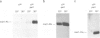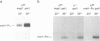Abstract
The developmental programme of fission yeast brings about a transition from mitotic cell division to the dormant state of ascospores. In response to nitrogen starvation, two cells of opposite mating type conjugate to form a diploid zygote, which then undergoes meiosis and sporulation. This differentiation process is characterized by a transcriptional induction of the mating-type genes. Conjugation can also be induced in pat1-ts mutants by a shift to a semi-permissive temperature. The pat1 gene encodes a protein kinase, which also functions further downstream in the developmental pathway controlling entry into meiosis. We have analysed transcriptional induction of mating-type genes in various strains--with and without a pat1-ts allele. In wild-type cells of P-mating type derepression occurs in two rounds. First, the mat1-Pc gene is induced in response to nitrogen starvation. Mutants in the map1 gene are defective in this process. In the following step the mat1-Pm gene is expressed in response to a pheromone signal generated by cells of M mating type. Both these controls are derepressed in the pat1-ts mutant at semipermissive temperature. Previous work has established that expression of the mating-type genes in the zygote leads to complete loss of pat1 protein kinase activity causing entry into meiosis. Thus, pat1 can promote its own inactivation. We suggest a model according to which a stepwise inactivation of pat1 leads to sequential derepression of the processes of conjugation and meiosis.
Full text
PDF

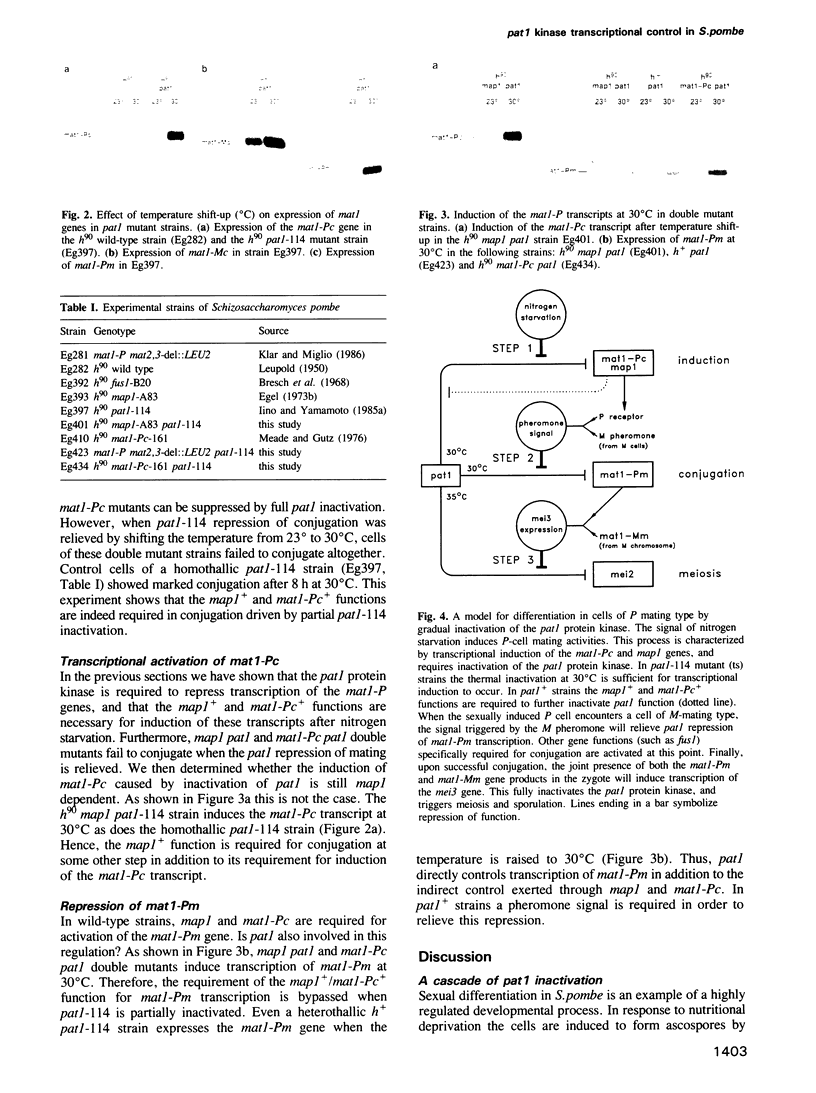



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beach D., Rodgers L., Gould J. ran1+ controls the transition from mitotic division to meiosis in fission yeast. Curr Genet. 1985;10(4):297–311. doi: 10.1007/BF00365626. [DOI] [PubMed] [Google Scholar]
- Bresch C., Müller G., Egel R. Genes involved in meiosis and sporulation of a yeast. Mol Gen Genet. 1968;102(4):301–306. doi: 10.1007/BF00433721. [DOI] [PubMed] [Google Scholar]
- Egel R., Egel-Mitani M. Premeiotic DNA synthesis in fission yeast. Exp Cell Res. 1974 Sep;88(1):127–134. doi: 10.1016/0014-4827(74)90626-0. [DOI] [PubMed] [Google Scholar]
- Egel R. Frequency of mating-type switching in homothallic fission yeast. Nature. 1977 Mar 10;266(5598):172–174. doi: 10.1038/266172a0. [DOI] [PubMed] [Google Scholar]
- Egel R. Genes involved in mating type expression of fission yeast. Mol Gen Genet. 1973 May 28;122(4):339–343. doi: 10.1007/BF00269434. [DOI] [PubMed] [Google Scholar]
- Fujioka H., Shimoda C. A mating-type-specific sterility gene map1 is required for transcription of a mating-type gene mat1-Pi in the fission yeast Schizosaccharomyces pombe. FEMS Microbiol Lett. 1989 Jul 1;51(1):45–48. doi: 10.1016/0378-1097(89)90075-x. [DOI] [PubMed] [Google Scholar]
- Fukui Y., Kaziro Y., Yamamoto M. Mating pheromone-like diffusible factor released by Schizosaccharomyces pombe. EMBO J. 1986 Aug;5(8):1991–1993. doi: 10.1002/j.1460-2075.1986.tb04454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskowitz I., Marsh L. Conservation of a receptor/signal transduction system. Cell. 1987 Sep 25;50(7):995–996. doi: 10.1016/0092-8674(87)90162-0. [DOI] [PubMed] [Google Scholar]
- Iino Y., Yamamoto M. Negative control for the initiation of meiosis in Schizosaccharomyces pombe. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2447–2451. doi: 10.1073/pnas.82.8.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M., Burke J., Smith M., Klar A., Beach D. Four mating-type genes control sexual differentiation in the fission yeast. EMBO J. 1988 May;7(5):1537–1547. doi: 10.1002/j.1460-2075.1988.tb02973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar A. J., Miglio L. M. Initiation of meiotic recombination by double-strand DNA breaks in S. pombe. Cell. 1986 Aug 29;46(5):725–731. doi: 10.1016/0092-8674(86)90348-x. [DOI] [PubMed] [Google Scholar]
- McLeod M., Beach D. A specific inhibitor of the ran1+ protein kinase regulates entry into meiosis in Schizosaccharomyces pombe. Nature. 1988 Apr 7;332(6164):509–514. doi: 10.1038/332509a0. [DOI] [PubMed] [Google Scholar]
- McLeod M., Beach D. Homology between the ran1+ gene of fission yeast and protein kinases. EMBO J. 1986 Dec 20;5(13):3665–3671. doi: 10.1002/j.1460-2075.1986.tb04697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod M., Stein M., Beach D. The product of the mei3+ gene, expressed under control of the mating-type locus, induces meiosis and sporulation in fission yeast. EMBO J. 1987 Mar;6(3):729–736. doi: 10.1002/j.1460-2075.1987.tb04814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade J. H., Gutz H. Mating-Type Mutations in SCHIZOSACCHAROMYCES POMBE: Isolation of Mutants and Analysis of Strains with an h or h Phenotype. Genetics. 1976 Jun;83(2):259–273. doi: 10.1093/genetics/83.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen O., Egel R. Mapping the double-strand breaks at the mating-type locus in fission yeast by genomic sequencing. EMBO J. 1989 Jan;8(1):269–276. doi: 10.1002/j.1460-2075.1989.tb03373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipiczki M. The role of sterility genes (ste and aff) in the initiation of sexual development in Schizosaccharomyces pombe. Mol Gen Genet. 1988 Aug;213(2-3):529–534. doi: 10.1007/BF00339626. [DOI] [PubMed] [Google Scholar]
- Sørensen M. A., Kurland C. G., Pedersen S. Codon usage determines translation rate in Escherichia coli. J Mol Biol. 1989 May 20;207(2):365–377. doi: 10.1016/0022-2836(89)90260-x. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Lino Y., Furuhata K., Shimoda C., Yamamoto M. The S.pombe mei2 gene encoding a crucial molecule for commitment to meiosis is under the regulation of cAMP. EMBO J. 1988 Mar;7(3):761–767. doi: 10.1002/j.1460-2075.1988.tb02873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]