with primary antibody (1 : 500 Aβ ab2539, Abcam, Cambridge, UK; 1 : 100 Ngb 13499‐1‐AP, Protein Tech, Manchester, UK; 1 : 100 polyclonal Cygb 31). Subsequently, sections were incubated with 1 : 200 goat anti‐rabbit antibody (E0432; Dako, Leuven (Heverlee), Belgium) and further developed with the ABC Kit (Vector Laboratories, Peterborough, UK). Images were captured using an Olympus BX51 standard research fluorescence microscope (Olympus, Berchem, Belgium) equipped with an Olympus DP71 digital camera. Intensities of staining patterns were analyzed with imagej software (Fiji, Madison, WI, USA) and represented as optical densities; OD = log(max intensity/mean intensity).
Oxidative damage
Total brain hemisphere lysate levels of malondialdehyde, an end product of lipid peroxidation, were studied using the lipid peroxidation (MDA) assay kit (Biovision, Milpitas, CA, USA) according to the manufacturer's instructions. The degree of lipid peroxidation was determined with reference to a malondialdehyde standard.
Data analysis
The relative mean transcript values for the different conditions were calculated from normalized Cq values by reference to the mean of the baseline group, being the 3‐month‐old wild‐type mice that were housed under normoxic conditions. Calculations were performed with qBase+ (Version 3.0; BioGazelle). For immunohistochemistry data, the mean value of the cortex of the normoxic 3‐month‐old wild‐type mice was considered as the baseline. graphpad prism software (v5.0; San Diego, CA, USA) was used for graphing and statistical calculations. Statistical comparisons were performed using repeated measures analysis of variance (one‐way ANOVA) with Bonferroni's post hoc testing or Student's t‐testing, as indicated. P‐values below 0.05 were considered statistically significant. All data are depicted as the mean ± standard error of the mean (SEM).
Results
Aβ accretion and lipid peroxidation impact neuroprotective globin levels
To be able to follow the progression of pathology, we studied amyloid immunoreactivity in the cortex and hippocampus of APP23 mice and wild‐type control littermates at the age of 3, 6, and 12 months. The hippocampus shows higher Aβ protein staining intensities than the cortex and the cerebral Aβ load significantly increases only in the hippocampus (t = 4.174 Bonferroni, Fig. 1D). Given lipid peroxidation to be an early event in AD development and to be a major product of free radical‐mediated damage 32, we also quantified the levels of malondialdehyde, an end product of lipid peroxidation (Fig. 1E). The severity of both these inductors of cellular stress was correlated with the cortical and hippocampal transcription level of Cygb and Ngb (as comparison). APP23 mice of 12 months of age showed a 2.43 ± 0.21‐fold increase in Cygb expression in the frontal cortex, similar to the upsurge in Ngb transcription (Fig. 1A). Such an effect was not detected at the age of 3 and 6 months, that is, before the onset of plaque formation (Fig. 1D) and the kick‐in of oxidative stress (Fig. 1E). In contrast to the frontal cortex, none of the studied neuroprotective transcripts was significantly upregulated in the hippocampus (Fig. 1B).
Figure 1.
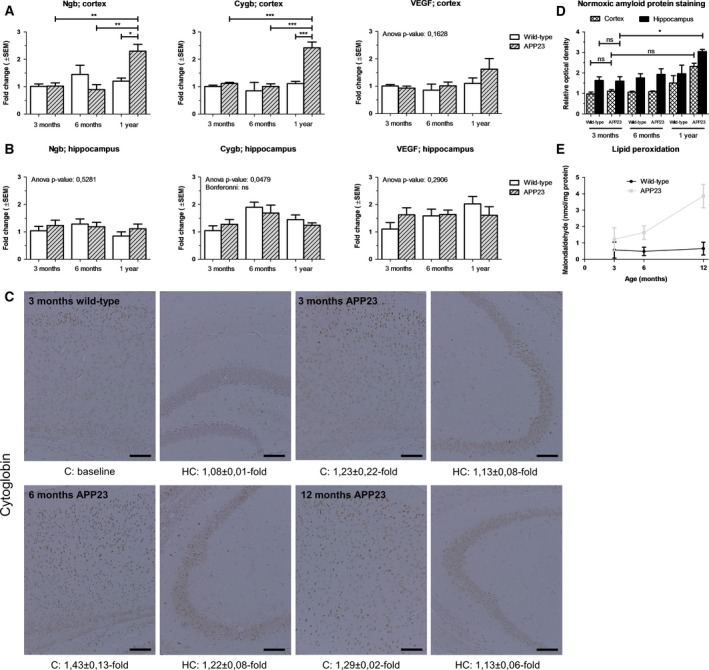
Influence of Aβ deposition and lipid peroxidation on increases in neuroprotective Ngb and Cygb. (A) Cortical and (B) hippocampal real‐time qPCR data of murine Cygb, Ngb, and VEGF expression levels of wild‐type mice (n = 4 per group) and APP23 mice (n = 4 per group) under normoxic conditions. (C) High‐power photomicrographs represent 20 × magnified pictures of Cygb immunohistochemistry. Optical densities relative to the 3‐month‐old wild‐type cortex are listed below. The scale bar represents 100 μm. Intensities show a disease progression‐dependent upregulation, that is, upon limited plaque burden (D, n ≥ 2) and in the presence of oxidative damage (E, n = 3). Data are depicted as the mean ± SEM. Statistical significance was tested using one‐way ANOVA with Bonferroni post hoc test; *P < 0.05, **P < 0.01, ***P < 0.001.
Next, we studied the relationship between the globins’ mRNA and protein levels, confirming that the Ngb protein staining pattern intensity increases at the onset of pathology (1.48 ± 0.19‐fold increase in cortices of 1‐year‐old APP23 mice, data not shown). However, we are the first too show that the optical density of the Cygb protein staining is also upregulated by 1.29 ± 0.02‐ and 1.13 ± 0.06‐fold in the cortex and hippocampus of 12‐month‐old APP23 mice, respectively. Of note, these elevations in Cygb protein levels correlate less with disease stage as those of Ngb, that is, they are not significantly different from those of the earlier 3 and 6 month time points (Fig. 1C).
Wild‐type animals show a more pronounced hypoxia‐counteracting transcriptional response
Next, we sought to elucidate whether hypoxic events could underlie the previously observed downregulation of Ngb expression in the later stages of AD pathology 14, 15. Moreover, we aimed to examine if a similar impact of experimentally induced hypoxia could be detected on Cygb levels in limited Aβ‐stressed brains. Hence, mice of both genotypes and different ages were subjected to a general hypoxic insult of 7% oxygen for 48 h. Wild‐type mice showed the expected increase in globin expression (10, 33, 34, Fig. 2A). In APP23 mice of 3‐ and 6‐months, however, Cygb and Ngb transcript levels stayed unaltered in comparison to the normoxic condition (Fig. 2A). Moreover, the globin expression levels were significantly lowered in the frontal cortex as compared to the normoxia group at 1 year (up to 0.62‐fold). Interestingly, mice of the same genotype but from different age groups did not display a difference in cerebral oxidative stress level upon the hypoxic insult, as can be deducted from the lipid peroxidation levels (Fig. 2D, ANOVA: P = 0.0572 for wild‐type mice and P = 0.1370 for APP23 mice). On the contrary, strong to very strong positive relationships were detected between VEGF and globin transcript levels across the different conditions (Table 1). These results indicate that the differences in globin transcription, that are perceived under hypoxia across the different age groups, are not driven by differences in lipid peroxidation levels, but rather can be attributed to other cellular dysregulations, for example, due to amyloid metabolism dysfunctions and VEGF signaling. Of note, although Cygb levels are lowered in the 1‐year‐old APP23 frontal cortices upon hypoxia induction, their levels are still retained above the level of 3‐month‐old, normoxic wild‐type mice (1.52‐fold, Fig. 2B). As for reinduction of globin expression after reoxygenation, the data of the hippocampus reveal a broader upregulation across the different conditions than the cortex (Fig. 2B).
Figure 2.
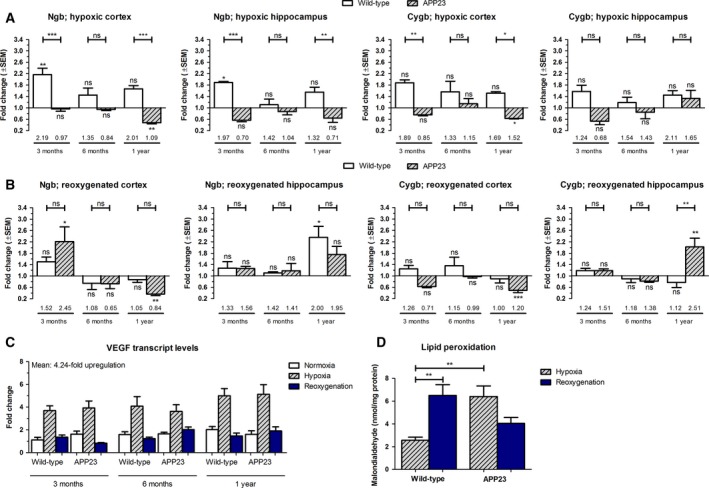
Withdrawal of Ngb expression upregulation upon hypoxia in APP23 brain. Cortical and hippocampal transcript (A,B) levels of murine Cygb and Ngb after (A) 48 h of hypoxia and (B) 48 h of hypoxia, followed by 6 h of reoxygenation. Data of wild‐type and APP23 mice (n = 4 per group) are depicted as the mean ± SEM and represent values relative to the age/genotype‐matched normoxic condition. Numbers above horizontal bars represent mean values relative to the baseline (values of 3‐month‐old, normoxic wild‐type mice). Significant differences with the baseline are defined as P < 0.05, according to Student's t‐test and depicted above rectangles. Other statistical significances were tested using one‐way ANOVA with Bonferroni post hoc test; *P < 0.05, **P < 0.01, ***P < 0.001. (C) Hippocampal expression levels of VEGF. (D) Quantitation of malondialdehyde (n = 3 per group), representing an end product of lipid peroxidation, in total brain hemisphere lysates. Given the lack of age‐dependent differences, the values of all wild‐type and APP23 mice were combined.
Table 1.
Pearson's correlation coefficients across the different oxygen availability conditions
| Correlation | VEGF: frontal cortex | VEGF: hippocampus | ||||
|---|---|---|---|---|---|---|
| Normoxia | Hypoxia | Reoxygenation | Normoxia | Hypoxia | Reoxygenation | |
| Cygb | 0.55 | 0.64 | 0.62 | 0.65 | 0.47 | 0.71 |
| Ngb | 0.38 | 0.66 | 0.74 | 0.59 | 0.74 | 0.63 |
r ≥ +0.70, very strong positive relationship; r = +0.40 to +0.69, strong positive relationship; r = +0.30 to +0.39, moderate positive relationship; r = +0.20 to +0.29, weak positive relationship; r = +0.01 to +0.19, no or negligible relationship.
In contrast to the transcriptional level where hypoxia reduces globin expression in APP23 mice, Ngb and Cygb protein levels are slightly elevated across all studied ages and in both the cortex and hippocampus of APP23 mice (Fig. 3A). The upregulation is paralleled with the lower degree of peroxidation as compared to the one of wild‐type mice (Fig. 2D). Of note, increases in protein staining after hypoxia and reoxygenation are higher for Cygb than for Ngb (Fig. 3A/B).
Figure 3.
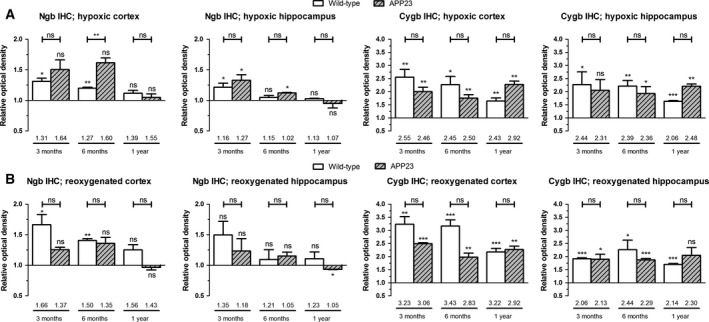
Increased globin protein levels upon and after hypoxia in wild‐type and APP23 brain. Cortical and hippocampal protein (A,B) levels of murine Cygb and Ngb after (A) 48 h of hypoxia and (B) 48 h of hypoxia, followed by 6 h of reoxygenation. Data of wild‐type and APP23 mice (n ≥ 2 per group) are depicted as the mean ± SEM and represent values taken relative to the age/genotype‐matched normoxic condition. Numbers above horizontal bars represent mean values relative to the baseline, that is, the mean value of the cortex of the normoxic 3‐month‐old wild‐type mice. Significant differences compared to baseline values are defined as P < 0.05, according to Student's t‐test and depicted above rectangles. Other statistical significances were tested using one‐way ANOVA with Bonferroni post hoc test; *P < 0.05, **P < 0.01, ***P < 0.001.
Influence of RE1‐silencing transcription factor expression on globin transcription
To further unravel the underlying mechanism, we searched for REST interactions with globin regulatory sequences. First, the regions upstream of the Ngb and Cygb promotors were scanned for predicted REST‐binding sites using the MA0138.2 REST‐binding profile on jaspar.genereg.net (Fig. 4A). This search revealed not only a REST transcription factor‐binding site before the Ngb promotor (Fig. 4B, chr12:87110424‐87110444), but also in the Cygb regulatory region (Fig. 4B, Chr11:116653849‐116653869). Moreover, ChIP‐seq data on the UCSC Genome Browser (Human Feb. 2009 GRCh37/hg19 Assembly) show REST binding to the regulatory region upstream of the Ngb promotor in H1‐hESC cells. Hence, we analyzed the expression levels of REST across the different experimental groups. Given that REST activation can be both gene transcription inducing and downregulating, Fig. 4 depicts the differences in REST expression levels between wild‐type and APP23 mice. REST expression does not differ between normoxic wild‐type and APP23 hippocampi across the different age groups (Fig. 4C). In the cortex, however, the difference in transcription between both genotypes increases with aging (Fig. 4C). This brain region‐specific expression alteration corresponds with the age‐dependent upsurge in cortical globin expression, which was not seen in the hippocampal area (Fig. 1A/B).
Figure 4.
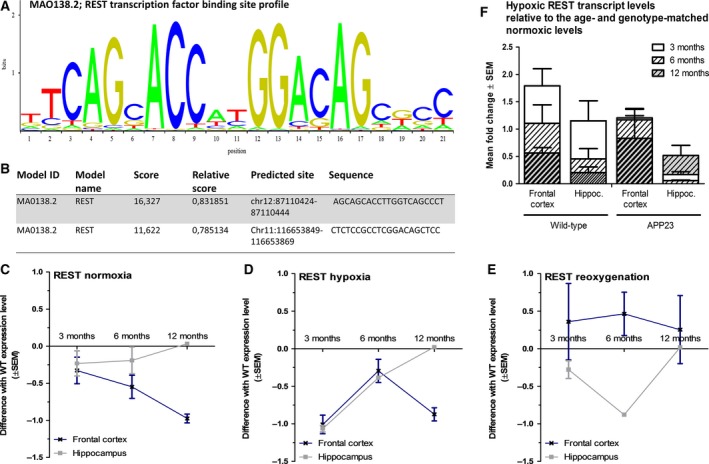
Linking RE1‐Silencing Transcription factor to globins. (A) Profile of the REST transcription factor‐binding sequence (ID: MA0138.2 from the JASPAR CORE database). (B) Predicted REST‐binding sequences and their positions in the regulatory regions of the Ngb and Cygb genes. Mean differences ± SEM between wild‐type and APP23 expression levels of REST under (C) normoxia, (D) hypoxia, and (E) after reoxygenation (n ≥ 3 per group). (F) Mean fold change values of wild‐type and APP23 mice (n = 3–4 mice per group) relative to the genotype‐ and age‐matched normoxic condition.
After the hypoxic insult, cortical REST expression levels of wild‐type and APP23 mice differ most clearly at the 3‐ and 12‐month time points (Fig. 4D), which is also observed for globin transcription levels (Fig. 2A, capped lines between rectangles). The difference in wild‐type and APP23 hippocampal expression of REST diminishes with age (Fig. 4D). Such a trend is seen for hippocampal Cygb expression as well, with the difference in fold change lowering from 1.04 over 0.34 to 0.13 (Fig. 2A). Ngb expression during hypoxia appears to deviate from this pattern, with the difference between genotypes being highest at the 3‐ and 12‐month time points, as was the case for the cortical area (Fig. 2A, capped lines between rectangles). Nonetheless, hypoxic REST transcript levels are generally lowered relative to the age‐ and genotype‐matched normoxic levels in both brain areas from 6 months onwards (Fig. 4F). After reoxygenation, expression levels of REST and globins correlate less.
Discussion
Over the past 15 years, the genetics, structure, evolution, and biological functions of Cygb and Ngb have been intensively studied across the phylogenetic tree. In addition to the maintenance of cellular oxygen supply, a scala of physiological implications has been proposed for both globins, including the involvement in signal transduction, the detoxification of reactive oxygen or nitrogen species and the counteraction of cytochrome c‐induced apoptosis 25. Moreover, as mentioned in the introduction, transgenic overexpression of Ngb in the APP Swedish‐Indiana double‐mutation mouse model reduced the Aβ(1–40) load, restored somal microdomain distribution, and ameliorated performance in the Y‐maze 11. Despite its neuroprotective traits, Ngb transcription is downregulated to baseline levels in cases of severe AD pathology 14, 15. As later stage AD is characterized by a lowered oxygen availability due to CAA 16, 22, 23, we questioned whether whole‐body hypoxia as a secondary insult could likewise downregulate Ngb and Cygb expression in young APP23 mice.
Upregulated cellular Ngb levels in AD‐affected cells have been described before. In particular, Ngb protein levels in patients with early and moderately advanced AD were detected to be increased by 23% in the superior temporal lobes 15. Ngb immunoreactivity further revealed the elevations in the cerebral cortex to be localized to both the cytoplasm of cell bodies and extracellular plaque‐like structures 14. To the best of our knowledge, this paper is the first to report an upregulation of Cygb levels in 12‐month‐old, normoxic APP23 cortices. In contrast to the upregulated globin expression in the cortex, we detected no alterations in APP23 hippocampal Ngb and Cygb transcription rates. As a trend of increased VEGF expression was also noticed in the cortex, but not in hippocampus, the latter could be attributed to the positive correlation between both globins and VEGF, which we detected across all studied conditions. Our observation of increased neuroprotective VEGF levels to be brain region specific is in line with the literature, which describes VEGF involvement to comprise the cerebral cortex and cerebrospinal fluid 35. Hence, Ngb and Cygb involvement in AD pathology could be strongly linked to Hif1 and VEGF expression. It is, however, likely that a variety of different transcription factors and upstream signals together elicit the observed globin expression patterns.
Binding sequences for REST, also known as Neuron‐Restrictive Silencer Factor, have been identified before in the regulatory region of Ngb, using both rVista 2.0 36 and MSCAN 37. To the best of our knowledge, we are the first to identify a recognition sequence in the regulatory region of Cygb as well. REST expression is induced during normal brain aging and its activation generally entails neuroprotection by downregulating targets involved in cell death 38. However, transcription of REST is markedly reduced in AD‐affected neuronal populations 38, 39. Moreover, REST increases transcription of genes related to neurotransmission in the early stages of AD. In later stages, these genes are suppressed 38. Given the dual and opposing effects of REST, no consensus is reached yet on whether upregulated REST levels are protective or detrimental 40. Hence, we opted to focus on differences in transcription levels of REST between normoxic wild‐type and APP23 mice. In doing so, we detected altered expressions in frontal cortices of 1‐year‐old APP23 mice for Ngb, Cygb, and REST, while hippocampal levels of all three genes remained relatively unchanged as compared to the wild‐type levels. These findings strengthen the hypothesis of REST/NRSF regulating globin expression together with Hif1/VEGF along the AD pathology.
In advanced AD stages, Ngb transcript levels in the CA1 region of the hippocampus were reported to return to values found in healthy control subjects 14. In the present study, we identified a comparable decrease to baseline expression in 3‐, 6‐, and 12‐month‐old APP23 mice when they were subjected to whole‐body hypoxia. Based on our observations, we postulate the following two hypotheses: (i) the Ngb promotor activity is downregulated when a threshold degree of cellular damage/dysregulation is reached and/or (ii) hypoxia and vast amyloid aggregation pathology effectuate a convergent pathway that interferes with the activity of an inducer of Ngb expression. Interestingly, hypoxia induces the miR‐106b~25 cluster that downregulates REST in LNCaP and PC3 prostate cancer cell lines 41. We identified REST expression levels to be decreased relative to age‐ and genotype‐matched normoxic levels, although this downregulation was only seen in animals aged ≥ 6 months. Hence, as similar for the normoxic groups, the globin expression patterns are more likely the result of a triad of upstream signals, for example, related to Hif1 as well.
In contrast to the downregulation of Ngb in hypoxic APP23 brains, Cygb transcript levels remain elevated relative to the normoxic condition. The reason as to why Cygb expression is able to overcome such negative regulation could be the confirmation of our previous finding of a strong positive relationship between Cygb and VEGF 34. VEGF was chosen over Hif1 as Hif1 is regulated to a large extent at the post‐mRNA level 42. The cross‐talk between Ngb and Hif1/VEGF, on the other hand, is indirect through the specificity protein 1 (Sp1) transcription factor 43. However, we also detected a generally lower transcriptional response of Cygb expression alteration in the 3‐month‐old APP23 mice as opposed to those of the 1‐year time point. It is possible that due to AD‐linked cellular dysregulations, some kind of prior history of hypoxia (e.g., amyloid angiopathy) is required to trigger an upregulation in Cygb transcription. Such phenomenon could be similar to hypoxic preconditioning in which sublethal stress endorses (neuro)protective actions against acute injuries 44. The latter would be consistent with the Cygb promotor containing a V‐ets avian erythroblastosis virus E26 oncogene homolog 1 (Ets‐1) site 45. Ets‐1 is upregulated in cortices and hippocampi of AD patients, surmounted by Ets‐1 being strongly connected with angiogenic markers as VEGF 46.
In contrast to their transcript levels, the Ngb and Cygb protein levels in APP23 mice are all elevated after hypoxia and reoxygenation, thereby paralleling lipid peroxidation levels. Sustained protein levels could be attributed to Aβ oligomers impairing the proteasome and, hence, favoring a low protein turnover. Decreased proteasome functioning already occurs in prepathological disease stages in 3xTg‐AD mice 47. In APP23 mice, we recently detected an age‐genotype interaction effect on expression levels of genes enriched for modules of the lysosomal pathway, the degradation by proteases and the proteasome (e.g., Psmb9) 48. The decrease in rodent proteasome activity is a good phenocopy of the human situation in which proteasomal inhibition reaches up to 48% in AD brains 49. Furthermore, AD is not only characterized by a lysosomal blockage caused by Aβ, but cathepsin D transcript and protein levels are both reduced in AD‐affected cells as well 50, 51. As a consequence, globin‐linked neuroprotection could be extended beyond stages with reduced transcript levels. We do not exclude that post‐transcriptional regulations take into effect, hence impacting globin protein levels as well. In particular, a discrepancy between Cygb/Ngb transcript and protein levels was observed before 52, 53. Moreover, the involvement of Cygb in AD pathology had not yet been described, with the exception of it being present in eosinophilic cytoplasmic inclusions 54. Ngb immunoreactivity in AD cortices also exhibits a punctate distribution outside cell bodies, in addition to being elevated within neurons 14. Hence, it should not be excluded that Cygb and Ngb become (in part) physiologically unavailable in later stages of AD pathology.
A combination of reduced protein turnover, REST activation patterns and Hif1 activation could underlie our observations, leading to a novel hypothesis concerning Ngb (post)transcriptional regulation as depicted in Fig. 5. Even though such a pathway seems to emerge from our obtained data, additional experiments will be required to fully support this hypothesis. Since REST has the potency to be both activating and inhibiting with regard to gene expression 38, 40, 55, its influence on globin expression requires further research to allow absolute expression levels of REST to be correlated with globin transcription. The latter would also require data on REST protein levels and knowledge on its subcellular localization. In cases of aggregation pathologies, for example, AD and frontotemporal lobar degeneration, REST appears trapped in cytoplasmic inclusions 38.
Figure 5.
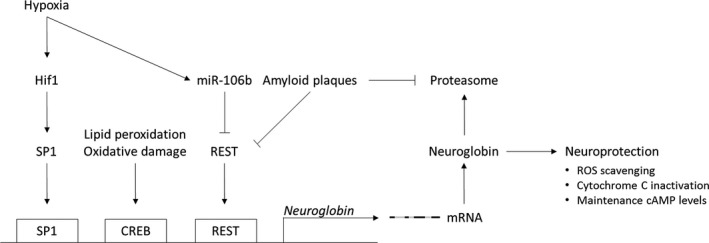
Scheme summarizing the proposed hypothesis for Ngb (post)transcriptional regulation. Hypoxia‐induced Ngb expression is hypothesized to work through the HIF1‐Sp1‐Ngb axis as the Ngb promotor lacks a direct‐binding site for HIF1, but contains a Sp1‐binding GC‐box 36, 43. Moreover, Sp1 is known to participate downstream of HIF1 in sequential gene activation upon cerebral ischemia 56. Another transcription factor regulating Ngb expression is REST. Its upregulation is paralleled with aging, largely ascribed to cell nonautonomous Wnt signaling. However, REST can get trapped with pathological misfolded proteins, including Aβ, losing its nuclear levels and downstream neuroprotective actions 38. REST is also downregulated by hypoxia‐induced miRNAs 41. Hence, we hypothesize the co‐occurrence of both nuclear REST‐depleting events in hypoxic APP23 brains to outweigh Ngb expression‐inducing signals. Of note, Aβ oligomers impair proteasome function as from prepathological disease stages, that is, 3 months in transgenic mice 47. The latter makes us postulate that the discrepancy between transcript and protein levels in hypoxic APP23 brains is caused by a blocked proteasomal function, favoring a low protein turnover. If still physiologically available, the sustained Ngb levels could entail a prolonged neuroprotective action being carried out. CREB, cAMP response element‐binding protein; Hif1, hypoxia‐inducible factor 1; REST, RE1‐silencing transcription factor; ROS, reactive oxygen species; SP1, specificity protein 1.
In conclusion, our data point for the first time to a possible widening of the neuroprotective traits of Cygb to AD pathology and to Cygb being strongly associated with VEGF angiopathogenic alterations. Moreover, we showed that hypoxia up‐ and downregulates globin expression in young, wild‐type, and APP23 mice, respectively. Given the genotype‐ and brain region‐specific expression of REST, we hypothesize REST activation patterns to also contribute to globin expression alterations.
Author contributions
ZPVA, WVL, EG, PPDD, DVD, and SD conceived and designed experiments. ZPVA, EL, and WVL performed experiments. ZPVA prepared the manuscript. All authors contributed to the further drafting and revision of the manuscript.
Acknowledgements
This work was supported by the Research Foundation‐Flanders (FWO), Interuniversity Poles of Attraction (IAP Network P7/16) of the Belgian Federal Science Policy Office, Methusalem excellence grant of the Flemish Government, agreement between Institute Born‐Bunge and University of Antwerp, the Medical Research Foundation Antwerp, the Thomas Riellaerts research fund, and Neurosearch Antwerp. ZPVA is supported by the University of Antwerp (2014BAPDOCPROEX153). EL holds a PhD fellowship from the FWO and WVL is a former PhD fellow of the agency for Innovation by Science and Technology (IWT). DVD is a former postdoctoral fellow of FWO.
Edited by Frances Edwards
References
- 1. Reitz C and Mayeux R (2014) Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol 88, 640–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bloom GS (2014) Amyloid‐beta and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol 71, 505–508. [DOI] [PubMed] [Google Scholar]
- 3. Morris GP, Clark IA and Vissel B (2014) Inconsistencies and controversies surrounding the amyloid hypothesis of Alzheimer's disease. Acta Neuropathol Commun 2, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Greenough MA, Camakaris J and Bush AI (2013) Metal dyshomeostasis and oxidative stress in Alzheimer's disease. Neurochem Int 62, 540–555. [DOI] [PubMed] [Google Scholar]
- 5. Federico A, Cardaioli E, Da Pozzo P, Formichi P, Gallus GN and Radi E (2012) Mitochondria, oxidative stress and neurodegeneration. J Neurol Sci 322, 254–262. [DOI] [PubMed] [Google Scholar]
- 6. Trent JT 3rd and Hargrove MS (2002) A ubiquitously expressed human hexacoordinate hemoglobin. J Biol Chem 277, 19538–19545. [DOI] [PubMed] [Google Scholar]
- 7. Burmester T, Weich B, Reinhardt S and Hankeln T (2000) A vertebrate globin expressed in the brain. Nature 407, 520–523. [DOI] [PubMed] [Google Scholar]
- 8. Fordel E, Thijs L, Martinet W, Schrijvers D, Moens L and Dewilde S (2007) Anoxia or oxygen and glucose deprivation in SH‐SY5Y cells: a step closer to the unraveling of neuroglobin and cytoglobin functions. Gene 398, 114–122. [DOI] [PubMed] [Google Scholar]
- 9. Reuss S, Saaler‐Reinhardt S, Weich B, Wystub S, Reuss MH, Burmester T and Hankeln T (2002) Expression analysis of neuroglobin mRNA in rodent tissues. Neuroscience 115, 645–656. [DOI] [PubMed] [Google Scholar]
- 10. Sun Y, Jin K, Mao XO, Zhu Y and Greenberg DA (2001) Neuroglobin is up‐regulated by and protects neurons from hypoxic‐ischemic injury. Proc Natl Acad Sci U S A 98, 15306–15311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khan AA, Mao XO, Banwait S, Jin K and Greenberg DA (2007) Neuroglobin attenuates beta‐amyloid neurotoxicity in vitro and transgenic Alzheimer phenotype in vivo. Proc Natl Acad Sci U S A 104, 19114–19119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li RC, Pouranfar F, Lee SK, Morris MW, Wang Y and Gozal D (2008) Neuroglobin protects PC12 cells against beta‐amyloid‐induced cell injury. Neurobiol Aging 29, 1815–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seal M, Uppal S, Kundu S and Dey SG (2015) Interaction of apoNeuroglobin with heme‐Abeta complexes relevant to Alzheimer's disease. J Biol Inorg Chem 20, 563–574. [DOI] [PubMed] [Google Scholar]
- 14. Sun F, Mao X, Xie L, Greenberg DA and Jin K (2013) Neuroglobin protein is upregulated in Alzheimer's disease. J Alzheimers Dis 36, 659–663. [DOI] [PubMed] [Google Scholar]
- 15. Szymanski M, Wang R, Fallin MD, Bassett SS and Avramopoulos D (2010) Neuroglobin and Alzheimer's dementia: genetic association and gene expression changes. Neurobiol Aging 31, 1835–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de la Torre JC (2002) Alzheimer disease as a vascular disorder: nosological evidence. Stroke 33, 1152–1162. [DOI] [PubMed] [Google Scholar]
- 17. Popa‐Wagner A, Buga AM, Popescu B and Muresanu D (2015) Vascular cognitive impairment, dementia, aging and energy demand. A vicious cycle. J Neural Transm (Vienna) 122(Suppl 1), S47–S54. [DOI] [PubMed] [Google Scholar]
- 18. Pluta R, Jablonski M, Ulamek‐Koziol M, Kocki J, Brzozowska J, Januszewski S, Furmaga‐Jablonska W, Bogucka‐Kocka A, Maciejewski R and Czuczwar SJ (2013) Sporadic Alzheimer's disease begins as episodes of brain ischemia and ischemically dysregulated Alzheimer's disease genes. Mol Neurobiol 48, 500–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA and Markesbery WR (1997) Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA 277, 813–817. [PubMed] [Google Scholar]
- 20. Iturria‐Medina Y, Sotero RC, Toussaint PJ, Mateos‐Perez JM and Evans AC (2016) Early role of vascular dysregulation on late‐onset Alzheimer's disease based on multifactorial data‐driven analysis. Nat Commun 7, 11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de la Torre JC (2004) Alzheimer's disease is a vasocognopathy: a new term to describe its nature. Neurol Res 26, 517–524. [DOI] [PubMed] [Google Scholar]
- 22. Kanekiyo T, Xu H and Bu G (2014) ApoE and Abeta in Alzheimer's disease: accidental encounters or partners? Neuron 81, 740–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Winkler DT, Bondolfi L, Herzig MC, Jann L, Calhoun ME, Wiederhold KH, Tolnay M, Staufenbiel M and Jucker M (2001) Spontaneous hemorrhagic stroke in a mouse model of cerebral amyloid angiopathy. J Neurosci 21, 1619–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Han BH, Zhou ML, Johnson AW, Singh I, Liao F, Vellimana AK, Nelson JW, Milner E, Cirrito JR, Basak J et al (2015) Contribution of reactive oxygen species to cerebral amyloid angiopathy, vasomotor dysfunction, and microhemorrhage in aged Tg2576 mice. Proc Natl Acad Sci U S A 112, E881–E890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Burmester T and Hankeln T (2014) Function and evolution of vertebrate globins. Acta Physiol (Oxf) 211, 501–514. [DOI] [PubMed] [Google Scholar]
- 26. Fordel E, Thijs L, Martinet W, Lenjou M, Laufs T, Van Bockstaele D, Moens L and Dewilde S (2006) Neuroglobin and cytoglobin overexpression protects human SH‐SY5Y neuroblastoma cells against oxidative stress‐induced cell death. Neurosci Lett 410, 146–151. [DOI] [PubMed] [Google Scholar]
- 27. Avivi A, Gerlach F, Joel A, Reuss S, Burmester T, Nevo E and Hankeln T (2010) Neuroglobin, cytoglobin, and myoglobin contribute to hypoxia adaptation of the subterranean mole rat Spalax . Proc Natl Acad Sci U S A 107, 21570–21575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sturchler‐Pierrat C, Abramowski D, Duke M, Wiederhold KH, Mistl C, Rothacher S, Ledermann B, Burki K, Frey P, Paganetti PA et al (1997) Two amyloid precursor protein transgenic mouse models with Alzheimer disease‐like pathology. Proc Natl Acad Sci U S A 94, 13287–13292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Calhoun ME, Wiederhold KH, Abramowski D, Phinney AL, Probst A, Sturchler‐Pierrat C, Staufenbiel M, Sommer B and Jucker M (1998) Neuron loss in APP transgenic mice. Nature 395, 755–756. [DOI] [PubMed] [Google Scholar]
- 30. Van Leuven W, Van Dam D, Moens L, De Deyn PP and Dewilde S (2013) A behavioural study of neuroglobin‐overexpressing mice under normoxic and hypoxic conditions. Biochem Biophys Acta 1834, 1764–1771. [DOI] [PubMed] [Google Scholar]
- 31. Geuens E, Brouns I, Flamez D, Dewilde S, Timmermans JP and Moens L (2003) A globin in the nucleus!. J Biol Chem 278, 30417–30420. [DOI] [PubMed] [Google Scholar]
- 32. Bradley‐Whitman MA and Lovell MA (2015) Biomarkers of lipid peroxidation in Alzheimer disease (AD): an update. Arch Toxicol 89, 1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Haines B, Demaria M, Mao X, Xie L, Campisi J, Jin K and Greenberg DA (2012) Hypoxia‐inducible factor‐1 and neuroglobin expression. Neurosci Lett 514, 137–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fordel E, Geuens E, Dewilde S, Rottiers P, Carmeliet P, Grooten J and Moens L (2004) Cytoglobin expression is upregulated in all tissues upon hypoxia: an in vitro and in vivo study by quantitative real‐time PCR. Biochem Biophys Res Commun 319, 342–348. [DOI] [PubMed] [Google Scholar]
- 35. Barker R, Ashby EL, Wellington D, Barrow VM, Palmer JC, Kehoe PG, Esiri MM and Love S (2014) Pathophysiology of white matter perfusion in Alzheimer's disease and vascular dementia. Brain 137, 1524–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang W, Tian Z, Sha S, Cheng LY, Philipsen S and Tan‐Un KC (2011) Functional and sequence analysis of human neuroglobin gene promoter region. Biochem Biophys Acta 1809, 236–244. [DOI] [PubMed] [Google Scholar]
- 37. Laufs TL, Wystub S, Reuss S, Burmester T, Saaler‐Reinhardt S and Hankeln T (2004) Neuron‐specific expression of neuroglobin in mammals. Neurosci Lett 362, 83–86. [DOI] [PubMed] [Google Scholar]
- 38. Lu T, Aron L, Zullo J, Pan Y, Kim H, Chen Y, Yang TH, Kim HM, Drake D, Liu XS et al (2014) REST and stress resistance in ageing and Alzheimer's disease. Nature 507, 448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Orta‐Salazar E, Aguilar‐Vazquez A, Martinez‐Coria H, Luquin‐De Anda S, Rivera‐Cervantes M, Beas‐Zarate C, Feria‐Velasco A and Diaz‐Cintra S (2014) REST/NRSF‐induced changes of ChAT protein expression in the neocortex and hippocampus of the 3xTg‐AD mouse model for Alzheimer's disease. Life Sci 116, 83–89. [DOI] [PubMed] [Google Scholar]
- 40. Baldelli P and Meldolesi J (2015) The transcription repressor REST in adult neurons: physiology, pathology, and diseases(1,2,3). eNeuro 2, e0010–e0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liang H, Studach L, Hullinger RL, Xie J and Andrisani OM (2014) Down‐regulation of RE‐1 silencing transcription factor (REST) in advanced prostate cancer by hypoxia‐induced miR‐106b~25. Exp Cell Res 320, 188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Belaiba RS, Bonello S, Zahringer C, Schmidt S, Hess J, Kietzmann T and Gorlach A (2007) Hypoxia up‐regulates hypoxia‐inducible factor‐1alpha transcription by involving phosphatidylinositol 3‐kinase and nuclear factor kappaB in pulmonary artery smooth muscle cells. Mol Biol Cell 18, 4691–4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wystub S, Ebner B, Fuchs C, Weich B, Burmester T and Hankeln T (2004) Interspecies comparison of neuroglobin, cytoglobin and myoglobin: sequence evolution and candidate regulatory elements. Cytogenet Genome Res 105, 65–78. [DOI] [PubMed] [Google Scholar]
- 44. Stetler RA, Leak RK, Gan Y, Li P, Zhang F, Hu X, Jing Z, Chen J, Zigmond MJ and Gao Y (2014) Preconditioning provides neuroprotection in models of CNS disease: paradigms and clinical significance. Prog Neurobiol 114, 58–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Guo X, Philipsen S and Tan‐Un KC (2006) Characterization of human cytoglobin gene promoter region. Biochem Biophys Acta 1759, 208–215. [DOI] [PubMed] [Google Scholar]
- 46. Jantaratnotai N, Ling A, Cheng J, Schwab C, McGeer PL and McLarnon JG (2013) Upregulation and expression patterns of the angiogenic transcription factor ets‐1 in Alzheimer's disease brain. J Alzheimers Dis 37, 367–377. [DOI] [PubMed] [Google Scholar]
- 47. Tseng BP, Green KN, Chan JL, Blurton‐Jones M and LaFerla FM (2008) Abeta inhibits the proteasome and enhances amyloid and tau accumulation. Neurobiol Aging 29, 1607–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Janssen L, Dubbelaar ML, Holtman IR, de Boer‐Bergsma J, Eggen BJ, Boddeke HW, De Deyn PP and Van Dam D (2016) Aging, microglia and cytoskeletal regulation are key factors in the pathological evolution of the APP23 mouse model for Alzheimer's disease. Biochem Biophys Acta 1863, 395–405. [DOI] [PubMed] [Google Scholar]
- 49. Keller JN, Hanni KB and Markesbery WR (2000) Impaired proteasome function in Alzheimer's disease. J Neurochem 75, 436–439. [DOI] [PubMed] [Google Scholar]
- 50. Urbanelli L, Emiliani C, Massini C, Persichetti E, Orlacchio A, Pelicci G, Sorbi S, Hasilik A and Bernardi G (2008) Cathepsin D expression is decreased in Alzheimer's disease fibroblasts. Neurobiol Aging 29, 12–22. [DOI] [PubMed] [Google Scholar]
- 51. Lipinski MM, Zheng B, Lu T, Yan Z, Py BF, Ng A, Xavier RJ, Li C, Yankner BA, Scherzer CR et al (2010) Genome‐wide analysis reveals mechanisms modulating autophagy in normal brain aging and in Alzheimer's disease. Proc Natl Acad Sci U S A 107, 14164–14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Oliveira KC, da Conceicao RR, Piedade GC, de Souza JS, Sato MA, de Barros Maciel RM and Giannocco G (2015) Thyroid hormone modulates neuroglobin and cytoglobin in rat brain. Metab Brain Dis 30, 1401–1408. [DOI] [PubMed] [Google Scholar]
- 53. Li WD, Sun Q, Zhang XS, Wang CX, Li S, Li W and Hang CH (2014) Expression and cell distribution of neuroglobin in the brain tissue after experimental subarachnoid hemorrhage in rats: a pilot study. Cell Mol Neurobiol 34, 247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hedley‐Whyte ET, Goldman JE, Nedergaard M, Friedman A, Han X, Schmidt RE and Powers JM (2009) Hyaline protoplasmic astrocytopathy of neocortex. J Neuropathol Exp Neurol 68, 136–147. [DOI] [PubMed] [Google Scholar]
- 55. Kim HJ, Denli AM, Wright R, Baul TD, Clemenson GD, Morcos AS, Zhao C, Schafer ST, Gage FH and Kagalwala MN (2015) REST regulates non‐cell‐autonomous neuronal differentiation and maturation of neural progenitor cells via Secretogranin II. J Neurosci 35, 14872–14884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Woo SK, Kwon MS, Geng Z, Chen Z, Ivanov A, Bhatta S, Gerzanich V and Simard JM (2012) Sequential activation of hypoxia‐inducible factor 1 and specificity protein 1 is required for hypoxia‐induced transcriptional stimulation of Abcc8. J Cereb Blood Flow Metab 32, 525–536. [DOI] [PMC free article] [PubMed] [Google Scholar]


