Abstract
Background
Hepatitis C (HCV) can only be eradicated if annual rates of cure (SVR) are consistently and significantly higher than new HCV infections, across many countries. In 2016, the WHO called for a 90% reduction in new HCV infection by 2030. Direct-acting antivirals (DAA) can cure the majority of those treated, at around 90% in most populations, at potentially very low prices. We compared the net annual change in epidemic size across 91 countries using data on SVR, new HCV infections, and deaths. In a further 109 countries, we projected this figure using regional averages of epidemic size.
Methods
Epidemiological data for 2016 were extracted from national reports, publications and the Polaris Observatory. There were 91/210 countries with data on SVR, HCV-related deaths and new infections available for analysis; 109 countries had net change in epidemic size projected from the regional prevalence of HCV, extrapolated to their population size. ‘Net cure’ was defined as the number of people with SVR, minus new HCV infections, plus HCV-related deaths in 2016.
Results
For the 91 countries analysed, there were 57.3 million people with chronic HCV infection in 2016. In the remaining 109 countries, the projected epidemic size was 12.2 million, giving a global epidemic size of 69.6 million. Across the 91 countries, there was a fall from 57.3 to 56.9 million people in 2017, a 0.7% reduction. The projected global net change was from 69.6 to 69.3 million, a 0.4% reduction. Ten countries had at least five times more people reaching SVR than new HCV infections, including Egypt and USA. In 47/91 countries, there were more HCV infections than SVR in 2016.
Conclusion
Very few countries are on target to achieve elimination of HCV as a public health problem by 2030. While the North American, North African/Middle East and Western European regions have shown small declines in prevalence, the epidemic is growing in sub-Saharan Africa and Eastern Europe. Far higher rates of DAA treatment are required for worldwide elimination of HCV.
Keywords: hepatitis C, epidemiology, direct acting antivirals, sustained virological response
Introduction
Low costs of treatment are necessary for its scale-up to eliminate HCV as a public health problem by 2030. WHO targets are to treat 80% of the HCV epidemic by 2030 and reduce new infections by 90% by 2030 [1]. Increasing levels of treatment can lead to lower infection rates, due to a reduction in the number of people who can transmit the infection. This was seen in the Netherlands, where in 2015 unrestricted access to DAA for all patients newly infected with HCV was introduced [2]. This led to a 51% reduction in new HCV infections among men who have sex with men (MSM). This was the first reduction in new infections of HCV in this group in over 10 years [2].
Scaling up HCV treatment using DAAs could be achieved with the use of generics, with competition between generic manufacturers facilitating reductions in cost. Previous studies have estimated the minimum cost of production of sofosbuvir to be $68–136 for a 12-week course if scaled up to mass treatment [3]. Generic versions of DAAs are being manufactured in various settings, including India and Egypt. Country-level procurement has largely been through licensed manufacturers to licensed countries. Individual-level procurement has predominantly been through internet-based buyers’ clubs.
The WHO estimates that around 500,000 people living with HCV in Egypt were treated with generic sofosbuvir made available for $153 per 12-week treatment course in January–September 2016 [4]. Egypt can be used as a model to adopt in other countries with significant populations living with HCV, especially in low- and middle-income countries.
This study aims to assess the net cure of HCV infection in 2016, based on the number of new infections, number of cures and HCV-related deaths worldwide.
Methods
Epidemiology of hepatitis C
Data were collected from 91 countries worldwide to evaluate the number of people living with HCV, the number of new infections each year, the number of deaths related to HCV and the number of people treated annually. Sources of information included online databases of HCV infection and national government reports, as well as published literature on best estimates of HCV infection [4–8].
Epidemiological data were extracted from an online database – the Polaris Observatory, run by the Center for Disease Analysis (CDA) ( www.polarisobservatory.org/polaris/hepC.htm) [8].
The Polaris Observatory database uses a model to estimate epidemic sizes based upon literature reviews by country and expert opinion. The model was developed through literature searches of the epidemiology of HCV, searches of unpublished data and through consultation with experts. In situations where no data were available for a country, expert opinion was consulted and countries with similar risk factors or healthcare practices were used as analogue input data. The precise methodology used to create the model is described in detail elsewhere [9].
The number of new infections each year was also modelled by the CDA, if the reported number was not available. The model estimated the average number of new infections per year. This considered the main drivers of the HCV epidemic by country, for example: intravenous drug use (IVDU), unsafe hospital practices leading to nosocomial infections, and immigration from high-risk countries where HCV is endemic. Reduction in risk factors through blood screening and needle exchange programmes were considered as features that reduced the number of new infections.
The data on epidemic size and numbers treated was used to estimate the annual net cure for each country. This was calculated by subtracting the total number cured and HCV-related deaths from the number of new infections, as a percentage of the country's epidemic size. All input data were from 2016.
An annual net cure of 7% was used as a target to reach the WHO target of elimination of HCV as a public health issue by 2030. If 7% of the epidemic in 2016 is cured each year (net), >90% of people infected in 2016 should be cured by 2030.
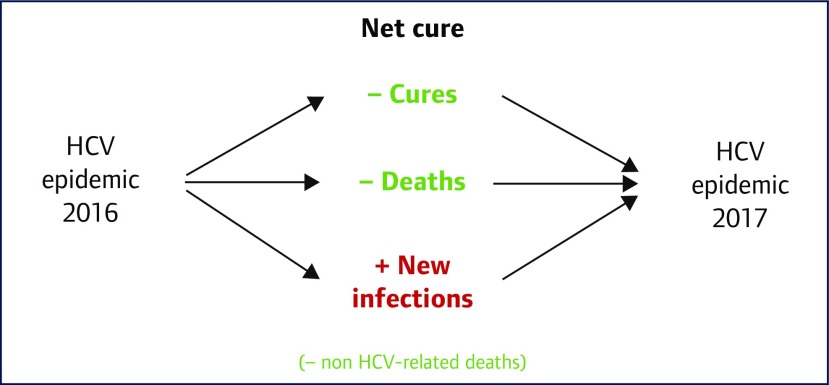
Net cure was also used to estimate the HCV epidemic size in 2017, for both the 91 countries analysed, and as a worldwide estimate. The method is shown in Figure 1. Non-HCV-related deaths were not included in this analysis.
Figure 1.
Diagram showing how the epidemic size of HCV for 2017 was estimated. This takes into account the number of cures, HCV-related deaths and new infections in 2016. Non-HCV-related deaths were not included for analysis
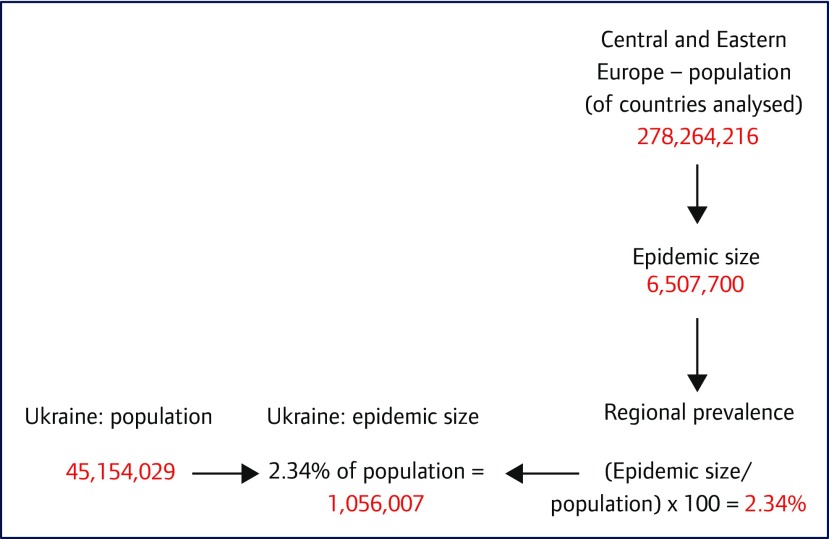
Data on epidemic size, cures, new infections and deaths from missing countries were estimated using regional prevalence data. The epidemic size in each region was divided by the regional population size of countries included in the analysis. This was scaled up to the population size of each country, using World Bank data [9]. The estimated change in epidemic size of Ukraine is shown as an example (Figure 2).
Figure 2.
Flowchart showing how the HCV epidemic size of Ukraine, a country with missing data, was estimated. This takes into account the regional population of the 91 countries analysed, the known epidemic size in this region and the population of Ukraine
Countries were analysed individually as well as being grouped into seven geographical regions: Asia and Pacific, sub-Saharan Africa, North Africa and Middle East, Central and Eastern Europe, Western Europe, North America and Latin and South America. Where available, data were cross-referenced with estimates from other public sources of data, including the 2016 Polaris Observatory HCV Collaborators review on global prevalence of HCV [7], WHO Global Report of Access to Hepatitis C treatment [4], findings presented at the International Liver Congress 2016 [10,11] and national government health reports [12–15].
Results
Number of people with hepatitis C infection
The literature search provided estimates of HCV epidemiology for 91 countries worldwide; there were 109 countries and territories excluded from the analysis, due to a lack of reliable data. Only 8/44 countries that make up sub-Saharan Africa were included in the analysis. All data were taken from the Polaris Observatory and cross referenced for reliability.
In total, 57.3 million people are estimated to be living with HCV in these 91 countries, versus a global estimate of 71 million (81% of global burden). This is an updated estimate of prevalence based upon viraemic infection, contrasting with previous studies that used HCV antibody prevalence. The geographical distribution of those living with HCV sees more than half living in the Asia and Pacific region (29.6 million) and the smallest population living within the Western Europe region (2.4 million). There are thought to be significant populations living with HCV in sub-Saharan Africa [16]. Estimates of HCV epidemiology from missing countries have been used to produce a global estimate of net cure for 2016, but have not been included in the main analysis.
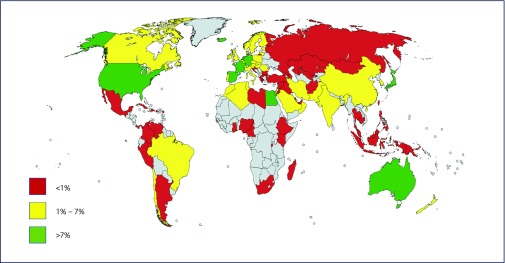
China has the largest HCV epidemic at 9.8 million, followed by Pakistan (7.1 million) and India (6.2 million). Figure 3 shows HCV prevalence by country. Four of the top 10 countries with the highest prevalence of HCV are located within the Asia and Pacific region.
Figure 3.
Map showing the percentage treatment rate by country for HCV. Countries shaded in grey were excluded from analysis due to a lack of reliable data
Percentage of people treated with DAAs in 2016
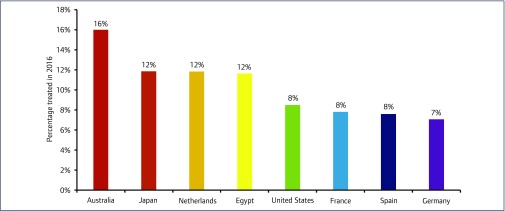
The percentage of people with HCV who were treated with DAAs in 2016 ranged from 8.1% in North America and North Africa/Middle East to 0.1% in sub-Saharan Africa (Figure 3). The percentage of those treated by country ranged from 0.0015% in Kenya to 50% in Iceland. For countries with an epidemic size >1000, Australia had the highest percentage of those treated at 16.0%. Ten countries in total had a treatment rate of over 7%, of which eight had an epidemic size >1000 (Figure 4). Four of these countries are in the Western European region. There were 44 countries with a treatment rate of below 1%. All eight countries in sub-Saharan Africa had a treatment rate of below 1%.
Figure 4.
Graph showing the eight countries treating the most people by percentage in 2016. Countries with a viraemic population <1000 (Iceland and Qatar) were excluded from this analysis
Net cure
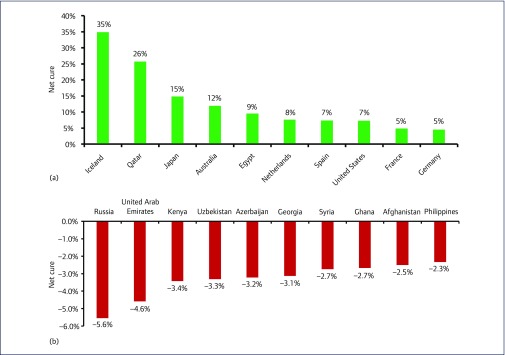
The net cure by country represents how well a country is dealing with the HCV epidemic. In Australia, there were 29,160 cures, 830 HCV-related deaths and 5,900 new infections in the year 2016. The net cure was therefore (29,160+830)−5900=24,090. As a percentage of the number viraemic, this equates to a 11.9% reduction in the total epidemic size (Figure 5a). Net cure ranged from +34.8% in Iceland to −5.6% in Russia (Figure 5b). A negative value indicates that the epidemic is increasing in size.
Figure 5.
(a) Bar chart showing the 10 countries with the highest net cure in 2016. (b) Bar chart showing the 10 countries with the lowest net cure in 2016
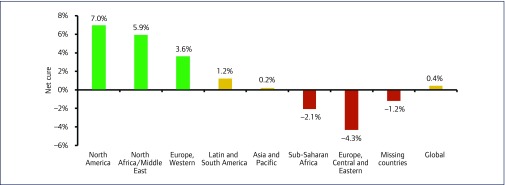
Regionally, net cure ranged from 7.0% in North America to −4.3% in Central and Eastern Europe. Estimated data from missing countries was included to provide a global estimate (Figure 6).
Figure 6.
Bar chart showing the net cure by region in 2016. Extrapolated data based on regional calculations of net cure were used to estimate the rate for missing countries and a global estimate. A negative net cure indicates that the epidemic size in the region is increasing
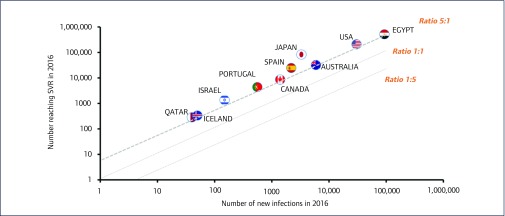
When restricting the analysis to numbers achieving SVR versus new infections (excluding deaths), 10 countries had five times more people achieving SVR than new infections: Egypt, USA, Australia, Japan, Spain, Canada, Portugal, Israel, Iceland and Qatar (Figure 7). Japan had the highest proportion of cures to new infections at 28.4.
Figure 7.
Scatter plot showing the 10/91 countries where there were >5 times more people reaching SVR than there were new infections in 2016. The guideline represents the mark of five people reaching SVR for every single new HCV infection
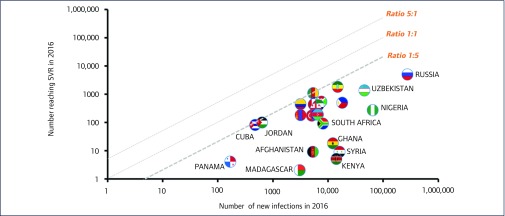
Twenty-three countries had five times fewer people with SVR than new infections in 2016 (Figure 8). This includes all eight countries that were analysed in the sub-Saharan Africa region: Cameroon, Ethiopia, Burundi, South Africa, Nigeria, Ghana, Kenya and Madagascar.
Figure 8.
Scatter plot showing the 23/91 countries where there were 5 times fewer people reaching SVR than there were new infections in 2016. The guideline represents the mark of one person reaching SVR for every five new HCV infections in 2016
Sub-Saharan Africa had 34.4 times more new infections than cures in 2016 and Central and Eastern Europe had 12.4 times more new infections than cures. Worldwide, 54/91 countries had more new infections than cures in 2016.
Table 1 provides a summary of epidemic size, new infections, cures and HCV-related deaths by region in 2016. This figure includes estimated data from missing countries. The net change in epidemic size between 2016 and 2017 is shown for all regions, with the sum of regions providing a global estimate.
Table 1.
Table showing the regional breakdown of epidemic size, new infections, number cured and HCV-related deaths. Using the data, the net change in epidemic size between 2016 and 2017 has been calculated. Estimated data is used for countries missing from the analysis. A global estimate for the burden of HCV is included as the sum of all regions, including missing countries
| Region | HCV Epidemic 2016 | New HCV infections | Number cured | HCV-related deaths | HCV Epidemic 2017 | Net change |
|---|---|---|---|---|---|---|
| Asia and Pacific | 29,564,900 | 574,330 | 456,552 | 179,810 | 29,502,868 | −62,032 |
| Central and Eastern Europe | 6,507,700 | 322,800 | 26,110 | 15,505 | 6,788,885 | +281,185 |
| Latin and South America | 3,477,400 | 27,537 | 47,859 | 21,496 | 3,435,582 | −40,548 |
| North Africa and Middle East | 7,399,470 | 156,660 | 542,724 | 51,944 | 6,961,462 | −438,008 |
| North America | 2,955,600 | 31,870 | 216,731 | 20,829 | 2,749,910 | −205,690 |
| Sub-Saharan Africa | 5,069,000 | 130,800 | 3,805 | 21,540 | 5,174,455 | +105,455 |
| Western Europe | 2,364,430 | 35,440 | 105,821 | 14,951 | 2,279,098 | −85,332 |
| 91 country subtotal | 57,338,500 | 1,279,437 | 1,399,602 | 326,075 | 56,892,260 | −446,240 |
| Missing countries | 12,216,308 | 318,375 | 113,157 | 57,923 | 12,363,603 | +147,295 |
| Global estimate | 69,554,808 | 1,597,812 | 1,512,759 | 383,998 | 69,255,863 | − 298,945 |
Discussion
This analysis shows large differences between countries in how hepatitis C is being treated. Some countries are treating a high percentage of patients, with significant annual reductions in HCV epidemic size. By contrast other countries have very low rates of treatment, and new HCV infections are driving the HCV epidemics.
For the 91 countries analysed with data available, it is estimated the HCV epidemic size will decrease from 57.3 million in 2016 to 56.9 million in 2017, a fall of less than 1%. Including global estimates, there is a decrease from 69.6 million to 69.3 million – a reduction of 0.4%. This is despite the $56 billion that has been spent on HCV DAAs since their launch [17].
For 47 of the 91 countries with data available, there were more people newly infected with HCV than cured in the year 2016. While there was an overall net cure of 0.78% in the 91 countries, we estimate that with extrapolated data, the net cure was 0.43% worldwide in 2016. At this rate, elimination of HCV is not feasible.
WHO estimates for HCV epidemiology in 2015 [1] can be compared to findings of this analysis (Table 2). The data include estimates of HCV epidemiology from countries where data were not available. Treatment has increased significantly from 2015 to 2016; however, deaths and new infections remain consistent between this analysis and estimates from WHO.
Table 2.
Table showing the HCV epidemiology calculated from this analysis (2016 data) in comparison to WHO estimated data for 2015. Data for 2016 is a combination of the 91 countries where data were available and the 109 countries and territories where epidemiology data were estimated from WHO regional prevalence data
| WHO estimate (2015 data) | This analysis (2016 data) | |
|---|---|---|
| New infections | 1,700,000 | 1,597,812 |
| Cures | 843,000 | 1,512,759 |
| HCV-related deaths | 399,000 | 383,998 |
| Epidemic size | 71,000,000 | 69,554,808 |
There are 109 countries and territories without data available for this analysis. However, estimates of the global burden were used to indicate the global picture. The global estimate for people living with HCV is 71 million, which is 13 million more than this analysis estimates in 91 countries. In sub-Saharan Africa, eight countries represent the whole region.
The WHO sector strategy for HCV suggests a target for 3 million people on treatment for HCV by 2020 [18]. The estimate of 1.5 million people on treatment in 2016 suggests that with continued scale-up of treatment, this target is achievable. Nevertheless, the continued success of delivering HCV treatment where it is required is dependent on strong political will, international donor support and reduction in the stigma associated with hepatitis C in IVDU and MSM populations.
Limitations of the analysis
This analysis highlighted the problem of a lack of reliable data in assessing the number of people living with HCV and the number who are treated worldwide.
The use of modelling data and expert opinion from many sources forms a substantial part of this review. Although not ideal, we believe this to be the most accurate estimate currently available. Future work would benefit from up-to-date national and regional estimates of HCV prevalence and treatment, particularly in areas where data is sparse, such as sub-Saharan Africa. Tailored country plans for elimination of HCV would benefit from more accurate data and give countries an achievable target to reach in a global effort for elimination.
It was assumed that all treatment in 2016 was with DAAs. Where the number of people cured by country was not available, we assumed that 90% of those treated would be cured (SVR), in line with cure rates seen with DAAs. It is possible that a minority of patients worldwide continue to be treated with pegylated interferon and ribavirin, which has cure rates of 50% [19].
This data did not take into account the effect of harm reduction strategies, which are important to consider as an approach to reduce new infections. Needle and syringe programmes (NSP) and opioid substitution therapy (OST) have been implemented in countries such as Kenya in late 2014 [20]. The effectiveness of such programs thus may not yet be apparent.
Although injection safety has improved, IVDU and unsafe healthcare practices still remain the leading causes of HCV transmission [1]. It is estimated that 5% of all healthcare-related injections worldwide are unsafe, from 39% in 2000 [1]. It is important that harm reduction strategies and safer injection practices are implemented independently alongside increasing HCV cures.
The calculation of net cure did not take into account non-HCV-related deaths. This may lead to an underestimation of net cure. However, it should not be assumed that the HCV epidemic will decline by people dying from the disease. Curing HCV through treatment should continue to be a mainstay of plans to eliminate HCV.
Wider implications
From this analysis, it is apparent that the current levels of treatment for HCV are not high enough to eliminate the epidemic by 2030. Despite progress being made in Western Europe and individual countries such as the United States and Japan, many countries find access to treatment limited. This is regardless of the price reductions that have been put in place by drug companies to increase affordability of DAA in low- and middle-income countries.
This suggests that such reductions are not going far enough to reach those who are most in need of treatment. Gilead Sciences have made their branded Harvoni sofosbuvir/ledipasvir combination available to 105 low- and middle-income countries for $900 per 12-week course [21]. Competition between generic manufacturers and mass production is required to drive down the cost of treatment well below this $900 level.
Jensen et al. suggest that demand for generic DAA is greater than the ability of national governments to effectively regulate quality control and inspect factories [22]. Falsified versions of generic daclatasvir and sofosbuvir/ledipasvir were highlighted in a WHO medical product alert in 2016 [23]. However, analysis of results from buyers’ clubs using generic DAA has shown high levels of efficacy. Egypt treated 500,000 individuals in 2016 with generic DAAs and is one of the few countries on track for elimination by 2030.
Although important, the cost of DAAs is not the only factor that needs to be addressed in the strategy to eliminate HCV. Diagnosis forms a vital part of achieving elimination. This comes into greater focus in areas where treatment levels are high but there remains a substantial population who are living with undiagnosed HCV.
Health service capacity may also prove to be a stumbling block to effective treatment in low-income countries. Although treatment often lasts for only 12 weeks to achieve cure, intensive monitoring is required. This includes genotyping and HCV RNA testing on treatment and post treatment, with their associated costs and technology required. International donor support, together with strong political will, are strongly needed to aid low-income countries in the elimination of HCV.
Recommended outcomes
Despite voluntary licensing helping to bring down treatment costs, WHO prequalification of generic DAAs would help to achieve the target prices for DAAs of $90 per 12-week treatment course. Prequalification assures quality, efficacy and safety of drugs [24]. This would allow bulk procurement of DAAs by donor agencies and national health departments for mass treatment programmes, with economies of scale allowing target prices to be met.
A tiered system of pricing can allow drug companies to sell DAAs at higher prices in high-income countries whilst ensuring access in low-income countries through price reductions. However, it has been argued that this is a short-term solution to increase access in low-income countries [25]. Subjective divisions between wealth of countries can also create very expensive prices in middle-income countries [25].
The Australian system of uncapped treatment numbers for a set price negotiated with drug manufacturers could be a solution for high-income countries. This ‘all you can treat’ model has allowed Australia to treat 32,400 people in 2016 at an average price of $5,799 per treatment course for sofosbuvir, ledipasvir and daclatasvir combinations – the lowest price for all high-income countries [26]. A similar arrangement in other high-income countries could prevent rationing of treatment to those with more severe disease.
A plan for elimination should be put in place for countries approaching elimination to overcome these barriers. For example, screening programs that target at-risk populations such as prisoners, people who inject drugs and those who are HIV positive can be introduced to diagnose as many people as possible in order to treat more of the epidemic.
A reduction in incidence of HCV infection is vital alongside treatment. The focus to reduce drivers of new infection, such as unsafe injection practices, should be put on the areas where they are most prevalent, such as the WHO Eastern Mediterranean Region [1]. This can discourage needle reuse and unnecessary injection, which alongside greater access to affordable generic DAA treatment can help to achieve elimination.
Despite the marginal gains in treatment of HCV in a few countries, more work needs to be done overall to increase access to generic DAA to combat the threat of HCV. Political will and international donor support are required to build on these recent gains, to work alongside increased access to generic forms of DAA.
Conclusions
Fifty-four of the 91 countries analysed had more people newly infected with HCV than the number of people cured. Only 8/91 countries have a net cure of >7%. The estimate of net cure worldwide is 0.43%. Globally, the level of treatment must be increased considerably to be on course for elimination by 2030.
The inflated prices of branded DAAs pose a barrier to treatment in many countries. List prices of sofosbuvir, daclatasvir and ledipasvir in the USA are significantly higher than their generic counterparts, despite generic forms of DAA treating more people in 2016. The minimum costs of production of these drugs suggest prices should be further driven down by mass generic production of DAA.
Further efforts to reduce price of DAAs should focus on different strategies for high-income and low- and middle-income countries. For low- and middle-income countries, voluntary licences are important to contain costs. These could allow the manufacture or import of cheaper generic DAA to treat national epidemics on a nationwide level.
High-income countries could use the Australian system of a negotiated price for uncapped treatment numbers, preventing a situation where treatment is rationed to the most severely affected. Alternatively, unit prices of treatment should be lowered to a level that allows elimination of HCV epidemics within an affordable health budget for each country.
References
- 1. World Health Organization Global Hepatitis Report 2017. Available at: apps.who.int/iris/bitstream/10665/255016/1/9789241565455-eng.pdf?ua=1/ ( accessed June 2017).
- 2. Aidsmap. HIV and AIDS Information New hepatitis C infections among HIV-positive gay men drop by half after direct-acting antiviral roll-out in Netherlands. 2017. Available at: www.aidsmap.com/New-hepatitis-C-infections-among-HIV-positive-gay-men-drop-by-half-after-direct-acting-antiviral-roll-out-in-Netherlands/page/3118696/ ( accessed June 2017).
- 3. Ven N, Fortunak J, Simmons B et al. Minimum target prices for production of direct-acting antivirals and associated diagnostics to combat hepatitis C virus. Hepatology 2015; 61: 1174– 1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organisation Global Report on Access to Hepatitis C Treatment. Focus on overcoming barriers. Available at: apps.who.int/iris/bitstream/10665/250625/1/WHO-HIV-2016.20-eng.pdf?ua=1/ ( accessed June 2017.
- 5. Alliance for Public Health Expanding Access to Hepatitis C Treatment – Alliance for Public Health. 2016. Available at: aph.org.ua/en/news/expanding-access-to-hepatitis-c-treatment/ ( accessed June 2017).
- 6. Maistate L, Golovin S, Deineka O, Khan T.. Hepatitis C in Eastern Europe and Central Asia. Civil Society Response to the Epidemic. 2015. Available at: www.aidsalliance.org.ua/ru/news/pdf/28.10.2015/EECA HCV EN.pdf ( accessed June 2017).
- 7. Blach S, Zeuzem S, Manns M et al. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol 2017; 2: 161– 176. [DOI] [PubMed] [Google Scholar]
- 8. CDA Foundation, Polaris Observatory Viremic HCV infections. Polaris Observatory. 2017. Available at: polarisobservatory.org/polaris/map.htm ( accessed June 2017).
- 9. The World Bank Population, total DataBank. The World Bank Group 2017. Available at: databank.worldbank.org/data/reports.aspx?source=2&series=SP.POP.TOTL&country= ( accessed June 2017).
- 10. Hatzakis A. National perspectives in Europe: common themes and challenges. In: International Viral Hepatitis Elimination (IVEHM) 2016. Amsterdam; 2016 Available at: regist2.virology-education.com/2016/IVHEM/02_Hatzakis.pdf ( accessed June 2017).
- 11. Tata Marinho R, Rodrigues J, Martins J et al. Evidence of impressive real-world SVR from Portuguese ledipasvir/sofosbuvir and sofosbuvir universal coverage program to eradicate (eliminate) hepatitis C. International Liver Congress. April 2016. Barcelona, Spain.
- 12. Public Health England Annual hepatitis C in the UK report. 2016. Available at: www.gov.uk/government/news/annual-hepatitis-c-in-the-uk-report ( accessed June 2017).
- 13. Centers for Disease Control and Prevention New Hepatitis C Infections Nearly Tripled over Five Years. 2017. Available at: www.cdc.gov/nchhstp/newsroom/2017/Hepatitis-Surveillance-Press-Release.html ( accessed June 2017).
- 14. European Centre for Disease Prevention and Control Annual Epidemiological Report 2016: Hepatitis C. 2016. Available at: ecdc.europa.eu/en/healthtopics/hepatitis-b/Pages/Annual-epidemiological-report-2016.aspx [PubMed]
- 15. Kirby Institute Hepatitis B and C in Australia Annual Surveillance Report Supplement 2016. Available at: kirby.unsw.edu.au/sites/default/files/hiv/resources/Hepatitis B and C in Australia Annual Surveillance Report Supplement 2016_0.pdf ( accessed June 2017).
- 16. Rao VB, Johari N, Cros P et al. Hepatitis C seroprevalence and HIV co-infection in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Infect Dis 2015; 15: 819– 824. [DOI] [PubMed] [Google Scholar]
- 17. Freeman J, Khwairakpam G, Dragunova J et al. 94% SVR with parallel imported generic direct acting antiviral treatment for hepatitis C. International Liver Congress. Amsterdam, Netherlands. April 2017. Abstract PS-097. [DOI] [PMC free article] [PubMed]
- 18. World Health Organization Global Health Sector Strategy on Viral Hepatitis 2016–2021. Available at: apps.who.int/iris/bitstream/10665/246177/1/WHO-HIV-2016.06-eng.pdf?ua=1 ( accessed June 2017).
- 19. Petta S, Craxì A.. Current and future HCV therapy: do we still need other anti-HCV drugs? Liver Int 2015; 35 ( Suppl 1): 4– 10. [DOI] [PubMed] [Google Scholar]
- 20. Abdool R. Policy change towards implementing harm reduction in Sub-Saharan Africa. Int J Drug Policy 2016; 30: 140– 142. [DOI] [PubMed] [Google Scholar]
- 21. Afghanistan, Algeria, Angola, Antigua B 5, Bangladesh, Benin. Chronic Hepatitis C Medicines Pricing Gilead offers its branded chronic hepatitis C medicines at a suggested government price of $300/bottle for Sovaldi and $400/bottle for Harvoni, in the 101. 2016. [ cited 2017 May 13]; Available at: https://www.gilead.com/~/media/files/pdfs/other/chronic%20hepatitis%20c%20medicines%20pricing%20%20030716.pdf ( accessed June 2017).
- 22. Jensen DM, Sebhatu P, Reau NS.. Generic medications for hepatitis C. Liver Int 2016; 36: 925– 928. [DOI] [PubMed] [Google Scholar]
- 23. WHO Medical Product Alert N° 3/2016. Falsified hepatitis C medicines circulating in South East Asia. Available at: www.who.int/medicines/publications/drugalerts/Alert32016_Fev_FalsifiedHepatitisCproducts_en.pdf?ua=1 ( accessed June 2017).
- 24. WHO Prequalification of medicines by WHO. Available at: www.who.int/mediacentre/factsheets/fs278/en/ ( accessed June 2017).
- 25. Moon S, Jambert E, Childs M, Schoen-Angerer T.. A win-win solution? A critical analysis of tiered pricing to improve access to medicines in developing countries. Available at: apps.who.int/medicinedocs/en/d/Js19031en/ ( accessed June 2017). [DOI] [PMC free article] [PubMed]
- 26. Dore GJ, Grebely J, Cunningham EB et al. Negotiating better discounts for DAA therapy is critical to achieve HCV elimination by 2030. J Hepatol 2017; 66: S513. [DOI] [PubMed] [Google Scholar]