Abstract
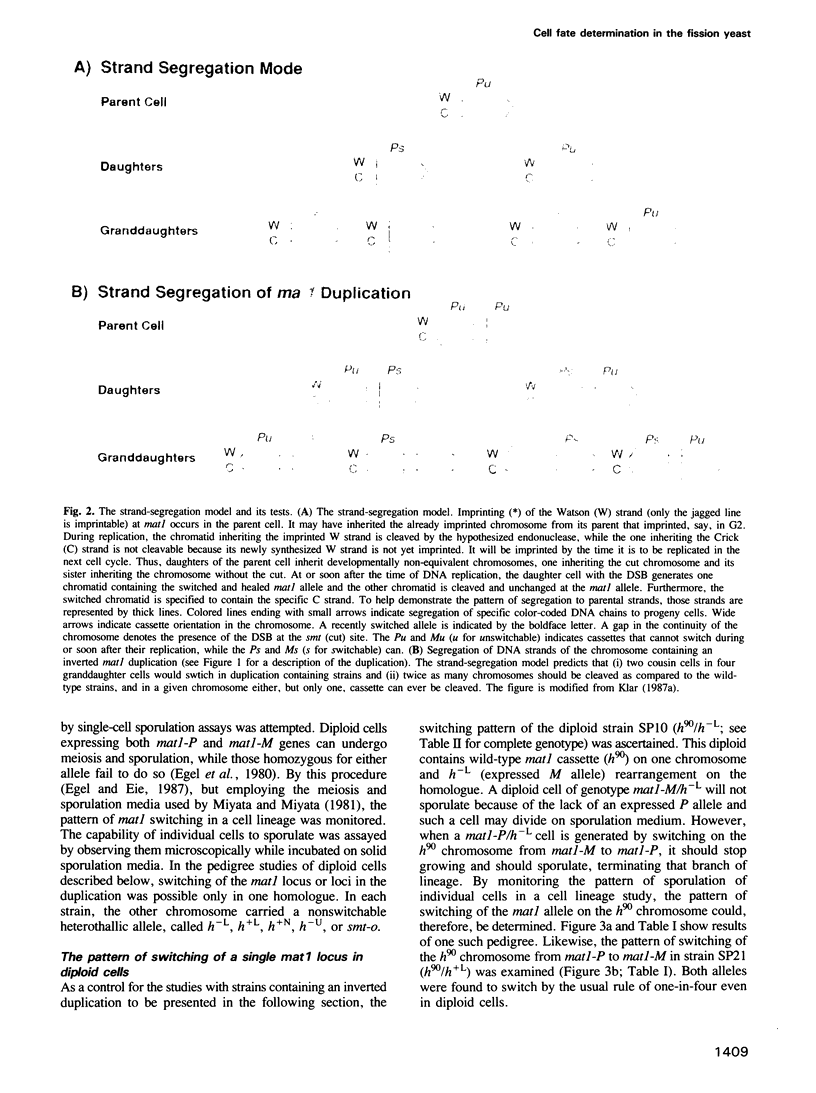
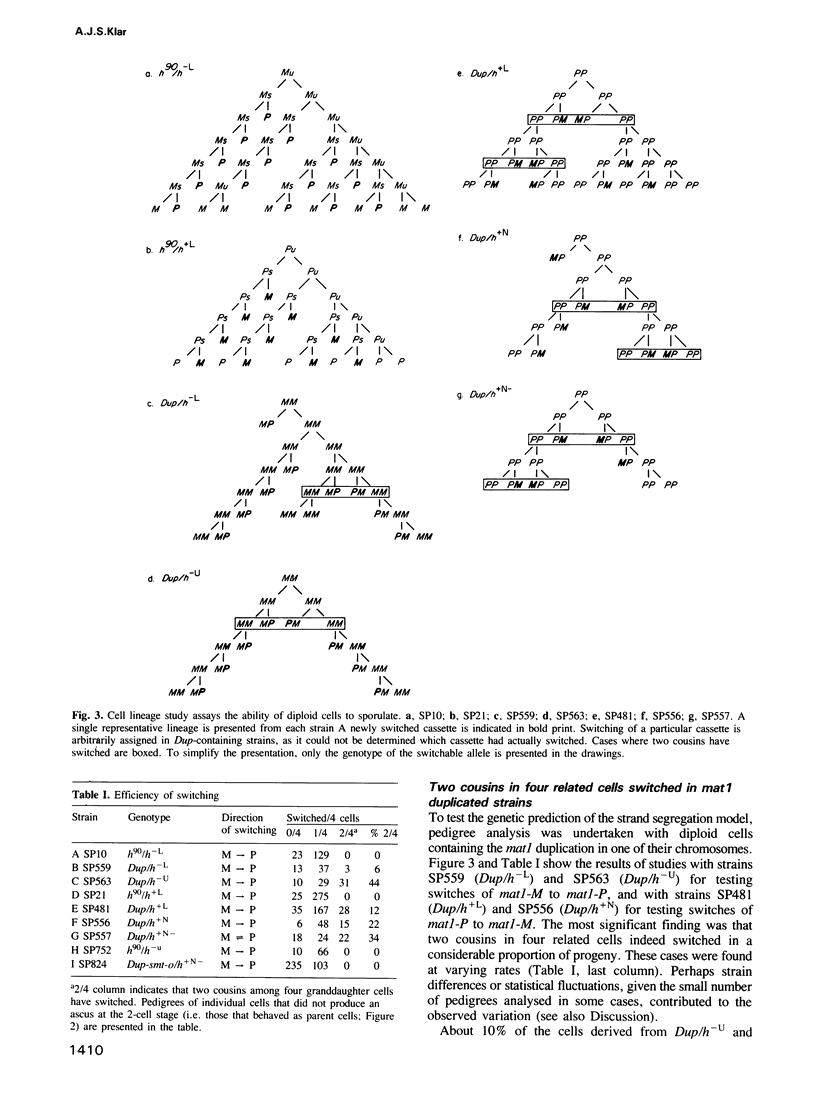
A key feature for development consists of producing sister cells that differ in their potential for cellular differentiation. Following two cell divisions, a haploid Schizosaccharomyces pombe cell produces one cell in four 'granddaughters' with a changed mating cell type, implying nonequivalence of sister cells in each of two consecutive cell divisions. The observed pattern of switching is analogous to the mammalian 'stem cell' lineage by which a cell produces one daughter like itself while the other daughter is advanced in its developmental program. It is tested here whether sisters differ because of unequal distribution of cytoplasmic and/or nuclear components to them or due to inheriting a specific parental DNA chain at the mating type locus. Only the DNA strand-segregation model predicts that those cells engineered to contain an inverted tandem duplication of the mating type locus should produce equivalent sisters. Consequently, two 'cousins' in four related granddaughter cells should switch. The results verified the prediction, thus establishing that all cells otherwise fully possess the potential to switch. Therefore, the program of cell type change in S.pombe cell lineages is determined by the pattern of DNA strand inheritance at the mating type locus. A specific DNA sequence present at the mating type locus is postulated to be the cause of developmental asymmetry between sister cells. A general model for cellular differentiation is proposed in which the act of DNA replication itself is hypothesized to produce developmentally nonequivalent sister genomes.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beach D. H., Klar A. J. Rearrangements of the transposable mating-type cassettes of fission yeast. EMBO J. 1984 Mar;3(3):603–610. doi: 10.1002/j.1460-2075.1984.tb01855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach D., Nurse P., Egel R. Molecular rearrangement of mating-type genes in fission yeast. Nature. 1982 Apr 15;296(5858):682–683. doi: 10.1038/296682a0. [DOI] [PubMed] [Google Scholar]
- Cairns J. Mutation selection and the natural history of cancer. Nature. 1975 May 15;255(5505):197–200. doi: 10.1038/255197a0. [DOI] [PubMed] [Google Scholar]
- Crouse H V. The Controlling Element in Sex Chromosome Behavior in Sciara. Genetics. 1960 Oct;45(10):1429–1443. doi: 10.1093/genetics/45.10.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egel R., Beach D. H., Klar A. J. Genes required for initiation and resolution steps of mating-type switching in fission yeast. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3481–3485. doi: 10.1073/pnas.81.11.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egel R. Frequency of mating-type switching in homothallic fission yeast. Nature. 1977 Mar 10;266(5598):172–174. doi: 10.1038/266172a0. [DOI] [PubMed] [Google Scholar]
- Egel R., Kohli J., Thuriaux P., Wolf K. Genetics of the fission yeast Schizosaccharomyces pombe. Annu Rev Genet. 1980;14:77–108. doi: 10.1146/annurev.ge.14.120180.000453. [DOI] [PubMed] [Google Scholar]
- Holliday R., Pugh J. E. DNA modification mechanisms and gene activity during development. Science. 1975 Jan 24;187(4173):226–232. [PubMed] [Google Scholar]
- Holliday R. The inheritance of epigenetic defects. Science. 1987 Oct 9;238(4824):163–170. doi: 10.1126/science.3310230. [DOI] [PubMed] [Google Scholar]
- Kelly M., Burke J., Smith M., Klar A., Beach D. Four mating-type genes control sexual differentiation in the fission yeast. EMBO J. 1988 May;7(5):1537–1547. doi: 10.1002/j.1460-2075.1988.tb02973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar A. J. Determination of the yeast cell lineage. Cell. 1987 May 22;49(4):433–435. doi: 10.1016/0092-8674(87)90442-9. [DOI] [PubMed] [Google Scholar]
- Klar A. J. Differentiated parental DNA strands confer developmental asymmetry on daughter cells in fission yeast. Nature. 1987 Apr 2;326(6112):466–470. doi: 10.1038/326466a0. [DOI] [PubMed] [Google Scholar]
- Klar A. J., Miglio L. M. Initiation of meiotic recombination by double-strand DNA breaks in S. pombe. Cell. 1986 Aug 29;46(5):725–731. doi: 10.1016/0092-8674(86)90348-x. [DOI] [PubMed] [Google Scholar]
- Klar A. J. The mother-daughter mating type switching asymmetry of budding yeast is not conferred by the segregation of parental HO gene DNA strands. Genes Dev. 1987 Dec;1(10):1059–1064. doi: 10.1101/gad.1.10.1059. [DOI] [PubMed] [Google Scholar]
- Meselson M., Stahl F. W. THE REPLICATION OF DNA IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1958 Jul 15;44(7):671–682. doi: 10.1073/pnas.44.7.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. M., Nasmyth K. A. Role of DNA replication in the repression of silent mating type loci in yeast. Nature. 1984 Nov 15;312(5991):247–251. doi: 10.1038/312247a0. [DOI] [PubMed] [Google Scholar]
- Monk M. Genomic imprinting. Genes Dev. 1988 Aug;2(8):921–925. doi: 10.1101/gad.2.8.921. [DOI] [PubMed] [Google Scholar]
- Nasmyth K. Molecular analysis of a cell lineage. Nature. 1983 Apr 21;302(5910):670–676. doi: 10.1038/302670a0. [DOI] [PubMed] [Google Scholar]
- Nasmyth K., Seddon A., Ammerer G. Cell cycle regulation of SW15 is required for mother-cell-specific HO transcription in yeast. Cell. 1987 May 22;49(4):549–558. doi: 10.1016/0092-8674(87)90457-0. [DOI] [PubMed] [Google Scholar]
- Nasmyth K., Stillman D., Kipling D. Both positive and negative regulators of HO transcription are required for mother-cell-specific mating-type switching in yeast. Cell. 1987 Feb 27;48(4):579–587. doi: 10.1016/0092-8674(87)90236-4. [DOI] [PubMed] [Google Scholar]
- Nielsen O., Egel R. Mapping the double-strand breaks at the mating-type locus in fission yeast by genomic sequencing. EMBO J. 1989 Jan;8(1):269–276. doi: 10.1002/j.1460-2075.1989.tb03373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. W. Strand segregation or recombination. Nature. 1987 Oct 22;329(6141):678–678. doi: 10.1038/329678b0. [DOI] [PubMed] [Google Scholar]
- Sternberg P. W., Stern M. J., Clark I., Herskowitz I. Activation of the yeast HO gene by release from multiple negative controls. Cell. 1987 Feb 27;48(4):567–577. doi: 10.1016/0092-8674(87)90235-2. [DOI] [PubMed] [Google Scholar]
- WATSON J. D., CRICK F. H. Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature. 1953 Apr 25;171(4356):737–738. doi: 10.1038/171737a0. [DOI] [PubMed] [Google Scholar]



