Abstract
Background
Results of numerous studies show that major depressive disorder (MDD) is associated with a chronic low-grade inflammation, but the underlying mechanism remains unclear.
Aim
To compare the results of blood cell analysis of MDD patients with healthy controls, and explore the potential value of it as an indicator of immune-inflammation in MDD, especially the mean platelet volume.
Methods
103 MDD patients and 106 healthy controls with matched age and gender were recruited.
We collected peripheral blood samples from both groups and gathered basic data. For comparison of normally distributed data (age, body mass index, lymphocyte count, white blood cell count, red blood cell count, hematocrit, platelet count and mean corpuscular volume) between groups, single t-test were used; and for comparison of non-normally distributed data (Neutrophil count, neutrophil count, platelet/ lymphocyte ratio, hemoglobin, red blood cell distribution width, mean platelet volume, mean hemoglobin concentration, mean hemoglobin and platelet distribution width), we used Mann-Whitney U-test.
Results
Compared with healthy controls, the MDD groups showed significantly higher white blood cell count (F=0.443, p=0.004), plateletcrit (F=8.3, p<0.001), neutrophil and lymphocyte ratio (Z=-6.063, p<0.001), neutrophil count (Z=-5.062, p<0.001), platelet/lymphocyte ratio (Z=-2.469, p=0.014), red blood cell distribution width (Z=-2.481, p=0.013) and mean platelet volume (Z=-2.668, p=0.008). In addition they had significantly lower hemoglobin (Z=-3.876, p<0.001), mean hemoglobin amount (Z=-3.005, p=0.003), red blood cell count (F=0.248, p<0.001), lymphocyte count (F=3.826, p=0.004) and hematocrit (F=0.000, p>0.001).
Conclusions
The results suggest that serum inflammatory response probably exists in people with MDD, and indicators of blood analysis especially mean platelet volume have a potential value as biomarker for inflammation.
Key words: major depressive disorder, mean platelet volume, inflammation, China
Abstract
背景
研究发现抑郁症存在免疫炎症反应,但是其炎 症反应的病理机制仍不完全清楚。
目标
探讨抑郁症血液炎症细胞标志物水平,观察平 均血小板体积水平能否反映抑郁症免疫炎症程度。
方法
选取103 例符合DSM-IV 诊断标准的抑郁症患 者作为研究对象,以106 例健康者作为对照。采集所 有参与者的外周血进行全血细胞分析,比较两组血细 胞指标水平的差异。非正态分布的数据(中性粒和淋 巴细胞比值、中性粒细胞计数、血小板/ 淋巴细胞比值、 血红蛋白、红细胞分布宽度、平均血小板体积、平均 血红蛋白浓度、平均血红蛋白量、血小板分布宽度) 采用Mann–Whitney U-test 进行分析。符合正态分布的 数据(如年龄、体重指数、淋巴细胞计数、白细胞计数、 红细胞计数、红细胞压积、血小板计数、平均红细胞 体积、血小板压积)采用独立样本t 检验。
结果
(1)抑郁症组白细胞计数(F=0.443, p=0.004)、 血小板压积(F=8.3, p<0.001)、中性粒和淋巴细胞比值 (Z=-6.063, p<0.001)、中性粒计数(Z =-5.062, p<0.001)、 血小板/ 淋巴细胞比值(Z =-2.469, p=0.014)、红细胞分 布宽度(Z=-2.481, p=0.013)、平均血小板体积(Z =-2.668, p=0.008) 高于正常对照组。(2)正常对照组血红蛋 白(Z=-3.876, p<0.001)、平均血红蛋白量(Z=-3.005, p=0.003)、红细胞计数(F=0.248, p<0.001)、淋巴细胞计 数(F=3.826, p=0.004)、红细胞压积(F=0.000, p<0.001) 高于抑郁症患者组。
结论
抑郁症存在血液炎症反应,平均血小板体积可 能是炎症状态的生物学指标。
1. Introduction
Major Depressive Disorder (MDD) is one of the most common mood disorders seen in clinical practice. Although various approaches to treatment have been explored, for patients treated with antidepressants there are still about 40% of patients who have almost no response to a first administration. [1,2,3] In addition, residual symptoms and functional damage were left on about one third of patients after treatment, for whom the opportunity to achieve total remission gets smaller and smaller with the extension of time. [4,5] A five-year follow-up study observed 102 patients with a diagnosis of MDD in a general hospital setting and found that patients spent 33.4% of the follow-up time in major depressive episodes (MDE), 24.2% in partial remission and 42.4% in full remission. [6,7] There are many factors that lead to poor curative efficacy of depression, including racial differences, incorrect diagnosis, genetic inheritance and comorbidity with physical health conditions.[8,9] Another study found that the incidence of MDD was highly correlated with the disturbance of neuro-immunological function, and that inflammation also was involved in its pathogenesis.[10] When an episode (MDE) arose, a series of abnormal changes occur in the concentration level of inflammatory factors and C-reactive protein (CRP), with an aberrant of neutrophil/lymphocyte ratio, platelet/lymphocyte ratio as well as the red cell distribution width. [11,12,13] A recent study suggests the concentration level of CRP has potential to be applied as a predictive factor for the curative efficacy of antidepressants. It showed that in patients with MDD with lower CRP (<1mg), the score of Montgomerie Scale for Depression (MADRS) for patients with 6-weeks intervention of escitalopram were 3 points higher on average than the group receiving 6-weeks treatment with nortriptyline. In contrast, in the patients with relatively higher CRP, the MADRS score of the nortriptyline group was higher than escitalopram by 3 points on average after 6-week administration.[14] Our previous work indicated that the serum level of inflammatory factors such as interleukin-8 (IL-8), macrophageinflammatoryprotein-1α(MIP-1α), monocyte chemoattractant protein 1(MCP-1) and stromal cell derived factor-1(SDF-1) are significantly greater in MDD patients and remain greater than normal controls even after an 8-week drug therapy program. These results suggest that MDD is strongly related to inflammation. [15]
Inflammatory markers could be used for monitoring the inflammation level of the body. First, clinical trials have proven that mean platelet volume, neutrophil/ lymphocyte ratio, platelet/lymphocyte ratio and red blood cell distribution width have the value to be used as biomarkers of inflammation in various diseases.[16,17,18] Neutrophil/lymphocyte ratio and platelet/lymphocyte ratio were already used for consequently evaluating inflammation in clinical practice.[19] But there is still a lack of studies on mean platelet volume. Research already demonstrated that mean platelet volume, neutrophil/ lymphocyte ratio, platelet/lymphocyte ratio and red blood cell distribution width are correlated with inflammation in chronic diseases (such as cardiovascular diseases, chronic nephropathies, neoplasms, cerebrovascular diseases and autoimmune diseases), and that they may have valuable potential to evaluate the inflammation in these diseases.[16,18,19,20] Moreover, researchers also found that mean platelet volume, neutrophil/lymphocyte ratio, platelet/lymphocyte ratio and red blood cell distribution width in people with MDD are significantly higher than in individuals who are mentally healthy.[21,22] Platelets are fractions produced by megakaryocytes, and the volume of platelets is inversely proportional to the amount.[23,24] Despite a previous study reporting the change of serum mean platelet volume also could be used in evaluation of inflammation in diseases,[20] the relationship between it and MDD is still unclear. Our study compared the inflammation biomarkers in MDD patients with healthy controls, and explored whether the level of serum mean platelet volume has the potential of being applied in inflammation detection in MDD.
2. Methods
2.1 Sample
The flow chart in figure 1 shows the inclusion procedure for the study. All participants enrolled into the MDD group were registered inpatients at the Sir Run Run Shaw Hospital affiliated with Zhejiang University staying in the hospital between December 2014 and April 2016. To eliminate possible confounding factors, our study only included patients 18 to 65 years old and who had not taken psychotropic medicine at least one month before registering in this study. [25,26] From 241 MDD patients who met the initial inclusion criteria listed about above, 70 patients declined to participate in this study and 171 patients provided informed consent. 103 patients met the final criteria for inclusion in this study, amongst which 46 were males and 57 were females with a mean (sd) age of 46.6 (13.4). Details of the final criteria are listed below:
Figure 1.
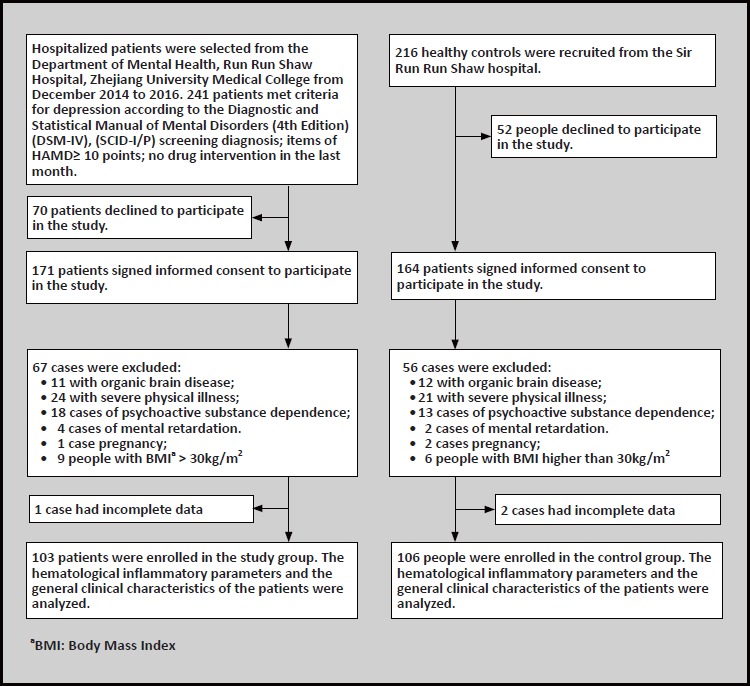
Flowchart of the study
Inclusion criteria for the MDD group were the following: (1) 18 to 65 years old; (2) provided written informed consent; (3) met the diagnostic criteria for MDD according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), and passed assessment using the Structured Clinical Interview for DSM-IV, Axis I Disorders (SCID-I/P);[27] (4) score on the Hamilton Depression Scale (HAMD) of greater than 10 points; (5) no psychotropic medications used in past one month. Exclusion criteria were the following: (1) having an autoimmune disease, infection, severe systemic diseases, epilepsy, diabetes mellitus, hypertension, or other kind of cardiovascular disease as well as liver and kidney diseases; (2) Mental retardation or neurodevelopment disorders, traumatic brain injuries and pregnancy; (3) history of dependence or abuse of alcohol, tobacco and other kinds of substances related disorders; (4) Body Mass Index (BMI) greater than 30kg/m2 or taking any kind of medication.
Healthy control group was the following: 216 people were recruited from the Health Examination Center, Sir Run Run Shaw Hospital, 52 of them declined to participate and 164 agreed to participate. Ultimately 106 people were selected, including 47 males and 59 females, with a mean (sd) age of 46.6(12.9) years. Inclusion criteria for the healthy control group was the following: (1) aged 18 to 65 years; (2) screened by SCIDI/ P assessment and no DSM-IV axis I mental disorders were found; (3) three generations of relatives without mental disorders or hereditary neurological diseases. Exclusion criteria were the following: (1) have a past or present history of any kind of substance dependence; (2) took any kind of psychotropic agents in the past one month; (3) having any kind of physical disease; (4) mental retardation or neurodevelopmental disorders, pregnancy and BMI>30 kg/m2.
No significant difference was found between participants in the MDD group and control group in any of the following areas: gender (x2=0.002, p=0.963), age (F=0.002, p=0.990), education (x2=2.614, p=0.106), marital status (x2=0.077, p=0.782) or BMI (F=5.307, p=0.5). All participants accepted a Complete Blood Count and blood analysis test at 6am on the first day after admission to the inpatient unit and gave 3 ml venous blood into a container containing anticoagulants of EDTA.
2.2 Assessment
Clinical symptoms were assessed by a team of trained psychometric specialists. They showed good reliability and consistency (average Kappa > 0.80), after evaluating 5 patients continuously. The Hamilton Depression Scale (HAMD, showed reliability from 0.88 to 0.99 and validity of 0.92 and the Hamilton Anxiety Scale (HAMA, reliability 0.83 to 1.00 and validity 0.36) was applied in the assessment.[28]
2.3 Laboratory
Following items of blood cytology were measured: neutrophil count, hemoglobin, red blood cell distribution width, mean platelet volume, mean hemoglobin contribution, mean hemoglobin content, lymphocyte count, leukocyte count, erythrocyte count, mean erythrocyte content, erythrocyte hematocrit, platelet count and plateletcrit. Neutrophil/lymphocyte ratio and platelet/lymphocyte ratio were calculated. All blood samples were sent to laboratory of Sir Run Run Shaw Hospital and analyzed through LH 780 Hematology Analyzer of Beckman Coulter Inc.
2.4 Statistical analysis
SPSS 16.0 was used for data analysis. Continuous data was described as x ±s. Chi-square test was used to analyze discreet data. Shapiro-Wilk test were used for the normality test of continuous data. Differences in normally distributed continuous data between groups were analyzed by independent sample t-test. For non-normally distributed continuous data, we used Mann- Whitney U-test. The spearman method was introduced for the need of correlation analysis. Results with p<0.05 were considered as significantly different.
3. Results
3.1 Comparison of clinical features between groups (gender, age, educational level, marriage status and BMI, see table 1)
Table 1.
Basic features of patients with major depressive disorder and normal healthy controls
| characteristic | MDD group (n=103) |
Control group (n=106) |
Statistic | p-value |
|---|---|---|---|---|
| age | ||||
| range | 18-65 | 18-65 | ||
| mean (standard deviation) | 46.58(13.38) | 46.56(12.94) | F=0.002 | 0.990 |
| BMI | 22.00(3.1) | 21.73(2.24) | F=5.307 | 0.500 |
| mean (standard deviation) | ||||
| Gender | ||||
| male/female | 46/57 | 47/59 | 0.002 | 0.963 |
| education level | 2.614 | 0.106 | ||
| 0-8 years | 41 | 54 | ||
| > 8 years | 62 | 52 | ||
| marriage | ||||
| married | 81 | 85 | ||
| unmarried | 22 | 21 | 0.077 | 0.782 |
significantly different: p<0.05.
Table 1 shows that there was no significant difference in gender, age, education, marital status and BMI between MDD patients and controls.
3.2 Comparison of cytological indicators of blood analysis between groups
Results are shown in table 2 and table 3. These results suggest that for MDD patients, the indicators of leukocyte count, neutrophils count, red blood cell distribution width, mean platelet volume, neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, plateletcrit were significantly higher than the healthy controls. For the control group, their lymphocyte count, hemoglobin, mean hemoglobin content, erythrocyte count and erythrocyte hematocrit were greater than the MDD group. Both differences were significant.
Table 2.
Comparison of blood analysis between depressive patients and normal controls, mean (sd)
| MDD group (n=103) | Control group(n=106) | F | p-value | |
|---|---|---|---|---|
| WBC count(109/L) | 6.11(1.12) | 5.67(0.98) | 0.443 | 0.004* |
| Lymphocyte count (109/L) | 1.67 (0.39) | 1.85(0.47) | 3.826 | 0.004* |
| Red blood cell count (1012/L) | 4.34(0.43) | 4.63(0.41) | 0.248 | <0.001* |
| Mean corpuscular volume (FL) | 90.19 (3.07) | 91.6(3.45) | 0.702 | 0.003* |
| Hematocrit (%) | 39.78(3.87) | 41.74(3.85) | 0.000 | <0.001* |
| Plateletcrit (%) | 0.22 (0.02) | 0.175(0.29) | 8.300 | <0.001* |
| Platelet count (109/L) | 217.7(42.92) | 209.48(39.76) | 0.010 | 0.166 |
*significantly different: p<0.05.
**single t-test used for normally distributional data
Table 3.
Comparison of blood analysis between depressive patients and normal controls, mean (sd)
| MDD group (n=103) | Control group(n=106) | Z | p-value | |
|---|---|---|---|---|
| platelet/lymphocyte ratio | 128.69(29.73) | 118.79(32.84) | -2.469 | 0.014* |
| neutrophil/lymphocyte ratio | 2.28 (0.62) | 2.0 (0.73) | -6.063 | <0.001* |
| the neutrophil count (109/L) | 3.85 (0.77) | 3.27(0.82) | -5.062 | <0.001* |
| platelet distribution width (%) | 15.80 (2.12) | 14.04 (3.97) | -1.789 | 0.074 |
| mean platelet volume (FL) | 9.39 (0.6) | 9.06(0.85) | -2.668 | 0.008* |
| mean hemoglobin (pg.) | 30.45 (1.30) | 30.98(1.26) | -3.005 | 0.003* |
| mean hemoglobin concentration (g/L) | 33.57 (0.85) | 33.61 (0.45) | -0.069 | 0.945 |
| red blood cell distribution width (%) | 13.29 (0.94) | 12.97 (0.49) | -2.481 | 0.013* |
| hemoglobin (g/dl) | 13.38 (1.33) | 14.22(2.17) | -3.876 | <0.001* |
*significantly different: p<0.05.
**Mann-Whitney test used for non-normally distributed data
3.3 Result of Spearman relativity analysis of mean platelet volume, total course of disease and HAMD score
Spearman correlation analysis was used to analyze the correlation between the platelet volume and total course of disease and HAMD score of the MDD group, respectively. The results did not show any correlation between platelet volume and total course of disease(r=0.031, p=0.768), and HAMD score (r=0.002, p=0.987).
4. Discussion
4.1 Main findings
Clinical trials have found that inflammation is involved in the etiology of MDD,[10] and inflammatory biomarkers can reflect the status of inflammation response in systemic diseases. This study compared blood cell markers of inflammation in depressed patients and healthy individuals. The results showed that the white blood cell count, neutrophil count, neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, erythrocyte distribution width, mean platelet volume and plateletcrit were higher in the depressive group than in the control group. While the lymphocyte count, hemoglobin, mean hemoglobin, red blood cell count and hematocrit of patients with depression were lower than those of normal healthy controls.
Chronic inflammatory response may impair cognitive function in patients with mood disorders, and affects sleep, mood and mental status.[29] Animal studies have also confirmed that immune and inflammatory responses to the nervous system also cause psychiatric symptoms.[30-34] An example of this is that an injection of exogenous inflammatory cytokines in animals will be accompanied by feelings of loneliness and depressed mood.[32] MDD patients often have comorbid autoimmune inflammatory diseases, such as diabetes, malignancies, autoimmune hypothyroidism, multiple sclerosis, rheumatoid arthritis, etc.[31-33, 35-37] At the same time, suffering from autoimmune diseases can increase the risk of MDD incidence.[30-32] All this evidence suggests that immune and inflammatory reactions affect the brain’s neurological function.[30-32] Further studies have confirmed that the inflammatory response is through the inflammatory cytokines that affect the central nervous system function. Inflammatory cytokines can cause changes in the neurotransmitters of the central nervous system and cause synaptic plasticity dysfunction, such as impacting brain 5-HT neurotransmitter generation and metabolism.[32] Moreover, inflammatory cytokines also trigger the hypothalamus-pituitary-adrenal axis, and decrease the sensitivity of it to glucocorticoid.[37] Indicating that the inflammatory response can lead to dysfunction of the central nervous system and abnormality of brain structure.[38]
It is possible to use serum contribution levels of inflammatory markers to assess a disease’s inflammatory response. In a Major Depressive Episode, the concentration of white blood cells, neutrophils, C-reactive protein levels in the peripheral blood escalate, and can increase the symptoms of depression.[39] The neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, and red blood cell distribution width are inflammatory markers that monitor the inflammatory state of a disease.[16-18] In this study, we found that the neutrophil/ lymphocyte ratio, platelet/lymphocyte ratio and red blood cell distribution width of depressive patients were significantly higher than those of the normal controls. These results are consistent with previous research.[21,22] Furthermore, we found that the blood inflammatory cells (i.e. white blood cell count, neutrophil count) of patients with depression were significantly higher than those of the normal control group, which indicates that there was an inflammatory reaction in MDD. However, their lymphocyte counts were lower than those in the normal controls, which may be inflammatory or chronic stress-induced cellular immunosuppression, resulting in elevated neutrophils and leukocytes and a relatively reduced lymphocyte count.[10,40,41] It has been reported in previous research that the mean platelet volume levels in some inflammatory diseases is elevated and can be used as a potential inflammatory marker. [18,42] The results of this study found that the mean platelet volume in patients with MDD was significantly higher than those of the healthy control group, suggesting that mean platelet volume, as well as neutrophil/lymphocyte ratio and platelet/lymphocyte ratio, may also be useful as markers of inflammation in MDD patients. In addition, our study also found that in patients with MDD, hemoglobin, mean hemoglobin, red blood cell count and hematocrit were lower in the MDD group than the control group. These changes are probably related to the inflammatory state that patients in the MDD group were in, and that systemic inflammation inhibited the erythropoiesis, resulting in inflammatory anemia in MDD patients.[43] But this may also be a result of long-term poor appetite seen in the individuals with MDD, which leads to decreased hemoglobin.[44,45]
There was no correlation between mean platelet volume and total course of disease and HAMD scores. This result is consistent with previous findings that there is no correlation between the severity of depressive symptoms and the immunoinflammatory response.[46-48] However, research has found that severe depressive symptoms and immunosuppression are related.[49] Another study suggested that the severity of depressive symptoms is associated with changes in autoimmune system functioning, and the lymphocyte count decreases as depressive symptoms increase.[50] Results of our study found no correlation between the mean platelet volume and the severity of depressive symptoms. Possible reasons could be small sample size, or the heterogeneity of inflammatory responses that exist in MDD. For example, Yirmiya and colleagues[51] concluded that activation or attenuation of microglia could also be a potential cause of depression. Further studies are required.
4.2 Limitations
This study has several major limitations that should be considered when interpreting the results. First, the sample size was quite small. Second, we did not include inflammatory cytokines associated with immunological function in the statistical analysis and did not perform correlation analysis with the HPA axis, therefore the data is not sufficient to use mean platelet volume as an independent marker for MDD. Moreover, this is a retrospective study so no causal relationship can be drawn between mean platelet volume and MDD. Further studies require follow-up analysis with larger samples of patients with MDD.
4.3 Implications
Our results suggests that the concentration of inflammatory cells in blood analysis (white blood cells, neutrophils, platelet, mean platelet volume) and ratio between counts (neutrophil/lymphocyte ratio, platelet/lymphocyte ratio) were significantly higher in MDD patients than in healthy controls, implying that inflammation probably plays an important role in the pathogenesis of MDD, and may lead to its recurrence and affect the therapeutic effect of pharmacological treatments. Mean platelet volume may also serve as a biomarker of the inflammatory state of depression. This provides a basis for future studies of MDD treatment. Recurrent episodes and poor treatment of MDD may be due to immune and inflammatory response.
Acknowledgement
This study was supported by the National Natural Science Foundation of China (81371490), the Key Project of Medical and Health Platform of Zhejiang Province (2015ZDA016) and the Science and Technology Development Plan Project of Hangzhou Municipality (number 20160533B57), the Medical and Health Science and Technology Plan Project of Hangzhou Municipality (number 2015A56), the Medical and Health Science and Technology Plan Project of Zhejiang Province (number 2017185411).
Biography

Liqiang Cai obtained a bachelor’s degree in medicine from the Anhui University of Traditional Chinese Medicine in July 2009, and a master’s degree in Clinical Medicine from the Zhejiang University School of Medicine in June 2012. Since 2012 he has been working in the Department of Mental Health at Sir Run Run Shaw Hospital of Zhejiang University. His research interests are bipolar disorder, sleep disorders and cognitive disorders.
Footnotes
Funding
This study was supported by the National Natural Science Foundation of China (81371490), the Zhejiang Province Health and Science Key Project (2015ZDA016), the Science and Technology Development Plan Project of Hangzhou Municipality (number 20160533B57), the Medical and Health Science and Technology Plan Project of Hangzhou Municipality (number 2015A56), the Medical and Health Science and Technology Plan Project of Zhejiang Province (number 2017185411).
Conflict of interest statement
The authors declare no conflict of interest.
Informed consent
Each participant in this study signed a consent form or provided oral consent at the beginning of the study.
Ethics approval
This study has been approved by the Ethics Committee of Run Run Shaw Hospital, Zhejiang University School of Medicine.
Authors’ contributions
CL was the principal investigator and was responsible for the collection of cases and data, statistical analysis and writing of the manuscript; XL and WL participated in the case collection and data processing; CW was in charge of project design, writing of the paper and editing.
References
- 1.American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder (3rd ed.). 2010; American Psychiatric Publishing, Inc. [Google Scholar]
- 2.Arroll B, Macgillivray S, Ogston S, Reid I, Sullivan F, Williams B, et al. Efficacy and tolerability of tricyclic antidepressants and SSRIs compared with placebo of or treatment of depression in primary care: a meta-analysis. Ann Fam. Med. 2005; 3: 449-456. doi: http://dx.doi.org/10.1370/afm.349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruhe HG, Huyser J, Swinkels JA, Schene AH. Switching antidepressants after a first selective serotonin reuptake inhibitor in major depressive disorder: a systematic review. J Clin Psychiatry. 2006; 67:1836-1855. doi: http://dx.doi.org/10.4088/jcp.v67n1203 [DOI] [PubMed] [Google Scholar]
- 4.Baghai TC, Moller HJ, Rupprecht R. Recent progress in pharmacological and non-pharmacological treatment options of major depression. Curr Pharm Des. 2006; 12: 503-515. doi: http://dx.doi.org/10.2174/138161206775474422 [DOI] [PubMed] [Google Scholar]
- 5.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am J Psychiatry. 2006; 163(11): 1905-1917. doi: http://dx.doi.org/10.1176/ajp.2006.163.11.1905 [DOI] [PubMed] [Google Scholar]
- 6.Wang SL, Qian MC, Zhong H, Song GH, Lu MJ, Feng R, et al. Comparison of the effecitveness of duloxeitne in depressed paitents with and without a family history of affecitve disorders in ifrst-degree relaitves. Shanghai Arch Psychiatry. 2015; 27(4): 237-245. doi: http://dx.doi.org/10.11919/j.issn.1002-0829.215080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riihimäki KA, Vuorilehto MS, Melartin TK, Isometsä ET. Five-year outcome of major depressive disorder in primary health care. Psychol Med. 2014; 44(7): 1369-1379. doi: http://dx.doi.org/10.1017/s0033291711002303 [DOI] [PubMed] [Google Scholar]
- 8.McIntyre RS, Filteau MJ, Martin L, Patry S, Carvalho A, Cha DS, et al. Treatment-resistant depression: definitions, review of the evidence, and algorithmic approach. J Affect Disord. 2014; 156: 1-7. doi: http://dx.doi.org/10.1016/j.jad.2013.10.043 [DOI] [PubMed] [Google Scholar]
- 9.McMahon FJ, Buervenich S, Charney D, Lipsky R, Rush AJ, Wilson AF, et al. Variation in the gene encoding the serotonin 2A receptor is associated with outcome of antidepressant treatment. Am J Hum Genet. 2006; 78(5): 804-814. doi: http://dx.doi.org/10.1086/503820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008; 9(1): 46-56. doi: http://dx.doi.org/10.1038/nrn2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflam¬mation and the pathogenesis of depression. Trends Immunol. 2006; 27: 24-31. doi: http://dx.doi.org/10.1016/j.it.2005.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janssen DG, Caniato RN, Verster JC, Baune BT. A psychoneuroimmunological review on cytokines involved in antidepressant treat¬ment response. Hum Psychopharmacol. 2010; 25: 201-215. http://dx.doi.org/10.1136/jnnp-2011-301779 [DOI] [PubMed] [Google Scholar]
- 13.Krishnadas R, Cavanagh J. Depression: An inflammatory illness? J Neurol Neurosurg Psychiatry. 2012; 83: 495-502. doi: http://dx.doi.org/10.1136/jnnp-2011-301779 [DOI] [PubMed] [Google Scholar]
- 14.Uher R, Tansey KE, Dew T, Maier W, Mors O, Hauser J, et al. An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortriptyline. Am J Psychiatry. 2014; 171(12): 1278-1286. doi: http://dx.doi.org/10.1176/appi.ajp.2014.14010094 [DOI] [PubMed] [Google Scholar]
- 15.Shen YD, Lu PL, Wei LL, Cai LQ, Hu XY, Chen W. Fluoxetine treatment for major depression decreases the plasma levels of cytokines. Afr J Biotechnol. 2010; 9(43): 7346-7351 [Google Scholar]
- 16.Kuyumcu ME, Yesil Y, Ozturk ZA, Kizilarslanoğlu C, Etgül S, Halil M, et al. The evaluation of neutrophil-lymphocyte ratio in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2012; 34(2): 69-74. doi: http://dx.doi.org/10.1159/000341583 [DOI] [PubMed] [Google Scholar]
- 17.Boyraz I, Koç B, Boyaci A, Tutoğlu A, Sarman H, Ozkan H. Ratio of neutrophil/lymphocyte and platelet/lymphocyte in patient with ankylosing spondylitis that are treating with anti-TNF. Int J Clin Exp Med. 2014; 7(9): 2912-2915. doi: http://dx.doi.org/10.15824/actamedica.19182 [PMC free article] [PubMed] [Google Scholar]
- 18.Kilincalp S, Coban S, Akinci H, Hamamcı M, Karaahmet F, Coşkun Y, et al. Neutrophil/lymphocyte ratio, platelet/ lymphocyte ratio, and mean platelet volume as potential biomarkers for early detection and monitoring of colorectal adenocarcinoma. Eur J Cancer Prev. 2015; 24(4): 328-333. doi: http://dx.doi.org/10.1097/cej.0000000000000092 [DOI] [PubMed] [Google Scholar]
- 19.Balta S, Demirkol S, Kucuk U. The platelet lymphocyte ratio may be useful inflammatory indicator in clinical practice. Hemodial Int. 2013; 17(4): 668-669. doi: http://dx.doi.org/10.1111/hdi.12058 [DOI] [PubMed] [Google Scholar]
- 20.Kopuz A, Turan V, Ozcan A, Kopuz Y, Toz E, Kurt S. A novel marker for the assessment of the treatment result in pelvic inflammatory disease. Minerva Ginecol. 2016; 68(2): 117-123 [PubMed] [Google Scholar]
- 21.Demir S, Atli A, Bulut M, ibiloğlu AO, Güneş M, Kaya MC, et al. Neutrophil-lymphocyte ratio in patients with major depressive disorder undergoing no pharmacological therapy. Neuropsychiatr Dis Treat. 2015; 11: 2253-2258. doi: http://dx.doi.org/10.2147/ndt.s89470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demircan F, Gözel N, Kılınç F, Ulu R, Atmaca M. The impact of red blood cell distribution width and neutrophil/lymphocyte ratio on the diagnosis of major depressive disorder. Neurol Ther. 2016; 5(1): 27-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson SR, Carter JM. Platelet volume: laboratory measurement and clinical application. Blood Rev. 1993; 7(2): 104-113. doi: http://dx.doi.org/10.1016/s0268-960x(05)80020-7 [DOI] [PubMed] [Google Scholar]
- 24.Threatte GA. Usefulness of the mean platelet volume. Clin Lab Med. 1993; 13(4): 937-950 [PubMed] [Google Scholar]
- 25.Janssen DG, Caniato RN, Verster JC, Baune BT. A psychoneuro immunological review on cytokines involved in antidepressant treatment response. Hum Psychopharmacol. 2010; 25: 201–215 [DOI] [PubMed] [Google Scholar]
- 26.Hannestad J, DellaGioia N, Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology. 2011; 36: 2452–2459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.First MB, Spitzer RL, Miriam G, Williams JBW. User’s Guide for the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I, clinician version). 1996; American Psychiatric Press Inc [Google Scholar]
- 28.Zhang MY. [Handbook of Psychiatric Rating Scale]. Changsha: Hunan Science and Technology Press; 1998. Chinese [Google Scholar]
- 29.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009; 65(9): 732-741. doi: http://dx.doi.org/10.1016/j.biopsych.2008.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Najjar S, Pearlman DM, Alper K, Najjar A, Devinsky O. Neuroinflammation and psychiatric illness. J Neuroinflammation. 2013; 10: 43. doi: http://dx.doi.org/10.1186/1742-2094-10-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008; 9(1):46-56. doi: http://dx.doi.org/10.1038/nrn2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laske C, Zank M, Klein R, Stransky E, Batra A, Buchkremer G, et al. Autoantibody reactivity in serum of patients with major depression, schizophrenia and healthy controls. Psychiatry Res. 2008; 158(1): 83-86. doi: http://dx.doi.org/10.1016/j.psychres.2006.04.023 [DOI] [PubMed] [Google Scholar]
- 33.Benros ME, Nielsen PR, Nordentoft M, Eaton WW, Dalton SO, Mortensen PB. Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register study. Am J Psychiatry. 2011; 168(12): 1303-1310. doi: http://dx.doi.org/10.1176/appi.ajp.2011.11030516 [DOI] [PubMed] [Google Scholar]
- 34.Leboyer M, Soreca I, Scott J, Frye M, Henry C, Tamouza R, et al. Can bipolar disorder be viewed as a multi-system inflammatory disease? J Affect Disord. 2012; 141(1): 1-10. doi: http://dx.doi.org/10.1016/j.jad.2011.12.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tremblay M, Majewska AK. A role for microglia in synaptic plasticity? Commun Integr Biol. 2011; 4: 220-222. doi: http://dx.doi.org/10.4161/cib.4.2.14506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McNally L, Bhagwagar Z, Hannestad J. Inflammation, glutamate, and glia in depression: a literature review. CNS Spectr. 2008; 13(6): 501-510. doi: http://dx.doi.org/10.1017/s1092852900016734 [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Dunn AJ. Mouse interleukin-6 stimulates the HPA axis and increases brain tryptophan and serotonin metabolism. Neurochem Int. 1998; 33(2): 143-154. doi: http://dx.doi.org/10.1016/s0197-0186(98)00016-3 [DOI] [PubMed] [Google Scholar]
- 38.Stertz L, Magalhaes PV, Kapczinski F. Is bipolar disorder an inflammatory condition? The relevance of microglial activation. Curr Opin Psychiatry. 2013; 26(1): 19-26. doi: http://dx.doi.org/10.1097/yco.0b013e32835aa4b4 [DOI] [PubMed] [Google Scholar]
- 39.Kiecolt-Glaser JK, Marucha PT, Malarkey WB, Mercado AM, Glaser R. Slowing of wound healing by psychological stress. Lancet. 1995; 346: 1194-1196. doi: http://dx.doi.org/10.1016/s0140-6736(95)92899-5 [DOI] [PubMed] [Google Scholar]
- 40.Seidel A, Arolt V, Hunstiger M, Rink L, Behnisch A, Kirchner H. Cytokine production and serum proteins in depression. Scand J Immunol. 1995; 41: 534-538. doi: http://dx.doi.org/10.1111/j.1365-3083.1995.tb03604.x [DOI] [PubMed] [Google Scholar]
- 41.Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, et al. The relationship of depression and stressors to immunological assays: a meta analytic review. Brain Behav Immun. 2001; 15: 199-226. doi: http://dx.doi.org/10.1006/brbi.2000.0597 [DOI] [PubMed] [Google Scholar]
- 42.Topal E, Celiksoy MH, Catal F, Karakoç HT, Karadağ A, Sancak R. The platelet parameters as inflammatory markers in preschool children with atopic eczema. Clin Lab. 2015; 61(5-6): 493-496. doi: http://dx.doi.org/10.7754/clin.lab.2014.140930 [PubMed] [Google Scholar]
- 43.Wang Y, Huang F. N-3 polyunsaturated fatty acids and inflammation in obesity: local effect and systemic benefit. Biomed Res Int. 2015; 2015: 581469. doi: http://dx.doi.org/10.1155/2015/581469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trevisan C, Veronese N, Bolzetta F, De Rui M, Correll CU, Zambon S, et al. Low hemoglobin levels and risk of developing depression in the elderly: Results from the prospective PRO.V.A. study. J Clin Psychiatry. 2016; doi: http://dx.doi.org/10.4088/JCP.15m10270 [DOI] [PubMed] [Google Scholar]
- 45.Vulser H, Wiernik E, Hoertel N, Thomas F, Pannier B, Czernichow S, et al. Association between depression and anemia in otherwise healthy adults. Acta Psychiatr Scand. 2016; 134(2): 150-160. doi: http://dx.doi.org/10.1111/acps.12595 [DOI] [PubMed] [Google Scholar]
- 46.Andreoli A, Keller SE, Rabaeus M, Marin P, Bartlett JA, Taban C. Depression and immunity: age, severity, and clinical course. Brain Behav Immun. 1993; 7: 279-292. doi: http://dx.doi.org/10.1006/brbi.1993.1028 [DOI] [PubMed] [Google Scholar]
- 47.Schleifer SJ, Keller SE, Bartlett JA, Eckholdt HM, Delaney BR. Immunity in young adults with major depressive disorder. Am J Psychiatry. 1996; 153: 477-482. doi: http://dx.doi.org/10.1176/ajp.153.4.477 [DOI] [PubMed] [Google Scholar]
- 48.Ravindran AV, Griffiths J, Merali Z, Anisman H. Circulating lymphocyte subsets in major depression and dysthymia with typical or atypical features. Psychosom Med. 1998; 60: 283-289. doi: http://dx.doi.org/10.1097/00006842-199805000-00013 [DOI] [PubMed] [Google Scholar]
- 49.Mittwoch-Jaffe T, Shalit F, Srendi B, Yehuda S. Modification of cytokine secretion following mild emotional stimuli. Neuroreport. 1995; 6: 789-792. doi: http://dx.doi.org/10.1097/00001756-199503270-00021 [DOI] [PubMed] [Google Scholar]
- 50.Miller GE, Cohen S, Herbert TB. Pathways linking major depression and immunity in ambulatory female patients. Psychosom Med. 1999; 61: 850-860. doi: http://dx.doi.org/10.1097/00006842-199911000-00021 [DOI] [PubMed] [Google Scholar]
- 51.Yirmiya R, Rimmerman N, Reshef R. Depression as a microglial disease. Trends Neurosci. 2015; 38(10): 637-658. doi: http://dx.doi.org/10.1016/j.tins.2015.08.001 [DOI] [PubMed] [Google Scholar]


