Abstract
The Zika virus (ZIKV) was first isolated in 1947 in Uganda. While it took 60 years for this virus to cause major outbreaks, an important shift in its ability to cause epidemics took place in the first and second decades of the this century: in 2007 in Yap Island, Micronesia, followed by French Polynesia in 2013 and, finally in 2015 and 2016, when ZIKV infections occurred throughout South America, Central America and the Caribbean, spreading rapidly to reach North America in just a single year. No licensed prophylactic vaccine is yet available but recent efforts towards the development of a vaccine have been remarkable from both the private and public sectors and include new candidate vaccines ranging from the classical live-attenuated or inactivated vaccines to more sophisticated approaches such as mRNA or genetically engineered viral platforms. Previous successes with licensed flavivirus vaccines indicate that a protective ZIKV vaccine should be an achievable goal. Nevertheless, numerous pre- and post-licensure challenges need to be taken into account, such as the interaction of vaccine-induced immune responses with other flaviviruses, in particular with dengue, where antibody-dependent enhancement could become an issue, and the importance of a rapid induction of protective responses during pregnancy.
Keywords: Zika virus, flavivirus, vaccine
Introduction
The Zika virus (ZIKV) is a single-stranded positive-sense RNA arbovirus that belongs to the Flaviviridae family, which is transmitted to humans by Aedes mosquitoes, mainly Aedes aegypti, which is considered to be responsible for the current outbreaks [1]. Recently, sexual transmission has also been suggested to play a role in the epidemics. The illness caused in humans by ZIKV was first recognised in Nigeria in 1953 [2], but only a few cases were reported over many years. Notably, in 2007 an outbreak took place in the islands of Yap State in the Federated States of Micronesia with an estimated 5000 infections among a population of 6700 [3]. Subsequently, another outbreak in 2013–2014 in French Polynesia affected 32,000 people and uncovered an association between ZIKV infection and Guillain–Barré syndrome [4,5]. More recently, in 2015 and 2016, in the Brazilian state of Pernambuco, a sporadic outbreak occurred over a period of 8–10 months. By September 2015, an increase in the number of infants born with microcephaly and other congenital defects was noted in the same areas in which ZIKV had been first reported, and by mid-February 2016, more than 4300 cases of microcephaly had been recorded [6]. The link between ZIKV infection, Guillain–Barré syndrome and microcephaly led the World Health Organization (WHO) to declare ZIKV a public health emergency in February 2016 [7].
There are important challenges associated with the clinical development of ZIKV preventative vaccines with lessons learned from other flavivirus vaccines. The cross-reactivity elicited by other flaviviruses will need to be addressed and the impact of vaccine-induced antibodies on ZIKV and dengue virus (DENG) infections will have to be examined closely. ZIKV is structurally and antigenically closely related to other flaviviruses such as DENG, yellow fever (YF), and Japanese encephalitis (JEV), and cross-reactive, non-neutralising antibodies have the potential to exacerbate secondary flavivirus infections through antibody-dependent enhancement (ADE) mechanisms [8]. Therefore, it is important to consider the possibility of ADE in the development of a ZIKV vaccine and select candidates unlikely to produce cross-reactivity with undesirable effects, in particular with DENG, where ADE can lead to a fatal outcome. Additional challenges for the development of a ZIKV vaccine are determined by the vaccine's target product profile (TPP). For instance, the TPP of this vaccine is mainly aimed at women of reproductive age and may include pregnant women, as well as adult men who can transmit the virus. A vaccine delivered during or near pregnancy will require additional safety checks. A vaccine with attenuated virus may not be ideal in this scenario [9] due to the potential reversion to virulence and transmission from vaccinees to uninfected people. In addition, other types of vaccines delivering ZIKV genes, such as DNA or recombinant viral vectors may require proof that gene expression in the host does not harm the fetus. Reproductive toxicology studies could be useful in this regard. ZIKV sexual transmission may create the need for a vaccine to stop viral spreading via this route. Therefore, vaccine platforms and delivery routes supporting mucosal immunity will be highly valuable to prevent ZIKV epidemics. Finally, the accurate diagnosis and detection of new ZIKV infections will certainly affect the development of a vaccine, due to the fact that approximately 80% of cases are asymptomatic, thereby adding a layer of complications in the follow-up of volunteers during field trials.
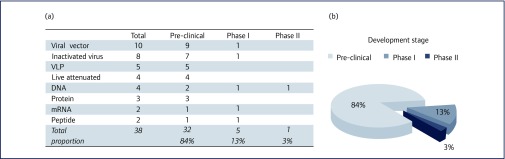
The rapid development of a safe and efficacious vaccine against ZIKV is unequivocally an important goal. Such vaccines should prevent the acquisition of infection and protect against the severe sequelae caused by the virus. These issues are currently being addressed by multiple vaccine platforms, such as DNA, mRNA, whole-inactivated and live-attenuated organisms, viral vectors, virus-like particles (VLPs), proteins and peptides. Approximately 38 ZIKV candidate vaccines have been reported to the World Health Organization (WHO) as being under development, 32 are in early pre-clinical assessment, five have progressed to Phase I trials and plans for one candidate are underway for a Phase II trial, see Figure 1(a) and (b). Among these, viral-vectored candidates are the most common platforms under development.
Figure 1.
(a) Vaccines reported by the World Health Organization to be in various stages of development, from pre-clinical to Phase I and II trials. (b) Chart indicating the proportion of Zika vaccine candidates at various stages of development, from pre-clinical to clinical trials. (Information from www.who.int/immunization/research/vaccine_pipeline_tracker_spreadsheet/en/)
The purpose of this review is to analyse the current trends of ZIKV-vaccine development, with a particular emphasis on novel strategies used to ensure safety and efficacy for at-risk populations.
Whole-inactivated and live-attenuated vaccines
The classical approach in the development of vaccines includes the use of inactivated and attenuated organisms. A major advantage of the live-attenuated vaccines is that they have been successful for other flavivirus vaccines, such as the JEV vaccine and the YF 17D live-attenuated vaccine, the latter being considered a major milestone in the history of vaccinology [10]. Other advantages for this approach include the induction of strong humoral and cellular responses and a low cost compared with most other novel vaccine strategies [11].
The ZIKV genome is composed of a single open-reading frame encoding a polyprotein, which is processed into the capsid (C), the precursor membrane (prM), the envelope protein (Env) and seven non-structural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5), flanked by 5′ and 3′ untranslated regions [1].
Shan et al. have designed a live vaccine through deletions within the 3′ untranslated region of the viral genome [12]. 3′ UTR elements modulate mRNA stability and deletions in this region can induce repressive effects [13]. A cDNA clone of ZIKV Cambodian strain FSS13025 was used to prepare a panel of viruses containing different 3′ UTR deletions. The attenuated virus showed more sensitivity to interferon β inhibition and induced less weight loss and no mortality in immunodeficient A129 mice. Furthermore, similar pre-challenge neutralisation titres of antibodies were observed between mice infected with the wild type and mutant virus strains. After a challenge using a subcutaneous injection of 105 IFU of the ZIKV FSS13025 strain, neutralisation titres were equivalent to the pre-challenge ones, indicating a sterilising antibody response after a single immunisation. The attenuated vaccine with a deletion of 10 bp of 3′ UTR region was the best candidate and elicited higher T cell responses than the wild-type virus.
Live-attenuated vaccines can produce high levels of protection against ZIKV infection; however, these immunogens may be associated with severe vaccine-associated adverse events. Therefore the whole-inactivated virus vaccine candidates may be a preferred option due to the relative safety that supports their use in immunocompromised people, such as the elderly, newborns and pregnant women [9].
The US National Institute of Allergy and Infectious Diseases (NIAID) is supporting several projects aimed at the development of a ZIKV vaccine. One candidate, ZIKV purified inactivated virus (ZPIV) is being trialled with the support of the Biomedical Advanced Research and Development Authority (BARDA), also supported by Sanofi Pasteur and the Walter Reed Army Institute of Research (WRAIR) in Silver Spring, USA. Initial pre-clinical results [14] have demonstrated immunogenicity and protective efficacy of a ZPIV (or ZIKA PIV) vaccine derived from the Puerto Rico PRVABC59 strain when administered intramuscularly [14]. Subsequently, testing in rhesus monkeys has shown similar results with complete protection against viraemia after ZIKV challenge [15].
Sumathy et al. have developed an alum-adsorbed inactivated virus vaccine based on the African MR 766 strain [16]. The AG129 and BALB/C mice were completely protected with no detectable viral load after a subcutaneous challenge with 104 PFU of the FSS13025 or MR766 strains.
Finally, Takeda Pharmaceutical Company Ltd has received support from BARDA to develop a whole-inactivated virus vaccine candidate, which is in pre-clinical development and will soon advance the vaccine to clinical trials.
DNA-based vaccines
DNA-based vaccines offer several advantages over other commonly used vaccine platforms. They are easily engineered and manipulated, allowing testing of multiple candidate antigens in various contexts. Additionally, they are also relatively simple to manufacture; their thermostability supports handling and transporting without requiring cold-chain logistics [17]. However, DNA vaccines have yet to prove they can provide protection in humans against infectious diseases, and the eliciting of effective immunity relies on the use of methods like electroporation to help DNA enter cells, this is a limiting factor when using DNA vaccines.
Larocca et al. have designed a ZIKV vaccine expressing the M and Env immunogens based on the Brazil BeH815744 strain and optimised them for increased antigen expression [14]. A DNA vaccine using the M-Env elicited high Env-specific neutralising antibody (NAb) titres and good CD4 and CD8 T lymphocyte responses after a single immunisation and provided complete protection against ZIKV strains from Brazil (ZIKV-BR) and Puerto Rico (ZIKV-PR) in BALB/c, SJL and C57BL/6 mouse models [18]. However, in comparison with purified inactivated virus vaccines, DNA vaccines have induced a less potent immunological response [15].
A different DNA vaccine approach was developed by Dowd et al. [19], who have cloned the prM-Env sequence from a French Polynesian strain (H/PF/2013) similar to the one circulating in the Americas, and exchanged the prM signal sequence for the analogous region of JEV to improve expression. NIAID has developed two vectors VRC5383 and VRC5288 that only differ in the final 98 amino acids of Env comprising the stem and transmembrane region (ST/TM), with the VRC5283 construct encoding for the wild-type ZIKV sequence and the VRC5288 construct having the ST/TM region swapped with the corresponding sequence from JEV. Both vaccines elicited specific ZIKV NAbs after a single immunisation in BALB/c and C57BL/6 mice models. Subsequently, these vaccines were evaluated in rhesus macaque models, showing a successful induction of NAbs 2 weeks post-immunisation. Macaques who had received a single dose of VRC5288 had lower antibody titres than those who had received two doses of either 1 mg or 4 mg of VRC5283 or two, 4-mg doses of VRC5288. A subcutaneous challenge with ZIKV strain PRVABC59 resulted in better protection by VRC5383 as compared to VRC5288. A Phase 1 clinical trial of the first candidate, VRC5288, started in August 2016, and a Phase 1 clinical trial of the second candidate, VRC5283, began in December 2016. A Phase 2a clinical trial of VRC5283 began in March 2017 to expand safety and immunogenicity data on VRC5283 in flavivirus endemic regions and to evaluate dose and delivery regimens. A Phase 2b study is planned to begin in June 2017.
RNA-based vaccines
RNA-based vaccines consist of a genetic vector of relatively simple structural composition and excellent versatility. Instead of presenting the antigen directly as protein-based vaccines do, these vaccines supply the information needed to induce endogenous antigen expression. A major advantage of RNA vaccine technology is its ability to be easily modified in order to enhance immunogenicity or eliminate undesired side-effects [20]. From a regulatory perspective, RNA vaccines offer an additional advantage over DNA vaccines because the risk of possible integration of genetic material into the genome of the vaccine is prevented due to the fact that the RNA is directly translated in the cytoplasm. RNA vaccines have reached clinical testing in cancer [21], and are also being tested against infectious diseases such as cytomegalovirus [21]. Nevertheless, the success of RNA vaccines will depend on the development of novel platforms to deliver the RNA into organisms and target cells.
Chahal et al. have created an RNA replicon vector using the Asian ZIKV isolate Z1106033 encoding the prM and Env proteins as a single open-reading frame [22]. The C57BL/6 mice immunised with this formulation by intramuscular injection exhibited IgG reactivity against the ZIKV Env protein with good CD8 T cell responses against the Env-derived peptide IGVSNRDFV.
Richner et al. [23] have designed a modified mRNA, encoding a type 1 (N7mGpppAm) cap containing the signal sequence from human IgE and the full length prM and Env genes (IgESigprM-Env), based on an Asian ZIKV strain [23]. In addition, optimisation of the 5′ and 3′ untranslated sequences was performed to improve intracellular stability and translation efficiency. The modified mRNA was packaged into lipid nanoparticles (LNP) to increase the shuttling between cells, using a modified technique derived from the delivery of siRNA and subsequently tested in immunodeficient AG129 and BALB/c mice and immunocompetent C57BL/6 mice, where high and durable protective NAbs were induced for over 14 weeks after a boost in these animal models. After replacing the IgESig with the corresponding sequence from the Japanese encephalitis virus (JEVSig) to increase expression levels, the authors tested the mRNA LNP vaccines with mutations in the conserved E-DII-FL (JEVSigprM-E-FL). Because the fusion loop in domain II (DII-FL) in the flavivirus envelope protein is immunodominant in humans, mutations abolish the reactivity of monoclonal and polyclonal antibodies targeting this region and thus reduce the risk of ADE induction during DENG infection. However, a lower level in NAb titres was observed. A Phase 1 study of the ZIKV mRNA vaccine candidate began enrolment in December 2016.
Pardi et al. have developed a mRNA vaccine using the prM-E glycoproteins of ZIKV containing nucleoside 1-methylpseudouridine as a modification to prevent innate immune sensing and enhance RNA translation [24]. The mRNA is encapsulated into lipid nanoparticles (mRNA-LNPs) to support prolonged protein expression. Mice were protected against ZIKV challenges shortly or long after immunisation, at 2 weeks or 5 months, respectively, with one dose of 30 μg of the immunogen. Similarly, rhesus macaques were protected against a ZIKV challenge by the immunisation with 50 μg of the nucleoside-modified ZIKV prM-E mRNA-LNP. Of note, titres of PRNT50 NAbs elicited by a single dose of mRNA-LNP were 50–100 times higher than those induced by a single dose of PIV or DNA vaccines in mice achieved in the study by Larocca et al. [14] and 0.05 mg of mRNA-LNP achieved 50 times higher NAb titres than 1 mg of DNA in macaques [19]. Titres, however, were apparently similar to those induced in macaques by a recombinant adenovirus, albeit different testing assays were used. Nevertheless, it is obvious that mRNA vaccines are highly immunogenic, even when used at low doses.
Finally, despite the lack of published experimental data, NIAID has announced that in collaboration with GlaxoSmithKline (GSK), a new ZIKV vaccine will be developed, using a self-amplifying mRNA vaccine platform, which could enter clinical trials in late 2017 [17].
Viral-vectored vaccination against ZIKV
Recombinant adenoviral vectors are emerging as promising genetic vaccine carriers whose applicability has increased in recent years due to their reliable safety profile, easy manufacturing process, and induction of broad and strong immune responses [25]. Using these platforms, Kim et al. have generated recombinant E1/E3-deleted adenovirus serotype 5-based vectors containing a codon-optimised extracellular portion of the ZIKV BeH815744 gene fused with the T4 fibritin foldon trimerisation domain (Ad5.ZIKV-Efl) [26]. Furthermore, in order to increase yield and facilitate downstream purification, the ZIKV-Efl vector was engineered to contain a polyhistidine-tag and a tobacco etch virus (TEV) protease cleavage sequence. The Ad5.ZIKV-Efl was inoculated subcutaneously in immunocompetent C57BL/6 mice with two doses of 1011 VP at 14 days interval, and induced high levels of anti-ZIKV Env NAbs as early as 2 weeks after the first immunisation, together with effective protection of neonatal mice against a lethal ZIKV DAKARA41542 strain challenge [26]. In a study by Abbink et al. rhesus adenovirus vectors were used to test efficacy in macaques. The adenoviruses from serotypes derived from chimpanzees or macaques have the advantage of minimal anti-vector immunity elicited by NAbs in human populations exposed to human adenovirus [18]. The rhesus RhAd52 vector expressing prM-Env was tested in rhesus monkeys. Robust protection was elicited after subcutaneous challenge with 106 VP of ZIKV-BR at week 2 following initial priming with RhAd52 vaccine and at week 4 after a boost with the DNA prM-Env vaccine.
Virus-like particles
Virus-like particles (VLPs) are self-assembling platforms that have become highly attractive as vaccines due to their capacity to induce strong antibody responses, prompted by the presentation of high densities of immunogens, arranged in a well-ordered matrix to mimic viral repetitive structure [27]. Boigard et al. have created an immunogenic vaccine using Zika-like virus particles [28]. Formation of these VLPs was accomplished by co-expression of the viral structural proteins C-prM-Env with a truncated form of the protease NS3Pro linked to its cofactor NS2B, a constituent of the viral NS2B/NS3Pro protease complex. Thus, purified VLPs resembled wild-type ZIKV in size, morphology and antigenic composition. The Zika VLP immunogen was evaluated in an immunodeficient Balb/c model with and without an adjuvant and in two different dose protocols. High serum NAb titres against ZIKV FSS13025 and MR-766 strains were observed and responses enhanced by the use of an adjuvant. Remarkably, when an ADE test to dengue-virus type 2 (DENV2) infection was performed, antibody responses to VLP immunisation did not enhance DENV2 infection [28].
Peptide vaccines
Immuno-informatic approaches are becoming of interest in the development of ZIKV vaccines, as they can support the recognition of conformational or linear epitopes to prime ZIKV-vaccine responses through the stimulation of B cell or cytotoxic T cell responses [29]. In a study addressing this approach, epitopes for both B and T cells have been predicted for the ZIKV Env and non-structural regions NS3 and NS5. This in silico approach is currently paving the way for epitope vaccine discovery and development of peptide vaccines.
Conclusions
Successful vaccines against related flaviviruses, such as YF virus, tick-borne JEV and DENG have been developed, This, coupled with the fact that ZIKV does not present with a high diversity or multiple serotypes, and a robust vaccine pipeline that involves private, academic and state-funded institutions acting alone or in collaboration and producing novel vaccine technologies, suggests that the development of a safe and protective ZIKV vaccine is feasible and will become a reality in the near future. While the technical part of the development may look relatively straightforward, ZIKV-vaccine candidates will face challenges during pre- and post-licensure when the time comes to assess the interaction of vaccine-induced antibody responses with other flaviviruses, particularly DENG where ADE can lead to severe disease and death. An important population to protect with a prophylactic vaccine will be women of reproductive age in order to prevent microcephaly and other congenital defects in newborns and while vaccines could ideally be administered before pregnancy, a vaccine suitable to be used during pregnancy would be ideal; however, not all platforms are suitable in this setting. It is highly likely that ZIKV will become endemic in regions that have presented outbreaks and circulate with other arboviruses such as DENG and Chikungunya virus, with the risk of future outbreaks. Hence, the search for a ZIKV vaccine should continue even in the absence of a global emergency in order to provide at-risk populations with a tool to prevent the complications caused by the ZIKV.
References
- 1. Wang A, Thurmond S, Islas L et al. . Zika virus genome biology and molecular pathogenesis. Emerg Microbes Infect 2017; 6: e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. MacNamara FN. Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg 1954; 48: 139– 45. [DOI] [PubMed] [Google Scholar]
- 3. Duffy MR, Chen TH, Hancock WT et al. . Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 2009; 360: 2536– 2543. [DOI] [PubMed] [Google Scholar]
- 4. Oehler E, Watrin L, Larre P et al. . Zika virus infection complicated by Guillain–Barré syndrome: case report, French Polynesia. Euro Surveill 2014; 19: 20720. [DOI] [PubMed] [Google Scholar]
- 5. Cao-Lormeau VM. Zika virus, French Polynesia, South Pacific, 2013 (Letter). Emerg Infect Dis 2014; 20: 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Petersen LR, Jamieson DJ, Powers AM, Honein MA.. Zika virus. N Engl J Med 2016; 374: 1552– 1563. [DOI] [PubMed] [Google Scholar]
- 7. World Health Organization WHO statement on the first meeting of the International Health Regulations (2005) (IHR 2005) Emergency Committee on Zika virus and observed increase in neurological disorders and neonatal malformations. WHO, 2016. Available at: www.who.int/mediacentre/news/statements/2016/1st-emergency-committee-zika/en/ ( accessed June 2017).
- 8. Dejnirattisai W, Supasa P, Wongwiwat W et al. . Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat Immunol 2016; 17: 1102– 1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fernandez E, Diamond MS. Vaccination strategies against Zika virus. Curr Opin Virol 2017; 23: 59– 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heinz FX, Stiasny K.. Flaviviruses and flavivirus vaccines. Vaccine 2012; 30: 4301– 4306. [DOI] [PubMed] [Google Scholar]
- 11. Minor PD. Live attenuated vaccines: historical successes and current challenges. Virology 2015; 479–480: 379– 392. [DOI] [PubMed] [Google Scholar]
- 12. Shan C, Muruato AE, Nunes BTD et al. . A live-attenuated Zika virus vaccine candidate induces sterilizing immunity in mouse models. Nat Med 2017; 23 : 763– 767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brinton MA, Basu M.. Functions of the 3′ and 5′ genome RNA regions of members of the genus flavivirus. Virus Res 2015; 206: 108– 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Larocca RA, Abbink P, Peron JPS et al. . Vaccine protection against Zika virus from Brazil. Nature 2016; 536: 474– 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abbink P, Larocca RA, De La Barrera RA et al. . Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science 2016; 353: 1129– 1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sumathy K, Kulkarni B, Gondu RK et al. . Protective efficacy of Zika vaccine in AG129 mouse model. Sci Rep 2017; 7: 46375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morrison C. DNA vaccines against Zika virus speed into clinical trials. Nat Rev Drug Discov 2016; 15: 521– 522. [DOI] [PubMed] [Google Scholar]
- 18. Abbink P, Maxfield LF, Ng’ang’a D et al. . Construction and evaluation of novel rhesus monkey adenovirus vaccine vectors. J Virol 2015; 89: 1512– 1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dowd KA, Ko S-Y, Morabito KM et al. . Rapid development of a DNA vaccine for Zika virus. Science 2016; 354: 237– 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kramps T, Probst J.. Messenger RNA-based vaccines: progress, challenges, applications. Wiley Interdiscip Rev RNA 2013; 4: 737– 749. [DOI] [PubMed] [Google Scholar]
- 21. Sebastian M, Papachristofilou A, Weiss C et al. . Phase Ib study evaluating a self-adjuvanted mRNA cancer vaccine (RNActive) combined with local radiation as consolidation and maintenance treatment for patients with stage IV non-small cell lung cancer. BMC Cancer 2014; 14: 748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chahal JS, Fang T, Woodham AW et al. . An RNA nanoparticle vaccine against Zika virus elicits antibody and CD8+ T cell responses in a mouse model. Sci Rep 2017; 7: 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Richner JM, Himansu S, Dowd KA, et al. . Modified mRNA vaccines protect against Zika virus infection. Cell 2017; 169: 176. [DOI] [PubMed] [Google Scholar]
- 24. Pardi N, Hogan MJ, Pelc RS et al. . Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 2017; 543: 248– 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Capone S, D’Alise AM, Ammendola V et al. . Development of chimpanzee adenoviruses as vaccine vectors: challenges and successes emerging from clinical trials. Expert Rev Vaccines 2013; 12: 379– 393. [DOI] [PubMed] [Google Scholar]
- 26. Kim E, Erdos G, Huang S et al. . Preventative vaccines for Zika virus outbreak: preliminary evaluation. EBioMedicine 2016; 13: 315– 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shirbaghaee Z, Bolhassani A. Different applications of virus-like particles in biology and medicine: vaccination and delivery systems. Biopolymers 2016; 105: 113– 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boigard H, Alimova A, Martin GR et al. . Zika virus-like particle (VLP) based vaccine. PLoS Negl Trop Dis 2017; 11: e0005608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Usman Mirza M, Rafique S, Ali A et al. . Towards peptide vaccines against Zika virus: immunoinformatics combined with molecular dynamics simulations to predict antigenic epitopes of Zika viral proteins. Sci Rep 2016; 6: 37313. [DOI] [PMC free article] [PubMed] [Google Scholar]