Abstract
Objective
To assess the predictive value of observed‐to‐expected lung‐to‐head ratio (O/E LHR) for survival and chronic lung disease (CLD) in survivors of left‐sided congenital diaphragmatic hernia (CDH) in an era of standardized neonatal treatment, and to evaluate the predictive value of the O/E LHR trajectory for survival.
Methods
This retrospective cohort study was performed in two high‐volume CDH centers in the Netherlands in prenatally detected, isolated left‐sided CDH patients born between 2008 and 2014. O/E LHR and liver position were determined using 2D‐ultrasonography at three time points during gestation from 19 weeks onwards. Ultrasound measurements were performed on stored ultrasound data by one single experienced operator blinded to postnatal outcome.
Results
Of the 122 included cases, 77.9% survived of whom 38.9% developed CLD. A significant association was found between the first measured O/E LHR and survival and development of CLD in survivors. Prenatal liver position did not have additional predictive value. No significant association was found between the trajectory of the O/E LHR and survival.
Conclusion
In an era of standardized neonatal treatment for neonates with CDH, the first measured O/E LHR per patient significantly predicts survival and development of CLD in survivors in isolated left‐sided CDH infants. © 2017 The Authors. Prenatal Diagnosis published by John Wiley & Sons, Ltd.
Introduction
Congenital diaphragmatic hernia (CDH) occurs in approximately 1 in 2200 live births.1 Although the survival rate has significantly increased to about 70–80%,2, 3 CDH is still a life‐threatening congenital anomaly.4 Various parameters are related to a worse prognosis like a right‐sided CDH, intrathoracic liver herniation, and associated congenital and/or chromosomal malformations.5, 6, 7
Metkus et al. were the first (1996) who described the predictive value of lung‐to‐head ratio (LHR) in fetuses with CDH.8 Because the LHR increases exponentially with gestation in healthy fetuses,9 the observed‐to‐expected LHR (O/E LHR) was introduced in 2007 by Jani et al. after a multicenter study in 354 isolated CDH fetuses.10 Thereafter, several studies have demonstrated that the O/E LHR is a useful predictor of postnatal outcome.11, 12, 13, 14
In fetuses with left‐sided severe CDH (O/E LHR < 25%) and fetuses with moderate CDH (O/E LHR 25–34.9% or O/E LHR 35–44.9% with intrathoracic liver herniation), the benefit of fetoscopic endotracheal occlusion (FETO) is currently being investigated in randomized controlled studies (NCT01240057 and NCT00763737). Groups are based on survival rates according to Deprest et al. 15 and Jani et al.,10 which is the largest study to date.10 However, in that period, there was still a lack of postnatal standardization of treatment, which has proven to influence postnatal outcome, reaching survival rates up to over 80%.3 Secondly, in their study, each of the participating centers provided the data. Information concerning inter‐observer reproducibility was not available and variability in prenatal ultrasound measurements may have influenced the results. Thirdly, up to date, there has not been a longitudinal evaluation of the O/E LHR per individual patient during pregnancy.
From 2008 onwards, all patients born in participating centers of the CDH EURO Consortium have been treated according to a standardized neonatal treatment protocol which was published in 2010 and recently actualized.16, 17 Subsequent high survival rates might influence validity of the ‘original’ cut‐offs and their value for prenatal counseling. Therefore, we evaluated the predictive value of the prenatally measured O/E LHR on postnatal survival and development of chronic lung disease (CLD), when neonates receive standardized treatment in the two Dutch CDH designated centers with extracorporeal membrane oxygenation (ECMO) availability. Moreover, we performed longitudinal analyses of the O/E LHR measurements per patient during gestation.
Methods
All patients with a prenatal diagnosis of CDH, born between January 2008 and December 2014, and treated in the Erasmus University Medical Centre, Rotterdam, The Netherlands or the Radboud University Medical Centre, Nijmegen, The Netherlands were included in this observational retrospective cohort study. Because all infants from the Netherlands with a CDH are referred to one of the two CDH centers, this represents a nationwide cohort. Both centers are high‐volume centers (defined as >10 CDH patients per year).18 Exclusion criteria were defined as: right‐sided CDH, termination of pregnancy, premature birth <30 weeks gestational age (GA), FETO, and associated major structural or chromosomal anomalies. Because subjects are not being submitted to any handling, nor are there rules of human behavior being imposed, Institutional Review Board approval was waived by the ethical committee of the Erasmus Medical Center, Rotterdam, The Netherlands (MEC‐2015‐517).
The original ultrasound image of a transverse plane of the fetal chest at the level of the four‐chamber view of the heart was retrieved from patient records and used for measurement of the contralateral (right) lung. The lung area was measured by manual tracing of the limits of the lung (mm2), if possible in multiple images per examination and preferably in an image recorded for measurement of lung area. Liver position (intrathoracic or intra‐abdominal) was determined by visual assessment in a transversal plane, as well as a coronal or sagittal plane if available. The head circumference (mm) was retrieved from medical records. The O/E LHR was then calculated as described by Jani et al. 19 Ultrasound measurements were performed on stored images reloaded on a GE Voluson E8 or E10 system (GE Medical Systems, Zipf, Austria) at the Department of Obstetrics and Gynecology, Division of Obstetrics and Prenatal Medicine at the Erasmus University Medical Centre. The original images where obtained using the GE Voluson 730/E8 system (GE Medical Systems, Zipf, Austria). All measurements were performed by one single operator (N. C. J. Peters) with 5 years of experience measuring the O/E LHR,20 who was unaware of postnatal outcome. If there was no image available meeting the requirements as described by Jani et al. 21 a measurement was regarded as missing. Ultrasound measurements were performed in the second and third trimester of pregnancy and were categorized as follows; ultrasound 1: 19+0–24+0 weeks' gestational age (GA), ultrasound 2: 24+1–29+6 weeks' GA, and ultrasound 3: ≥30+0 weeks' GA. Postnatal patient characteristics were retrieved from medical records. Patient demographics included: gestational age at birth, birth weight, gender, associated major structural or chromosomal anomalies, side of diaphragmatic hernia, liver position (intrathoracic/ intra‐abdominal) at surgical repair, diaphragmatic defect size (A/B/C/D, with ‘A’ being the smallest and ‘D’ being the largest defects),2 need for ECMO, survival, and presence of CLD in survivors. Survival was defined as survival after the first year of life. CLD was defined as oxygen dependency (>0.21) at day 28 of life.22 Since 2008, all patients have been treated according to a standardized neonatal treatment protocol, which was published in 2010 and recently updated in 2016.16, 17 ECMO therapy was available for patients born after >34 weeks of gestation, with a birth weight above 2000 grams during the complete study period, without a change in indications for ECMO during this period. Severity of CDH was divided according to the same groups as proposed by Deprest et al. 15: extreme CDH (O/E LHR <15%), severe CDH (O/E LHR 15–25%), moderate CDH (O/E LHR 26–35% or O/E LHR 36–45% with intrathoracic liver position), and mild CDH (O/E LHR 36–45% and liver down or O/E LHR ≥46%).
Statistical analysis
Patient characteristics were described as numbers (%) for categorical data, or median (interquartile range; IQR) for continuous data because they were not normally distributed. The first measured O/E LHR per patient was selected and used for all analyses, except for the longitudinal analyses for which all measurements per individual patient were evaluated. O/E LHR was compared between survivors and non‐survivors, and survivors with and without development of CLD, and comparison between centers using Mann–Whitney tests. Associations between O/E LHR and mortality, and CLD in survivors were evaluated using univariable logistic regression modeling. Multivariable logistic regression analyses with prenatal liver position and O/E LHR as independent variables were then used to evaluate their combined predictive value on survival and development of CLD in survivors. The calibration of the multivariable logistic regression models was assessed using the Hosmer–Lemeshow goodness‐of‐fit test. The association between O/E LHR and postnatal defect size was evaluated using the Jonckheere–Terpstra test, whereas the association between prenatal liver position and postnatal defect size was evaluated using a linear‐by‐linear association chi‐square test. Multivariable ordinal logistic regression analysis was used to determine the association between postnatal defect size (dependent variable) and the O/E LHR and prenatal position of the liver (independent variables). An univariable logistic regression analysis was performed to assess the association between the gestational age at diagnosis and survival. Receiver operating characteristic (ROC) curves were used to evaluate the prognostic value of O/E LHR for survival and development of CLD in survivors. Data were presented as areas under the ROC curves (AUC); [95% CI]. Optimal cut‐off values were determined by maximizing the Youden index (sensitivity plus specificity minus 1). Missing observations of O/E LHR could conceivably be missing not at random, 23 if the more severe cases of CDH are more likely to be detected early on during pregnancy. To test this hypothesis, univariable logistic regression analysis was repeated in a selection of patients in whom the CDH was detected before 24 weeks GA. Results from this group were compared with the results of the complete study group to assess whether the O/E LHR observations are missing at random.23 Then, for the evaluation of the predictive value of the trajectory of O/E LHR over time on survival, missing data of O/E LHR 19+0–24+0 weeks GA (n = 64 patients), O/E LHR between 24+1–29+6 weeks GA (n = 70), and O/E LHR >30+0 weeks GA (n = 11 patients), were imputed using multiple imputation by chained equations in SPSS with 100 imputations. Using the multiple imputation data set, a linear regression of the O/E LHR at the three time points was performed for each patient separately, with GA (coded as a continuous variable) as the only independent variable. The purpose of this analysis was to summarize the longitudinal data of O/E LHR using an estimated level (intercept in the linear regression) and time trend (slope in the linear regression). The resulting estimates of the intercept and slope in the linear regressions were used as independent variables in logistic regressions for survival and CLD in survivors. The linear regressions were performed using microsoft excel 2010, and all other analyses were performed using spss version 21.0 for Windows. A two‐sided p‐value of <0.05 was considered statistically significant.
Results
During the study period, 238 CDH patients were born alive with a CDH in one of the two CDH centers in the Netherlands. In 176 (74%) patients, the CDH was prenatally detected. Reasons for exclusion from the study are summarized in Figure 1. In total, 122 patients with a prenatal diagnosis of an isolated left CDH were included. In the study group, 95 (77.9%) patients survived, and 37 (38.9%) of the survivors developed CLD (Table 1). Thirty‐eight (31%) neonates received ECMO treatment, of which 21 died (55%). For 73 (60%) patients, more than one measurement was available, so 73 patients with a total of 190 measurements were available for the longitudinal analyses. The first measured O/E LHR and survival rate of patients with CDH were not significantly different between the two centers with survival rates of 78.2% at the Erasmus University Medical Center and 77.1% at the Radboud University Medical Center (p = 0.90).
Figure 1.
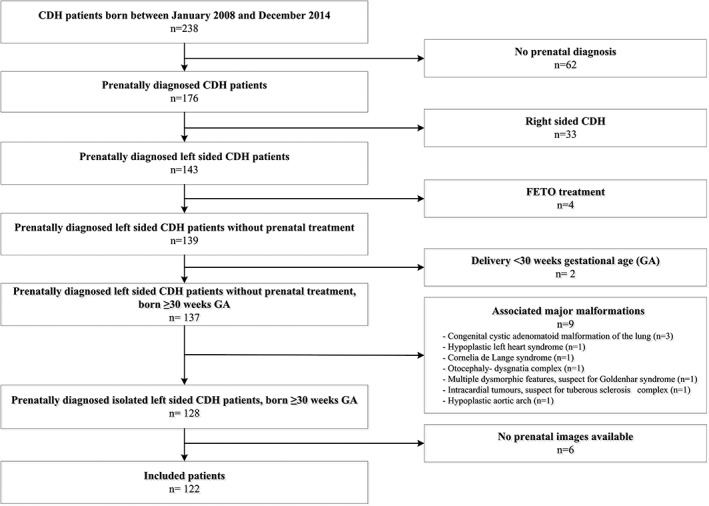
Flowchart of inclusion. CDH, congenital diaphragmatic hernia; FETO, fetoscopic endotracheal occlusion; GA, gestational age
Table 1.
Background characteristics of CDH patients
| Variables | CDH patients (n = 122) |
|---|---|
| Gestational age at delivery (weeks) | 38+2 (37+5–38+5) |
| Birth 30–34 weeks of GA | 5 (4.1%) |
| Birth 34–37 weeks of GA | 18 (14.8%) |
| Birth weight (grams) | 3000 (2700–3200) |
| Gender: male | 63 (51.6%) |
| Prenatal liver position | |
| Intra‐abdominal | 67 (54.9%) |
| Intrathoracic | 52 (42.6%) |
| Unknown/ missing | 3 (2.5%) |
| Postnatal liver position (during surgery) | |
| Intra‐abdominal | 65 (53.3%) |
| Intrathoracic | 50 (41.0%) |
| No repair | 5 (4.1%) |
| Unknown/ missing | 2 (1.6%) |
| Defect size2 | |
| A | 10 (8.2%) |
| B | 28 (23.0%) |
| C | 56 (45.9%) |
| D | 10 (8.2%) |
| No repair | 5 (4.0%) |
| Unknown/ missing | 13 (10.7%) |
| ECMO | 38 (31.1%) |
| Survival after first year of life | 95 (77.9%) |
| CLD (in survivors) | 37 (38.9%) |
GA, gestational age; ECMO, extracorporeal membrane oxygenation; CLD, chronic lung disease; CDH, congenital diaphragmatic hernia.
Data are presented as numbers (%) or median (interquartile range). Defect size was classified according to Lally et al. 2 with ‘A’ being defects entirely surrounded by muscle, ‘B’ defects having a small (<50%) and ‘C’ defects having a large (>50%) portion of the chest wall devoid of diaphragm tissue, and ‘D’ patients having complete or near complete absence of the diaphragm.
In non‐survivors and survivors, the median O/E LHR was 35.9% (IQR 29.2–43.0) and 45.7% (IQR 40.5–55.9), respectively (p < 0.001). The median of the O/E LHR was 44.0% (IQR 36.0–48.8) in survivors with CLD and 48.8% (IQR 41.8–57.7) in survivors without CLD (p = 0.03). Figure 2 shows the relationship between the O/E LHR and gestational age for each patient, stratified by survival status. There was no significant association between survival and GA at diagnosis (p = 0.30) nor between the first measured O/E LHR and the GA at diagnosis (p = 0.05).
Figure 2.
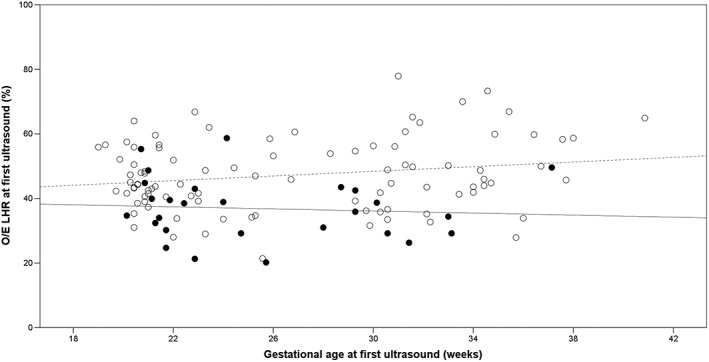
Relationship between the first measured observed to expected lung to head ratio (O/E LHR) and gestational age. This figure shows the O/E LHR measured at the first ultrasound examination per patient. The closed circles represent the neonates that died, and the open circles represent the survivors. The solid and dashed lines are linear regression lines for the neonates that died and the survivors, respectively
The relationship between O/E LHR and survival, stratified by prenatal liver position in each group is shown in Figure 3. None of our patients belonged to the extreme CDH group (O/E LHR < 15%). Only one of four patients (25%) with severe CDH (O/E LHR ≤ 25%) survived. In the moderate group (O/E LHR 26–35% or O/E LHR 36–45% with intrathoracic liver position) 29/43 patients (67.4%) survived. In the mild group (O/E LHR 36–45% and liver down or O/E LHR ≥ 46%), 65/75 patients (86.7%) survived. Fetuses with an O/E LHR of 36–45% with liver up show no statistically significant difference in survival rate in comparison with fetuses with an O/E LHR of 36–45% and liver down, respectively 82% and 76% (p = 0.63). In Figure 3, 42 instead of 43 patients are in the moderate group and 73 instead of 75 patients in the mild group. Those differences are explained by the fact that in the moderate group for one patient prenatal liver position was unknown, and in the mild group for two patients, the prenatal liver position was unknown.
Figure 3.
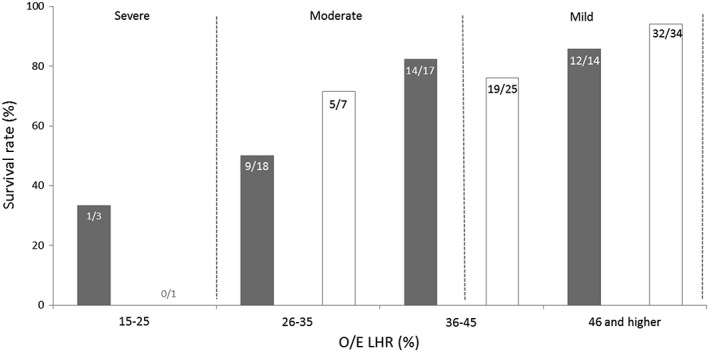
Survival rate according to the fetal observed to expected lung to head ratio (O/E LHR) and fetal liver position in fetuses with isolated left‐sided diaphragmatic hernia. The filled bars represent fetuses with intrathoracic herniation of the liver and the open bars represent those without herniation. The numbers inside the bars represent the absolute numbers of survived patients/total number of patients within that specific group. The areas between the dashed lines represent the division according to Deprest et al. into groups of estimated severity of pulmonary hypoplasia based on the O/E LHR in combination with liver position.24 The difference in patient numbers between this figure and the total group is explained by the fact that in the moderate group for one patient prenatal liver position was unknown and in the mild group for two patients the prenatal liver position was unknown
Univariable logistic regression analysis showed that a lower O/E LHR was significantly associated with mortality and with the development of CLD in survivors (Table 2). Multivariable logistic regression analysis with correction for prenatal liver position resulted in the same conclusions and showed that liver position was not of additional value for prediction of outcome (Table 2). P‐values of the Hosmer–Lemeshow test were larger than 0.05, indicating an adequate model calibration. Based on ROC analysis, mortality was predicted correctly by the O/E LHR with an optimal cut‐off value of 40.2% (sensitivity 0.76 and specificity 0.70, AUC 0.77; [0.666–0.866], p < 0.01) (Figure 4a). Development of CLD in survivors was predicted by the O/E LHR (AUC 0.64; [0.522–0.751], p = 0.03) with an optimal cut‐off value of 49.9% (sensitivity 0.48 and specificity 0.81) (Figure 4b).
Table 2.
Logistic regression analyses for the O/E LHR with outcomes survival and chronic lung disease in survivors
| Variable | Univariable analyses | Multivariable analyses | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p‐value | OR | 95% CI | p‐value | |
| Survival | Survival | |||||
| O/E LHR (%) | 1.11 | 1.052–1.168 | <0.001 | 1.11 | 1.048–1.172 | <0.001 |
| Liver position | 2.26 | 0.944–5.425 | 0.07 | 0.83 | 0.310–2.213 | 0.71 |
| CLD in survivors | CLD in survivors | |||||
| O/E LHR (%) | 0.96 | 0.919–0.997 | 0.04 | 0.94 | 0.902–0.988 | 0.01 |
| Liver position | 1.23 | 0.520–2.925 | 0.63 | 0.55 | 0.214–1.414 | 0.21 |
O/E LHR, observed‐to‐expected lung‐to‐head ratio; OR, odds ratio; CI, confidence interval; CLD, chronic lung disease.
For the multivariable analyses prenatal liver position (intrathoracic vs intra‐abdominal) was added into the model.
Figure 4.
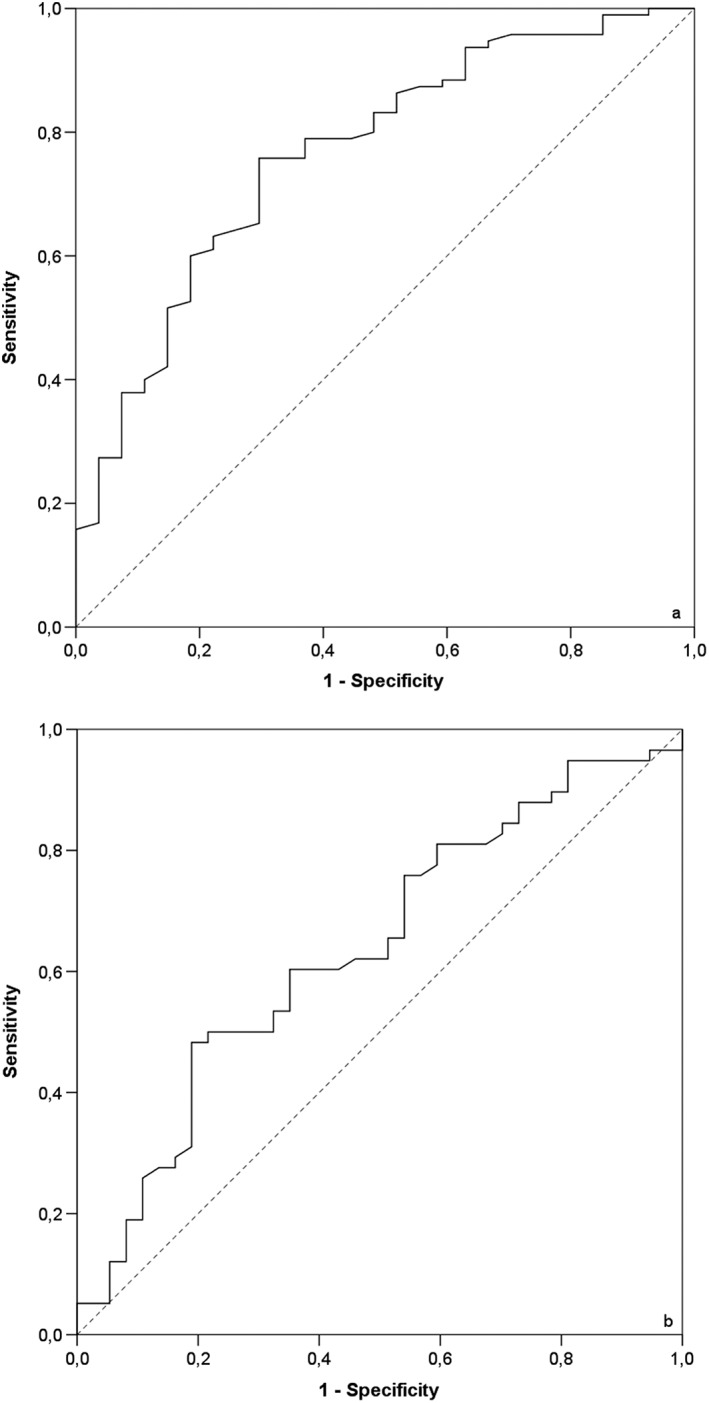
(a) Receiver operating characteristic (ROC) curve for survival. ROC curve for the prediction of survival in neonates with isolated left‐sided congenital diaphragmatic hernia according to cut‐off values of observed‐to‐expected lung area to head circumference ratio (continuous line). The dashed line is the reference line. Area‐under the curve: 0.77. (b) ROC curve for development of chronic lung disease in survivors. ROC curves for the prediction of chronic lung disease in surviving neonates with isolated left‐sided congenital diaphragmatic hernia according to cut‐off values of observed‐to‐expected lung area to head circumference ratio (continuous line). The dashed line is the reference line. Area under the curve: 0.64
In a separate univariable analysis in patients with a known postnatal defect size (n = 104), a statistically significant positive association was found between the O/E LHR and postnatal defect size (p = 0.02) as well as between the presence of CLD in survivors and postnatal defect size (p = 0.002). No statistically significant association was found between liver position and postnatal defect size (p = 0.25). The multivariable ordinal regression analyses showed that the addition of prenatal liver position was not relevant in the association between O/E LHR and postnatal defect size (p = 0.41).
The category of CDH severity based on O/E LHR measurements and prenatal liver position per patient remained stable for 58 patients (79.5%) of the 73 patients with at least two ultrasound measurements during the second and third trimester of pregnancy. In the univariable logistic regression analysis, no differences were found in predictive value of the O/E LHR on survival between the selected group of patients in whom the CDH was detected before 24 weeks of gestational age and the total patient population. Therefore, multiple imputation was performed. Longitudinal analyses of the trajectory of O/E LHR measurements during gestation showed no significant association with survival (p = 0.18).
Discussion
In this nationwide study performed in an era of standardized neonatal treatment, we demonstrated that the first prenatally measured O/E LHR per patient can predict survival in isolated left‐sided CDH infants. Survival within the different O/E LHR categories was comparable with data from Jani et al., the largest multicenter study concerning the predictive value of the O/E LHR,10 when no international consensus in standardization of postnatal therapeutic modalities had been reached and/or had been made available. A lower O/E LHR was significantly associated with development of CLD in survivors. The O/E LHR remained stable over time during gestation.
The rationale for the use of O/E LHR is that it provides an indirect assessment of contralateral lung volume, and therefore the likelihood of pulmonary hypoplasia.25 Adequate prenatal counseling, considering the indication for prenatal treatment (FETO) and expected postnatal prognosis, requires accurate prediction tools. Jani et al. retrospectively evaluated the predictive value of O/E LHR in a multicenter study of 354 isolated CDH patients (of whom 329 left‐sided), who were treated without a standardized protocol in a large number of centers with both high‐volume and low‐volume case load on a yearly base.18 A difference between the two studies is, next to different patient numbers and the presence of standardized treatment, a different inclusion period (2008–2014 in the present study vs 1996–2005 in the study by Jani et al.). The presented survival rates in the different categories (severe, moderate and mild) of O/E LHR in our study are comparable with the previous studies. Because inclusion criteria in the Tracheal Occlusion To Accelerate Lung (growth) trial (moderate CDH (NCT01240057) and severe CDH (NCT00763737)) are based on these previous studies, it is important that we can conclude that those criteria are still valid following a nationwide evaluation in The Netherlands in an era of standardized neonatal treatment protocol. Improving neonatal therapy is, however, a moving target explaining that may contribute to the different overall survival rates between the two study periods (76% in the present study vs 65% in the study by Jani et al.). Our data also show that fetuses with an O/E LHR of 36–45% have a survival chance equal to fetus in the mild group irrespective of liver position. Position of the liver seems more relevant in fetus with an O/E LHR between 26 and 35%. In the multivariable logistic regression analysis, however, prenatal liver position was not significantly associated with survival, postnatal defect size nor with development of CLD in survivors after adjustment for O/E LHR. In our study, we assessed the liver position as being a dichotomous variable (intrathoracic or not intrathoracic). Previous studies have shown, however, that quantification of the extent of liver tissue herniation by ultrasound or MRI and/or position of the stomach in the thorax are more predictive for survival, even independent of the O/E LHR.26, 27, 28, 29, 30 The primary aim of our study was to investigate the validity of the currently used O/E LHR for survival in an era of standardized neonatal treatment and quantification of herniated liver tissue was not part of the study design.
We found an AUC for survival of 0.77, which is comparable with previous studies (AUC 0.76 in the study by Jani et al.,10 AUC 0.78 in the study by Ruano et al.,31 and AUC 0.84 in the study by Kehl et al. 32). The relevance for clinical practice of the cut‐off values based on the Youden index is debatable, because the weight of a possible false positive or false negative prediction has not been taken into consideration Before assigning differential weights to the predictions, evaluation of parents preferences should, however, be further investigated.
In our study, we found a difference in the significance of the association between survival and GA at diagnosis (p0.30) and the association between the first measured O/E LHR and GA at diagnosis (p0.05). This indicates that a higher absolute difference in percentage O/E LHR at a later time during gestation does not immediately result in an overall higher survival. Deprest et al. 15 proposed a division of patients into categories (extreme/severe/moderate/mild). Because our data show that 80% of the patients remain in the same O/E LHR category during the second and third trimester of pregnancy, those categories rather than absolute percentages seem more suitable for prenatal counseling.
We found that a lower O/E LHR was significantly associated with development of CLD in survivors. The only studies that have also evaluated the prognostic value of the O/E LHR for the development of CLD10, 14 found the same result. In addition, we have shown that a lower O/E LHR is associated with a larger postnatal defect size as classified by the Boston scale and these larger defects are associated with the development of CLD in survivors. Therefore, it is likely that prenatally assessed size of the contralateral lung is not only a predictor of mortality, but also for pulmonary morbidity.
The strengths of this study are the inclusion of a large cohort of isolated left CDH patients in a relatively short inclusion period who were all treated according to the same standardized treatment protocol including the same ECMO protocols, in addition to standardized prenatal measurements. Because one single experienced operator performed all measurements on stored ultrasound data, inter‐observer variability could not have influenced our results. Cruz‐Martinez et al. showed that there is a learning curve for performing O/E LHR measurements, which emphasizes the importance of an experienced operator.20 We used the tracing method to calculate the O/E LHR which was shown to be superior to the anteroposterior diameter method in predicting postpartum survival in isolated left‐sided CDH.21, 32
A limitation of this study may be that, although O/E LHR was measured by one observer, measurements were performed on stored ultrasound images, which may not have been the perfect section of the cross‐sectional view of the fetal thorax at the level of the four‐chamber view of the heart. However, if an image did not meet the criteria for measurement of the lung area as described by Jani et al.,21 a measurement was regarded as missing.
Conclusions
In isolated left‐sided CDH patients, O/E LHR predicts survival and development of CLD in survivors in an era of a standardized neonatal treatment protocol, and the previously established categories of severe, moderate and mild CDH remain valid. Prenatal liver position (‘liver up’ vs ‘liver down’) was not significantly associated with survival nor with development of CLD in survivors after adjustment for O/E LHR.
Acknowledgements
We thank Dagmar de Bruijn from the Radboud University Medical Centre, Nijmegen, The Netherlands for her help with data collection.
WHAT'S ALREADY KNOWN ABOUT THIS TOPIC?
The O/E LHR ratio is currently used for prenatal prediction of postnatal outcome in fetuses with CDH.
WHAT DOES THIS STUDY ADD?
An evaluation of the predictive value of the O/E LHR ratio for survival and development of chronic lung disease in fetuses with CDH in an era of standardized neonatal treatment.
Insight into the predictive value of the O/E LHR trajectory throughout gestation.
Snoek, K. G. , Peters, N. C. J. , van Rosmalen, J. , van Heijst, A. F. J. , Eggink, A. J. , Sikkel, E. , Wijnen, R. M. , IJsselstijn, H. , Cohen‐Overbeek, T. E. , and Tibboel, D. (2017) The validity of the observed‐to‐expected lung‐to‐head ratio in congenital diaphragmatic hernia in an era of standardized neonatal treatment; a multicenter study. Prenat Diagn, 37: 658–665. doi: 10.1002/pd.5062.
Funding sources: None
Conflict of interest: None declared
References
- 1. Badillo A, Gingalewski C. Congenital diaphragmatic hernia: treatment and outcomes. Semin Perinatol 2014;38(2):92–96. [DOI] [PubMed] [Google Scholar]
- 2. Lally KP, Lasky RE, Lally PA, et al. Standardized reporting for congenital diaphragmatic hernia‐‐an international consensus. J Pediatr Surg 2013;48(12):2408–2415. [DOI] [PubMed] [Google Scholar]
- 3. van den Hout L, Schaible T, Cohen‐Overbeek TE, et al. Actual outcome in infants with congenital diaphragmatic hernia: the role of a standardized postnatal treatment protocol. Fetal Diagn Ther 2011;29(1):55–63. [DOI] [PubMed] [Google Scholar]
- 4. van den Hout L, Sluiter I, Gischler S, et al. Can we improve outcome of congenital diaphragmatic hernia? Pediatr Surg Int 2009;25(9):733–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hedrick HL, Danzer E, Merchant A, et al. Liver position and lung‐to‐head ratio for prediction of extracorporeal membrane oxygenation and survival in isolated left congenital diaphragmatic hernia. Am J Obstet Gynecol 2007;197(4):422 e1‐4. [DOI] [PubMed] [Google Scholar]
- 6. Mullassery D, Ba'ath ME, Jesudason EC, et al. Value of liver herniation in prediction of outcome in fetal congenital diaphragmatic hernia: a systematic review and meta‐analysis. Ultrasound Obstet Gynecol 2010;35(5):609–614. [DOI] [PubMed] [Google Scholar]
- 7. Werner NL, Coughlin M, Kunisaki SM, et al. Prenatal and postnatal markers of severity in congenital diaphragmatic hernia have similar prognostic ability. Prenat Diagn 2016;36(2):107–111. [DOI] [PubMed] [Google Scholar]
- 8. Metkus AP, Filly RA, Stringer MD, et al. Sonographic predictors of survival in fetal diaphragmatic hernia. J Pediatr Surg 1996;31(1):148–151 discussion 51‐2. [DOI] [PubMed] [Google Scholar]
- 9. Peralta CF, Cavoretto P, Csapo B, et al. Assessment of lung area in normal fetuses at 12‐32 weeks. Ultrasound Obstet Gynecol 2005;26(7):718–724. [DOI] [PubMed] [Google Scholar]
- 10. Jani J, Nicolaides KH, Keller RL, et al. Observed to expected lung area to head circumference ratio in the prediction of survival in fetuses with isolated diaphragmatic hernia. Ultrasound Obstet Gynecol 2007;30(1):67–71. [DOI] [PubMed] [Google Scholar]
- 11. Bebbington M, Victoria T, Danzer E, et al. Comparison of ultrasound and magnetic resonance imaging parameters in predicting survival in isolated left‐sided congenital diaphragmatic hernia. Ultrasound Obstet Gynecol 2014;43(6):670–674. [DOI] [PubMed] [Google Scholar]
- 12. Alfaraj MA, Shah PS, Bohn D, et al. Congenital diaphragmatic hernia: lung‐to‐head ratio and lung volume for prediction of outcome. Am J Obstet Gynecol 2011;205(1):43 e1‐8. [DOI] [PubMed] [Google Scholar]
- 13. Jani JC, Benachi A, Nicolaides KH, et al. Prenatal prediction of neonatal morbidity in survivors with congenital diaphragmatic hernia: a multicenter study. Ultrasound Obstet Gynecol 2009;33(1):64–69. [DOI] [PubMed] [Google Scholar]
- 14. Kastenholz KE, Weis M, Hagelstein C, et al. Correlation of observed‐to‐expected MRI fetal lung volume and ultrasound lung‐to‐head ratio at different gestational times in fetuses with congenital diaphragmatic hernia. AJR am J Roentgenol 2016;206(4):856–866. [DOI] [PubMed] [Google Scholar]
- 15. Deprest JA, Flemmer AW, Gratacos E, et al. Antenatal prediction of lung volume and in‐utero treatment by fetal endoscopic tracheal occlusion in severe isolated congenital diaphragmatic hernia. Semin Fetal Neonatal med 2009;14(1):8–13. [DOI] [PubMed] [Google Scholar]
- 16. Reiss I, Schaible T, van den Hout L, et al. Standardized postnatal management of infants with congenital diaphragmatic hernia in Europe: the CDH EURO Consortium consensus. Neonatology 2010;98(4):354–364. [DOI] [PubMed] [Google Scholar]
- 17. Snoek KG, Reiss IK, Greenough A, et al. Standardized postnatal management of infants with congenital diaphragmatic hernia in Europe: the CDH EURO Consortium Consensus −2015 Update. Neonatology 2016;110(1):66–74. [DOI] [PubMed] [Google Scholar]
- 18. Bucher BT, Guth RM, Saito JM, et al. Impact of hospital volume on in‐hospital mortality of infants undergoing repair of congenital diaphragmatic hernia. Ann Surg 2010;252(4):635–642. [DOI] [PubMed] [Google Scholar]
- 19. TOTAL TRIAL: TOTAL TRIAL ; 2016. [cited 2016 11th of February] Available from: http://totaltrial.eu/.
- 20. Cruz‐Martinez R, Figueras F, Moreno‐Alvarez O, et al. Learning curve for lung area to head circumference ratio measurement in fetuses with congenital diaphragmatic hernia. Ultrasound Obstet Gynecol 2010;36(1):32–36. [DOI] [PubMed] [Google Scholar]
- 21. Jani J, Peralta CF, Benachi A, et al. Assessment of lung area in fetuses with congenital diaphragmatic hernia. Ultrasound Obstet Gynecol 2007;30(1):72–76. [DOI] [PubMed] [Google Scholar]
- 22. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care med 2001;163(7):1723–1729. [DOI] [PubMed] [Google Scholar]
- 23. Rubin D. Inference and missing data. Biometrika 1976;63(3):581–592. [Google Scholar]
- 24. Deprest JA, Nicolaides K, Gratacos E. Fetal surgery for congenital diaphragmatic hernia is back from never gone. Fetal Diagn Ther 2011;29(1):6–17. [DOI] [PubMed] [Google Scholar]
- 25. Benachi A, Cordier AG, Cannie M, et al. Advances in prenatal diagnosis of congenital diaphragmatic hernia. Semin Fetal Neonatal med 2014;19(6):331–337. [DOI] [PubMed] [Google Scholar]
- 26. Cannie M, Jani J, Chaffiotte C, et al. Quantification of intrathoracic liver herniation by magnetic resonance imaging and prediction of postnatal survival in fetuses with congenital diaphragmatic hernia. Ultrasound Obstet Gynecol 2008;32(5):627–632. [DOI] [PubMed] [Google Scholar]
- 27. Werneck Britto IS, Olutoye OO, Cass DL, et al. Quantification of liver herniation in fetuses with isolated congenital diaphragmatic hernia using two‐dimensional ultrasonography. Ultrasound Obstet Gynecol 2015;46(2):150–154. [DOI] [PubMed] [Google Scholar]
- 28. Cordier AG, Jani JC, Cannie MM, et al. Stomach position in prediction of survival in left‐sided congenital diaphragmatic hernia with or without fetoscopic endoluminal tracheal occlusion. Ultrasound Obstet Gynecol 2015;46(2):155–161. [DOI] [PubMed] [Google Scholar]
- 29. Kitano Y, Okuyama H, Saito M, et al. Re‐evaluation of stomach position as a simple prognostic factor in fetal left congenital diaphragmatic hernia: a multicenter survey in Japan. Ultrasound Obstet Gynecol 2011;37(3):277–282. [DOI] [PubMed] [Google Scholar]
- 30. Victoria T, Bebbington MW, Danzer E, et al. Use of magnetic resonance imaging in prenatal prognosis of the fetus with isolated left congenital diaphragmatic hernia. Prenat Diagn 2012;32(8):715–723. [DOI] [PubMed] [Google Scholar]
- 31. Ruano R, Takashi E, da Silva MM, et al. Prediction and probability of neonatal outcome in isolated congenital diaphragmatic hernia using multiple ultrasound parameters. Ultrasound Obstet Gynecol 2012;39(1):42–49. [DOI] [PubMed] [Google Scholar]
- 32. Kehl S, Siemer J, Brunnemer S, et al. Prediction of postnatal outcomes in fetuses with isolated congenital diaphragmatic hernias using different lung‐to‐head ratiomeasurements. J Ultrasound med 2014;33(5):759–767. [DOI] [PubMed] [Google Scholar]


