Abstract
BACKGROUND
Bed bugs are a public health concern, and their incidence is increasing worldwide. Bed bug infestations are notoriously difficult to eradicate, further exacerbated by widespread resistance to pyrethroid and neonicotinoid insecticides. This study evaluated the efficacy of the newly developed fungal biopesticide Aprehend™, containing Beauveria bassiana, against insecticide‐resistant bed bugs.
RESULTS
Overall mortality for the Harold Harlan (insecticide‐susceptible) strain was high (98–100%) following exposure to Aprehend™ or Suspend SC (deltamethrin). The mean survival times (MSTs) for Harold Harlan bed bugs were 5.1 days for Aprehend™ and 4.8 and 3.0 days for the low and high concentrations of Suspend SC respectively. All three strains of pyrethroid‐resistant bed bugs were susceptible to infection by B. bassiana, resulting in MSTs of <6 days (median = 4 days) and >94% overall mortality. Conversely, mortality of the three insecticide‐resistant strains after exposure to Suspend SC was only 16‐40%.
CONCLUSION
These results demonstrate that Aprehend™ is equally effective against insecticide‐susceptible and insecticide‐resistant bed bugs and could provide pest control operators with a promising new tool for control of bed bugs and insecticide resistance management. © 2017 The Authors. Pest Management Science published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: Beauveria bassiana, Aprehend™, Suspend SC, entomopathogenic fungi, insecticide resistance
1. INTRODUCTION
Bed bugs, Cimex lectularius L. (Hemiptera: Cimicidae), are hematophagous ectoparasites that were all but eradicated from the United States and other industrialized nations after World War II. Their disappearance likely was due to the widespread use of dichlorodiphenyltrichloroethane (DDT) and other broad‐spectrum insecticides during the second half of the twentieth century.1 Still, while reports of bed bug infestations declined, evidence of resistance to insecticides, including DDT, were increasing.2, 3
Over the course of the past decades, bed bugs have re‐emerged on the global stage as an important public health pest. The cause(s) of the global resurgence remain unclear, but hypotheses include increased human movement via travel and migration, changes in pest management practices,1 the unavailability of effective residual insecticides4, 5, 6 and greater resistance to pyrethroid insecticides in reservoir or wild bed bug populations.4, 6, 7
Although pyrethroid insecticides are a mainstay of bed bug control owing to their broad‐spectrum activity, persistence in the environment and low cost, their efficacy is on the decline for bed bug control because of resistance.8 Laboratory studies consistently detect widespread insecticide resistance in field‐collected populations.4, 6, 9 Recent reports have provided compelling evidence that many bed bug populations have developed resistance to pyrethroid insecticides, and that resistance may lead to cross‐resistance to other classes of insecticides. Recently, high levels of resistance to four neonicotinoids (acetamiprid, imidacliprid, dinotefuran and thiamethoxam) were detected in field populations of bed bugs,10 including the Jersey City strain used in this study.
Various mechanisms contribute to resistance in bed bugs, and the geographic distribution of resistant bed bug populations is global. Two point mutations, V419L and L925I, have been identified as one mechanism for knockdown resistance (kdr) to deltamethrin in bed bug populations in New York.11 Subsequent studies found one or both mutations in bed bug populations throughout the United States,12 Paris,13 Central Europe,14 Australia15 and Israel,16 suggesting that kdr‐mediated resistance is a global phenomenon. Genome‐wide analysis of C. lectularius has revealed many resistance‐associated genes and mechanisms, including upregulation of P450s, esterases and ABC transporters.17, 18 Therefore, the ubiquitous evolution of resistance to pyrethroid and neonicotinoid insecticides in bed bug populations appears to involve multiple mechanisms, which further highlights the need for alternative approaches to control bed bugs.
Alternative approaches to bed bug control vary in complexity, cost of implementation and efficacy. The use of high temperatures to kill bed bugs has increased in popularity over the past decade.19 While effective, the use of volumetric heating can be expensive, ranging from $US 500 to $US 1000 per room.20 When alternative methods of control are not possible, placing infested items in a household freezer for several days to kill all life stages remains an option.21 Other approaches to bed bug control include the use of diatomaceous earth, various essential oils and detergents. Current methods of bed bug control often lack satisfactory efficacy, leaving the public seeking environmentally safe options that are new, innovative and effective.
Entomopathogenic fungi have demonstrated effectiveness against numerous public health pests, including malaria vectors,22 cockroaches23 and houseflies.24 Furthermore, fungal pathogens have been shown to be effective against insecticide‐resistant mosquito populations.25 Beauveria bassiana has been identified as a potential candidate for bed bug control.26 The aim of this study was to compare the efficacy of a new B. bassiana‐based product on an insecticide‐susceptible lab strain of C. lectularius and three field‐collected strains known to be resistant to pyrethroids. We also compared the mortality of the four bed bug strains after exposure to either a commercial deltamethrin‐based insecticide labeled for bed bug control or B. bassiana.
2. EXPERIMENTAL METHODS
2.1. Bed bug maintenance
The four strains of bed bugs used in these experiments were maintained in small plastic containers (Consolidated Plastics, Stow, OH) with plankton netting (BioQuip, Rancho Dominguez, CA) on the bottom for ventilation and through which bed bugs fed on blood. Harborages made of manila folders folded accordion style were used to provide shelter. Insects were maintained in environmental growth chambers at 27 ± 1 °C on a 12 h light:12 h dark cycle and 50 ± 5% relative humidity. Colonies were fed defibrinated rabbit blood (Quad Five, Ryegate, MT) using custom‐made glass feeders (Prism Research Glass, Raleigh, NC), and bed bugs were fed 24 h prior to the experiments.
The Harold Harlan strain (= Ft Dix strain) was collected in Fort Dix, New Jersey, in 1973 and has been maintained at North Carolina State University since December 2008; both the V419L and L925I mutations are absent in this strain. The Winston Salem No. 7 (collected in 2008 in North Carolina) and the Jersey City (collected in 2008 in New Jersey) strains are both resistant to pyrethroid insecticides (see Section 3), and have both the V419L and L925I mutations. Campus Courtyard No. 15 (collected in 2009 in North Carolina) has not yet been tested for kdr mutations, but is known to be resistant to pyrethroids (see Section 3). The Jersey City strain is also moderately resistant to neonicotinoid insecticides.10
2.2. Insecticides
Aprehend™ is a formulation of B. bassiana (GHA strain) containing 2% (w/v) active ingredient (AI) and a minimum concentration of 2.4 × 109 viable conidia mL−1 (ConidioTec LLC, State College, PA). The ready‐to‐use formulation was applied at approximately 2 μL cm−2 to white, Diamond Double Faced Quilt Fabric (Jo‐Ann Fabric and Craft Stores, Hudson, OH), chosen because this fabric is commonly used by manufacturers of box springs (beds). To check the volume applied, surfaces were weighed before and after spray application to determine the actual volume and number of applied conidia per cm2. The average volume applied was found to be 1.72 μL cm−2, equivalent to 4.48 × 106 viable conidia cm−2. The treated fabric was left to dry at room temperature and used for exposure of bed bugs within 2 weeks of the spray application.
Suspend SC (Bayer CropScience, Research Triangle Park, NC) contains 4.75% deltamethrin and is labeled for application at a maintenance rate of 0.03% (w/v) AI and a clean‐out rate of 0.06% (w/v) AI in water. These two treatments, referred to hereafter as the low and high concentrations respectively, were applied to white, Diamond Double Faced Quilt Fabric using a potter precision laboratory spray tower (Burkard Scientific, Uxbridge, UK) at a volume of 15 μL cm−2. Air pressure was provided by a carbon dioxide canister (R&D Sprayers, Opelousas, LA) at a pressure of 152 kPa. Treated fabric was permitted to dry overnight and used the following day for bed bug exposure.
2.3. Efficacy of B. bassiana on four bed bug strains (2014 experiment)
Twenty‐four hours after feeding to repletion, adult male bed bugs of unknown ages were randomly placed into either a control group (n = 50) or a treatment group (n = 50). Each of these groups was further divided into five replicates of ten bugs per strain. Bed bugs were then exposed for 15 min to dry fabric treated with B. bassiana in a 9 cm diameter petri dish (VWR, Radnor, PA). Control bed bugs were exposed to fabrics sprayed with water (dried overnight). Following exposure, bed bugs were transferred to clean petri dishes and sealed with Parafilm®. Bed bugs were housed in environmental growth chambers under the same conditions as described above for the duration of the experiment. Bed bugs were checked for mortality 24 h after exposure and then once daily for 14 days. Mortality was defined as the inability of a bed bug to right itself after being flipped on its back, and the lack of any visible muscle twitches over a 1 min period. Dead bed bugs were removed daily and dried over silica gel for 1 week. The dry cadavers were then placed individually into 30 mL plastic diet cups (Dart, Mason, MI) with moist, sterile cotton and allowed to incubate at ∼23 °C for 3 days. Each bed bug cadaver was assessed for the presence or absence of mycosis, based on the appearance of white B. bassiana conidia around the leg joints and intersegmental membranes.
2.4. Comparison of deltamethrin and B. bassiana efficacy on four bed bug strains (2015 experiment)
To evaluate the relative susceptibility of the four strains of bed bugs to deltamethrin in comparison with B. bassiana, a second bioassay was conducted using high and low concentrations of deltamethrin alongside the B. bassiana treatment. Bioassay procedures were identical to those used in the 2014 bioassays, except that Suspend SC was applied to the fabric swatches using a potter spray tower and allowed to dry overnight.
2.5. Statistical analysis
Data for survival analysis consisted of survival times for 50 individual bugs for each combination of year (2014 and 2015), strain (four strains) and treatment (four treatments). The period of observation was 14 days, and bed bugs that survived beyond the period of observation were coded as right censored. Kaplan–Meier non‐parametric comparisons between years within treatment indicated that survival differed significantly across years, but the effect was small in comparison with observed treatment effects (see Table 1). Accordingly, random year effects were included in a single proportional hazard regression, or Cox model, to investigate simple factorial effects of the treatment for fixed strains. The model was fitted using PROC PHREG.27 The Harold Harlan controls exhibited the most survivors and were taken as the baseline in the formulation of the proportional hazard model. The estimated log hazard ratios relative to this baseline were used for statistical separatation of treatments within each strain. Where appropriate, mean survival times (MSTs), median survival times and relative log hazard ratios were estimated.
Table 1.
Proportional hazard tests of the effects of strain and treatment on survival time, with year as a random effect
| Effect | Wald χ 2 | df | P > χ 2 | Adjusted df | Bonferroni adjusted P > χ 2 |
|---|---|---|---|---|---|
| Strain | 95.634 | 3 | <0.0001 | 2.9999 | <0.0001 |
| Treatment | 491.252 | 3 | <0.0001 | 2.9832 | <0.0001 |
| Strain × treatment | 277.244 | 9 | <0.0001 | 8.9997 | <0.0001 |
| Year | 6.362 | — | — | 0.8642 | 0.0092 |
3. RESULTS
The overall model indicated that the estimated variance component for the random effect (year) was statistically significant (P = 0.0092) but small (estimated variance component = 0.0308) in comparison with observed strain and treatment effects (Table 1). Therefore, a single proportional hazard regression was implemented with random year effects included to investigate the effects of the treatments for fixed strains. Survival curves for all treatments and bed bug strains are shown in Fig. 1. Estimated MSTs and median survival times for each bed bug strain and treatment are summarized in Table 2.
Figure 1.
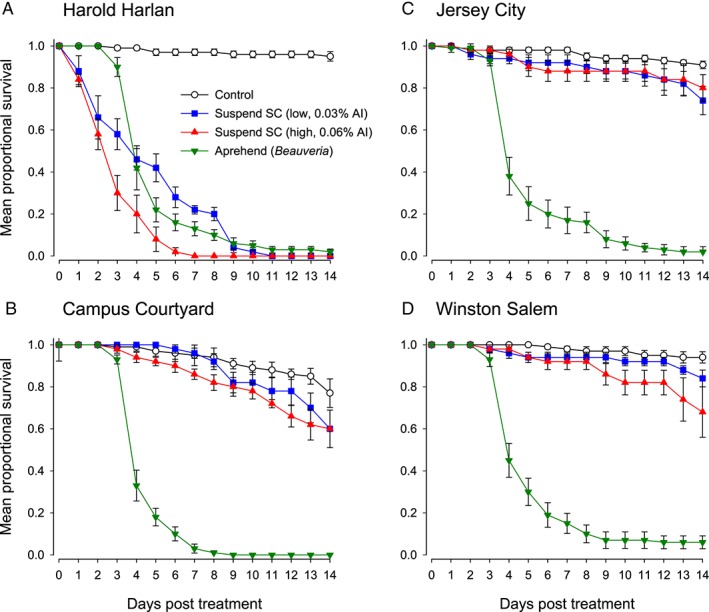
Proportional survival of fed adult bed bugs of four bed bug strains after 15 min exposure to fabric treated with B. bassiana (Aprehend™) at the recommended rate and deltamethrin (Suspend SC) at recommended high (0.06%) and low (0.03%) label rates. Control bed bugs are from the same strain as the respective treated bed bugs and were exposed to water‐treated fabric for 15 min. Bars represent SEM.
Table 2.
Kaplan–Meier estimates of mean survival time ± standard errors (not given in cases where substantial bias was caused by mortality below 50%), median survival times and log hazard ratio ± standard errors relative to baseline (Harold Harlan control). Fed adult males of four strains of bed bugs were exposed for 15 min to control (water‐treated), deltamethrin (Suspend SC)‐treated or B. bassiana (Aprehend™)‐treated surfaces. A proportional hazard model was applied to investigate factorial effects of the treatment, separately for each strain, using SAS PROC PHREG
| Strain | Treatmenta | Groupingb | Mortality | Mean survival time ± SE (days)c | Median survival time (days) |
Relative log hazard ratio ± SE |
|---|---|---|---|---|---|---|
| Harold Harlan | Control | A | 5/100 (5%) | — | ≥14 | 0.00 ± 0.00 |
| Suspend SC low | B | 50/50 (100%) | 4.8 ± 0.45 | 4 | 4.62 ± 0.48 | |
| Suspend SC high | B | 50/50 (100%) | 3.0 ± 0.22 | 3 | 5.56 ± 0.48 | |
| Aprehend™ | C | 98/100 (98%) | 5.1 ± 0.23 | 4 | 4.42 ± 0.46 | |
| Campus Courtyard | Control | A | 23/100 (23%) | — | ≥14 | 4.42 ± 0.46 |
| Suspend SC low | A | 20/50 (40%) | — | ≥14 | 4.42 ± 0.46 | |
| Suspend SC high | A | 20/50 (40%) | — | ≥14 | 4.42 ± 0.46 | |
| Aprehend™ | B | 100/100 (100%) | 4.6 ± 0.12 | 4 | 4.42 ± 0.46 | |
| Jersey City | Control | A | 9/100 (9%) | — | ≥14 | 0.61 ± 0.56 |
| Suspend SC low | A | 13/50 (26%) | — | ≥14 | 1.86 ± 0.53 | |
| Suspend SC high | A | 10/50 (20%) | — | ≥14 | 1.59 ± 0.55 | |
| Aprehend™ | B | 98/100 (98%) | 5.3 ± 0.25 | 4 | 4.38 ± 0.46 | |
| Winston Salem | Control | A | 6/100 (6%) | — | ≥14 | 0.18 ± 0.61 |
| Suspend SC low | AB | 8/50 (16%) | — | ≥14 | 1.33 ± 0.57 | |
| Suspend SC high | B | 16/50 (32%) | — | ≥14 | 2.08 ± 0.51 | |
| Aprehend™ | C | 94/100 (94%) | 5.3 ± 0.27 | 4 | 4.28 ± 0.46 |
The active ingredient in Suspend SC is deltamethrin, and in Aprehend™ it is B. bassiana. Low and high refer to the labeled rate for application at a maintenance rate of 0.03% (w/v) deltamethrin and a clean‐out rate of 0.06% (w/v) deltamethrin respectively.
Pairwise comparisons of survival of the four treatments within each strain with Bonferroni‐adjusted P‐values. Within a strain, treatments with the same letter do not differ significantly, based on a statistical comparison of log hazard ratios (P > 0.05).
A dash denotes that estimation could not be performed.
Two treatments were replicated in two independent experiments, in 2014 and 2015, the control treatment and the B. bassiana treatment. Relatively low mortality occurred in the control populations of bed bugs (exposed to fabric treated with water) across the four strains over the 14 day duration of the two experiments, with ≤8% mortality in 2014 (mean survival ± SEM: 95.5 ± 1.5%) and ≤44% mortality in 2015 (mean survival: 83.0 ± 2.7%). Overall survivorship across all four strains was 89.3 ± 1.5%. The estimation of mean survival time (MST) in Kaplan–Meier survival analysis requires that the majority of subjects die within the monitoring period in order to calculate accurate estimates. As a result, MSTs for each of the control populations could not be estimated (Table 2).
Mortality across all four bed bug strains exposed to B. bassiana‐treated substrates was 99.0 ± 0.7% in 2014 and 95.5 ± 1.4% in 2015 by the end of the 14 day monitoring period (overall survivorship: 2.5 ± 0.8%) (Fig. 1). Mean survival times were similar for all four strains, ranging from 4.6 days (Campus Courtyard) to 5.3 days (Winston Salem), with no significant differences among them according to the Wilcoxon test statistics computed from the Kaplan–Meier curves (χ 2 = 3.670, df = 3, P = 0.2994). Mortality in the B. bassiana‐treated bed bugs began on days 2 or 3 after exposure, and bed bugs in all four strains reached between 70 and 80% mortality by day 4 (Fig. 1). Mycosis was confirmed in 100% of cadavers in the B. bassiana treatments. Only one bed bug cadaver in the control group was found to have mycosis, and this individual was from the Harold Harlan strain.
The four strains of bed bugs were differentially affected by exposure to fabric treated with deltamethrin. Harold Harlan strain bed bugs were highly susceptible to both high and low concentrations of Suspend SC, with 100% mortality within 7 and 11 days respectively (Fig. 1A), and MSTs of 3.0 and 4.8 days (Table 2). There were no significant differences in survival of Harold Harlan bed bugs between the two deltamethrin treatments and B. bassiana (Table 2).
In contrast, the three field‐collected strains were highly resistant to deltamethrin, with only 16–40% mortality 14 days after exposure (Figs 1B to D). In two of these strains (Campus Courtyard and Jersey City) the survivorship was not significantly different from the control bed bugs, whereas in the Winston Salem strain only the high concentration of deltamethrin resulted in lower survivorship than in the controls (Table 2). For all three strains, deltamethrin‐treated bed bugs survived significantly longer than B. bassiana‐treated bed bugs (Table 2).
4. DISCUSSION
Our evaluations confirmed that the Harold Harlan strain was susceptible to deltamethrin, while the field‐collected Campus Courtyard, Jersey City and Winston Salem bed bug strains tolerated treatments with relatively high concentrations of deltamethrin. Although resistance has been shown to decline over time in some bed bug colonies following years of laboratory rearing without pyrethroid selection,28 these three populations retained sufficiently high pyrethroid resistance after 7–8 years in laboratory culture to experience low mortality after 15 min exposure even to a high concentration of Suspend SC.
Most importantly, this study corroborates that resistance to pyrethroid insecticides does not confer cross‐resistance to infection by B. bassiana. Similar results have been observed for Anopheles spp. mosquitoes, which demonstrated that resistance to permethrin, DDT and bendiocarb did not result in reduced infection by B. bassiana or M. anisopliae.25 This study also demonstrated that B. bassiana‐ and M. anisopliae‐infected mosquitoes with kdr mutations displayed increased susceptibility to chemical insecticides.25 While we have not investigated this combined effect, it is possible that a similar change in susceptibility could occur in deltamethrin‐resistant bed bugs and is worthy of further investigation.
Cuticular thickening appears to contribute to insecticide resistance in bed bugs and other insects, presumably by impeding insecticide penetration. This mechanism has been reported in insecticide‐resistant Triatomine kissing bugs,29 the housefly Musca domestica,30 the German cockroach Blattella germanica,31 Anopheles mosquitoes32 and bed bugs.33 Given that the mode of infection of B. bassiana is via germination of the conidia and direct penetration of the appresorium through the cuticle of the host insect, thickening of the cuticle might be expected to impede B. bassiana infection. Nevertheless, studies have shown that B. bassiana was effective on insecticide‐resistant insect populations, for example Anopheles mosquitoes,25 and even on pyrethroid‐resistant Triatoma infestans with thicker cuticles and greater amounts of cuticular lipids.29 Therefore, even if cuticle thickening is associated with insecticide resistance in any of our three bed bug strains, it would appear that B. bassiana infection was unaffected. It is important to identify the mechanisms that permit entomopathogenic fungi effectively to infect insects with a thicker cuticle. One mechanism, identified in T. infestans, involves degradation of cuticular hydrocarbons.29 Notably, however, detailed descriptions of the infection process by entomopathogenic fungi identify the mouthparts, intersegmental folds and spiracles as the primary sites of invasion.34 As such, generalized cuticular thickening might not interfere with infection unless it is accompanied with changes in thinner and softer areas of the cuticle, where conidia preferentially germinate and penetrate.
Aprehend™ is a ready‐to‐use oil formulation that has been developed to permit application at ultralow volume rates without the addition of water. Oil formulations of fungal conidia have been demonstrated to enhance efficacy35, 36 and facilitate the movement and accumulation of conidia into protected recesses on the insect body.37, 38, 39 Oil formulations create a favorable microenvironment for germination and infection and enhance the efficacy of mycoinsecticides at low (<50%) humidity,40 and certain oil‐based formulating components may disrupt the protective layer of epicuticular lipids, facilitating host penetration.38 Unlike most chemical insecticides, where the duration of exposure is key to adsorption of the active ingredient through the cuticle, B. bassiana relies on movement of the bed bugs over the surface to pick up spores on the tarsi and other body parts. Bed bugs that remain quiescent in one place collect fewer spores on their body surface, resulting in slower time to death, and in some cases survival because a lethal dose was avoided. We expect that longer exposure beyond 15 min would increase the likelihood of bed bug movement and hence greater efficacy of B. bassiana.
There are few effective classes of insecticides labeled for bed bug control, and pyrethroid insecticides, alone or in combination with neonicotinoid insecticides, have become a mainstay in bed bug interventions.41 However, the overuse of pyrethroid‐ and neonicotinoid‐based products and cross‐resistance have selected for the evolution of resistance in many bed bug populations.10 Pest management practices traditionally used in agricultural systems, including monitoring insecticide efficacy and managing resistance, are largely ineffective for bed bug management because (1) few active ingredients with different modes of action are available for use in rotations, and (2) reservoirs of insecticide‐susceptible bed bugs do not persist under the strong selection pressure and relatively closed spatial and genetic structure of bed bug populations.42 Alternative approaches such as whole‐building heat treatment and fumigation are helpful tactics when pyrethroid resistance is high. These approaches are expensive, however, and because they leave no residual insecticide, re‐infestations are likely from within the building or from outside sources. B. bassiana has a unique mode of action with no known resistance or cross‐resistance in bed bugs, and it is highly effective on pyrethroid‐resistant bed bugs, making it an excellent candidate for use in bed bug management programs. Entomopathogenic fungi are often considered slow acting compared with neuroactive insecticides. Although death due to B. bassiana infection was indeed slower in the insecticide‐susceptiple bed bug population, within 4 days after exposure its efficacy was similar to that of deltamethrin. Furthermore, the tendency of bed bugs to aggregate is likely to increase the dissemination of the fungus within the harborage and enhance overall population control.26
ACKNOWLEDGEMENTS
We thank Rick Santangelo for collecting and maintaining the bed bug colonies. Alexis Barbarin was supported by a National Science Foundation Postdoctoral Fellowship in Biology (award number 1308207). This work was conducted as part of USDA‐IR4 grant number 2012‐34383‐19759.
REFERENCES
- 1. Boase C, Bedbugs – back from the brink. Pestic Outlook 12:159–162 (2001). [Google Scholar]
- 2. Busvine JR, Insecticide‐resistance in bed‐bugs. Bull WHO 19:1041 (1958). [PMC free article] [PubMed] [Google Scholar]
- 3. Mallis A and Miller A, Prolonged resistance in the house fly and bed bug. J Econ Entomol 57:608–609 (1964). [Google Scholar]
- 4. Davies T, Field L and Williamson M, The re‐emergence of the bed bug as a nuisance pest: implications of resistance to the pyrethroid insecticides. Med Vet Entomol 26:241–254 (2012). [DOI] [PubMed] [Google Scholar]
- 5. Moore DJ and Miller DM, Field evaluations of insecticide treatment regimens for control of the common bed bug, Cimex lectularius (L.). Pest Manag Sci 65:332–338 (2009). [DOI] [PubMed] [Google Scholar]
- 6. Romero A, Potter MF, Potter DA and Haynes KF, Insecticide resistance in the bed bug: a factor in the pest's sudden resurgence? J Med Entomol 44:175–178 (2007). [DOI] [PubMed] [Google Scholar]
- 7. Myamba J, Maxwell C, Asidi A and Curtis C, Pyrethroid resistance in tropical bedbugs, Cimex hemipterus, associated with use of treated bednets. Med Vet Entomol 16:448–451 (2002). [DOI] [PubMed] [Google Scholar]
- 8. Zhu F, Gujar H, Gordon JR, Haynes KF, Potter MF and Palli SR, Bed bugs evolved unique adaptive strategy to resist pyrethroid insecticides. Sci Rep 3:1456 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Potter M, A bed bug state of mind: emerging issues in bed bug management. Pest Control Technol 33:82–85 (2005). [Google Scholar]
- 10. Romero A and Anderson TD, High levels of resistance in the common bed bug, Cimex lectularius (Hemiptera: Cimicidae), to neonicotinoid insecticides. J Med Entomol 53:727–731 (2016). [DOI] [PubMed] [Google Scholar]
- 11. Yoon KS, Kwon DH, Strycharz JP, Hollingsworth CS, Lee SH and Clark JM, Biochemical and molecular analysis of deltamethrin resistance in the common bed bug (Hemiptera: Cimicidae). J Med Entomol 45:1092–1101 (2008). [DOI] [PubMed] [Google Scholar]
- 12. Zhu F, Wigginton J, Romero A, Moore A, Ferguson K, Palli R et al, Widespread distribution of knockdown resistance mutations in the bed bug, Cimex lectularius (Hemiptera: Cimicidae), populations in the United States. Arch Insect Biochem Physiol 73:245–257 (2010). [DOI] [PubMed] [Google Scholar]
- 13. Durand R, Bouvresse S, Berdjane Z, Izri A, Chosidow O and Clark J, Insecticide resistance in head lice: clinical, parasitological and genetic aspects. Clin Microbiol Infect 18:338–344 (2012). [DOI] [PubMed] [Google Scholar]
- 14. Booth W, Balvín O, Vargo EL, Vilímová J and Schal C, Host association drives genetic divergence in the bed bug, Cimex lectularius . Mol Ecol 24:980–992 (2015). [DOI] [PubMed] [Google Scholar]
- 15. Dang K, Toi CS, Lilly DG, Bu W and Doggett SL, Detection of knockdown resistance mutations in the common bed bug, Cimex lectularius (Hemiptera: Cimicidae), in Australia. Pest Manag Sci 71:914–922 (2015). [DOI] [PubMed] [Google Scholar]
- 16. Palenchar DJ, Gellatly KJ, Yoon KS, Mumcuoglu KY, Shalom U and Clark JM, Quantitative sequencing for the determination of kdr‐type resistance allele (V419L, L925I, I936F) frequencies in common bed bug (Hemiptera: Cimicidae) populations collected from Israel. J Med Entomol 52:1018–1027 (2015). [DOI] [PubMed] [Google Scholar]
- 17. Koganemaru R, Miller DM and Adelman ZN, Robust cuticular penetration resistance in the common bed bug (Cimex lectularius L.) correlates with increased steady‐state transcript levels of CPR‐type cuticle protein genes. Pestic Biochem Physiol 106:190–197 (2013). [Google Scholar]
- 18. Benoit JB, Adelman ZN, Reinhardt K, Dolan A, Poelchau M, Jennings EC et al, Unique features of a global human ectoparasite identified through sequencing of the bed bug genome. Nat Commun 7:10165 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kells SA and Goblirsch MJ, Temperature and time requirements for controlling bed bugs (Cimex lectularius) under commercial heat treatment conditions. Insects 2:412–422 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Getty G, Taylor RL and Lewis VR, Evaluation of research on the use of heat to control bedbug infestations. Pest Control Technol 10:96–102 (2008). [Google Scholar]
- 21. Olson JF, Eaton M, Kells SA, Morin V and Wang C, Cold tolerance of bed bugs and practical recommendations for control. J Econ Entomol 106:2433–2441 (2013). [DOI] [PubMed] [Google Scholar]
- 22. Blanford S, Chan BH, Jenkins N, Sim D, Turner RJ, Read AF et al, Fungal pathogen reduces potential for malaria transmission. Science 308:1638–1641 (2005). [DOI] [PubMed] [Google Scholar]
- 23. Zurek L, Watson DW and Schal C, Synergism between Metarhizium anisopliae (Deuteromycota: Hyphomycetes) and boric acid against the German cockroach (Dictyoptera: Blattellidae). Biol Control 23:296–302 (2002). [Google Scholar]
- 24. Acharya N, Rajotte EG, Jenkins NE and Thomas MB, Potential for biocontrol of house flies, Musca domestica, using fungal biopesticides. Biocontrol Sci Technol 25:513–524 (2015). [Google Scholar]
- 25. Farenhorst M, Mouatcho JC, Kikankie CK, Brooke BD, Hunt RH, Thomas MB et al, Fungal infection counters insecticide resistance in African malaria mosquitoes. Proc Natl Acad Sci USA 106:17 443–17 447 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barbarin AM, Jenkins NE, Rajotte EG and Thomas MB, A preliminary evaluation of the potential of Beauveria bassiana for bed bug control. J Invertebr Pathol 111:82–85 (2012). [DOI] [PubMed] [Google Scholar]
- 27. SAS/STAT 9.3 User's Guide. SAS Institute Inc., Cary, NC: (2011). [Google Scholar]
- 28. Gordon JR, Goodman MH, Potter MF and Haynes KF, Population variation in and selection for resistance to pyrethroid–neonicotinoid insecticides in the bed bug. Sci Rep 4:3836 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pedrini N, Mijailovsky SJ, Girotti JR, Stariolo R, Cardozo RM, Gentile A et al, Control of pyrethroid‐resistant Chagas disease vectors with entomopathogenic fungi. PLoS Negl Trop Dis 3:e434 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. DeVries DH and Georghiou GP, Decreased nerve sensitivity and decreased cuticular penetration as mechanisms of resistance to pyrethroids in a (1R)‐trans‐permethrin‐selected strain of the house fly. Pestic Biochem Physiol 15:234–241 (1981). [Google Scholar]
- 31. Wu D, Scharf ME, Neal JJ, Suiter DR and Bennett GW, Mechanisms of fenvalerate resistance in the German cockroach, Blattella germanica (L.). Pestic Biochem Physiol 61:53–62 (1998). [Google Scholar]
- 32. Wood O, Hanrahan S, Coetzee M, Koekemoer L and Brooke B, Cuticle thickening associated with pyrethroid resistance in the major malaria vector Anopheles funestus . Parasit Vectors 3:1–7 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lilly DG, Latham SL, Webb CE and Doggett SL, Cuticle thickening in a pyrethroid‐resistant strain of the common bed bug, Cimex lectularius L. (Hemiptera: Cimicidae). PLoS ONE 11:e0153302 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Charnley A and Collins S, Entomopathogenic fungi and their role in pest control, in Environmental and Microbial Relationships, ed. by Kubicek C. and Druzhinina I. Springer, Berlin, Germany, pp. 159–187 (2007). [Google Scholar]
- 35. Prior C, Jollands P and Le Patourel G, Infectivity of oil and water formulations of Beauveria bassiana (Deuteromycotina: Hyphomycetes) to the cocoa weevil pest Pantorhytes plutus (Coleoptera: Curculionidae). J Invertebr Pathol 52:66–72 (1988). [Google Scholar]
- 36. Jenkins NE and Thomas MB, Effect of formulation and application method on the efficacy of aerial and submerged conidia of Metarhizium flavoviride for locust and grasshopper control. Pestic Sci 46:299–306 (1996). [Google Scholar]
- 37. Burges HD, Formulation of Microbial Biopesticides. Springer, Dordrecht, The Netherlands, 412 pp. (1998). [Google Scholar]
- 38. Ibrahim L, Butt T, Beckett A and Clark S, The germination of oil‐formulated conidia of the insect pathogen, Metarhizium anisopliae . Mycol Res 103:901–907 (1999). [Google Scholar]
- 39. Luz C, Rodrigues J and Rocha LF, Diatomaceous earth and oil enhance effectiveness of Metarhizium anisopliae against Triatoma infestans . Acta Trop 122:29–35 (2012). [DOI] [PubMed] [Google Scholar]
- 40. Bateman R, Carey M, Moore D and Prior C, The enhanced infectivity of Metarhizium flavoviride in oil formulations to desert locusts at low humidities. Ann Appl Biol 122:145–152 (1993). [Google Scholar]
- 41. Wang C, Singh N and Cooper R, Field study of the comparative efficacy of three pyrethroid/neonicotinoid mixture products for the control of the common bed bug, Cimex lectularius. Insects 6:197–205 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Booth W, Saenz VL, Santangelo RG, Wang C, Schal C and Vargo EL, Molecular markers reveal infestation dynamics of the bed bug (Hemiptera: Cimicidae) within apartment buildings. J Med Entomol 49:535–546 (2012). [DOI] [PubMed] [Google Scholar]


