Abstract
Background and purpose
A 1988 pilot study in Peru suggested an association between migraine and chronic exposure to high altitude. This study provides epidemiological evidence corroborating this.
Methods
In a cross‐sectional nationwide population‐based study, a representative sample of Nepali‐speaking adults were recruited through stratified multistage cluster sampling. They were visited at home by trained interviewers using a culturally adapted questionnaire. The altitude of dwelling of each participant was recorded.
Results
Of 2100 participants, over half [1100 (52.4%)] were resident above 1000 m and almost one quarter [470 (22.4%)] at ≥2000 m. Age‐ and gender‐standardized migraine prevalence increased from 27.9% to 45.5% with altitude between 0 and 2499 m and thereafter decreased to 37.9% at ≥2500 m. The likelihood of having migraine was greater (odds ratio, 1.5–2.2; P ≤ 0.007) at all higher altitudes compared with <500 m. In addition, all symptom indices increased with altitude across the range <500 m to 2000–2499 m, i.e. median attack frequency from 1.3 to 3.0 days/month (P < 0.001), median duration from 9 to 24 h (P < 0.001) and pain intensity [the proportion reporting ‘bad pain’ (highest intensity)] from 35.5% to 56.9% (P = 0.011). Each of these showed a downward trend above 2500 m.
Conclusions
Dwelling at high altitudes increases not only migraine prevalence but also the severity of its symptoms.
Keywords: altitude, epidemiology, Global Campaign against Headache, headache, hypoxia, migraine, prevalence, risk factors
Introduction
Migraine is the sixth highest cause of disability worldwide and the third most prevalent disorder 1. Its aetiology is poorly understood; studies of twins indicate involvement of environmental factors, but confirmation of this by other epidemiological methods has not been forthcoming 2. A pilot study in Peru in 1988 suggested an association between migraine and chronic exposure to high altitude 3. This pioneering investigation estimated migraine prevalence in two selected samples, one from a village at sea level and the other from a mining village at 4300 m. However, selection bias in the study undermines any conclusions.
The dramatic topographic landscape of Nepal provides a unique environment for resuming this line of research. The country is divided into three physiographic divisions: Terai, Hill and Mountain 4, rising from about 60 m above sea level to the Himalayas, including Mount Everest, the highest peak on Earth at 8848 m 5. We have recently estimated the prevalence of headache in Nepal, recruiting a large sample representative of the adult population of the entire country 6. The observed 1‐year prevalence of migraine was very high, at 34.7% (definite migraine, 17.5%; probable migraine, 17.2%) 6. Controlling for a wide range of potential confounding factors, we found that this was largely explained by an effect of altitude, i.e. living above 1000 m led to a 60% increased likelihood of having migraine. No similar association was observed between high‐altitude living and tension‐type headache (TTH) 6.
The aim of the present study was to explore the associations between migraine and altitude in greater detail, also looking at symptom indices of migraine (attack frequency, attack duration and pain intensity).
The study was conducted as a project within the Global Campaign against Headache, which is led by Lifting The Burden, a UK‐registered non‐governmental organization in official relations with the World Health Organization.
Methods
Ethics and Institutional Review Board approvals
The Nepal Health Research Council, Institutional Review Committee of Kathmandu University School of Medical Sciences, Dhulikhel Hospital (IRC‐KUSMS) and Regional Committee for Health and Research Ethics in central Norway all approved the study protocol.
All participants were informed about the nature and purpose of the study. Consent was obtained and recorded by signature or fingerprint in accordance with requirements of IRC‐KUSMS.
Study design
This was a cross‐sectional, nationwide population‐based survey using structured interviews administered by trained health workers making unannounced door‐to‐door visits to households in May 2013.
To ensure adequate representation from the country as a whole, we used multistage stratified cluster sampling. We divided the sample between Mountain, Hill and Terai physiographic divisions, according to their relative populations, in the proportions 8:44:48 (numerically 170, 930, 1000), and within each division equally between the country's five development regions (Far‐Western, Midwestern, Western, Central and Eastern). By randomly selecting one district in each region of each division, we sampled 15 districts out of the total of 75, spread across the country. We made one purposive change to the random selection, in the Central developmental region of the Hill division, replacing Sidhuli by Ramechap, to ensure that 25% of participants dwelt above 2000 m. We randomly selected households within districts, and one eligible adult (aged 18–65 years, Nepali‐speaking and living in Nepal) from each household. We only included subjects who had been residents of the household for at least 6 months. The details of the sampling and data‐collection procedure, including steps taken to ensure a very high percentage participation, have been published elsewhere 4. We estimated the sample size (n = 2100) assuming a migraine prevalence of ≥10% and absolute margin of error of 1.3% with 95% confidence interval 7.
Instruments
We used the Headache‐Attributed Restriction, Disability, Social Handicap and Impaired Participation (HARDSHIP) questionnaire developed by Lifting The Burden for similar studies 8. The English version was translated into Nepali according to Lifting The Burden's translation protocol for lay documents and adapted to fit the Nepalese culture 9. The questionnaire, published previously 4, asked personal and demographic questions of all participants, followed by a headache screening question (‘Have you had a headache in the last year?’). Those who answered ‘no’ were classified as headache‐free. Those who answered ‘yes’ were asked whether their headaches were of one or more types and, if more than one, to focus only on the most bothersome type; they were then asked diagnostic and burden questions relating to this headache 4.
We used culturally validated Nepali translations of the Hospital Anxiety and Depression Scale 10 and Eysenck Personality Questionnaire Neuroticism Short Form Revised version 11 to assess psychiatric comorbidity. We measured height, weight and waist circumference, and calculated body‐mass index. We measured blood pressure using a digital device (3BM1‐3®, Microlife, Taipei, Taiwan). We recorded the altitude of each household using a portable altimeter (SAL 7030®, Sunoh, Tokyo, Japan).
Headache diagnosis
Diagnoses were not made during interviews but later by an algorithm 8. Participants reporting headache on ≥15 days/month were first separated as a distinct group because they cannot be fully diagnosed by lay‐administered questionnaire. To all others, reporting headache on ≤14 days/month, the algorithm applied modified criteria of the International Classification of Headache Disorders (ICHD‐3 beta) 12 in the following order: definite migraine, definite TTH, probable migraine and probable TTH. We found that two additional adaptations were necessary. First, photophobia was reported in association with 75.8% of all headaches and therefore offered little discriminative value diagnostically. Accordingly, we treated photophobia as a missing value when diagnosing headache types. Second, attacks lasting <4 h when untreated may be compatible with a diagnosis of probable migraine when other ICHD‐3 beta criteria are met 12, but many of our participants could report attack durations only after taking acute medication, and some were very short. We disallowed a diagnosis of probable migraine (in favour of probable TTH) whenever headache duration was <1 h because so short a duration in adults, even with acute treatment, was very unlikely to be migraine (given that participants were asked to describe ‘usual’ attacks) 13. Cases of definite and probable migraine were combined in the estimations of prevalence and in analyses of associations. No attempt was made to diagnose migraine aura.
Statistics
We categorized household altitude as follows: <500, 500–999, 1000–1499, 1500–1999, 2000–2499 or ≥2500 m. We standardized the observed prevalence at each altitude for age and gender according to their general‐population distributions (within the range 18–65 years) taken from the 2011 population and housing census 14. We measured attack frequency in days/month and attack duration in hours, treating these data both as continuous and by categorizing them (defining categories so that as equal numbers as possible fell into each). We assessed intensity categorically as mild, moderate or severe. We treated blood pressure readings and Eysenck Personality Questionnaire Neuroticism Short Form Revised version and Hospital Anxiety and Depression Scale scores as continuous variables.
For continuous variables, medians with interquartile ranges are reported. Categorical variables are presented as absolute numbers and percentages. For prevalence, we used logistic regression analysis (calculating odds ratios and adjusted odds ratios, each with 95% confidence intervals) to quantify differences between altitude categories. We adjusted for age, gender, household consumption (as an indicator of the participant's economic wellbeing 4, 9), habitation (urban or rural), systolic and diastolic blood pressure and Eysenck Personality Questionnaire Neuroticism Short Form Revised version and Hospital Anxiety and Depression Scale scores. We used Mann–Whitney U‐test for comparing medians of continuous variables in two samples and Kruskal–Wallis test for more than two samples. We used chi‐square test for categorical variables. Significance was set at P < 0.05.
Statistical analyses were carried out using the Statistical Package for Social Science software (IBM SPSS Statistics 21, Chicago, IL, USA).
Results
The survey was completed by 2100 participants [861 (41.0%) male, 1239 (59.0%) female; mean age 36.4 ± 12.8 years]. There were nine refusals (participation proportion 99.6%). Almost two‐fifths [822 (39.1%)] were living in households with low economic wellbeing (consumption valued at < USD 950/year) and nearly two‐thirds [1328 (63.2%)] were from rural areas. Over half of the sample [1100 (52.4%)] were resident above 1000 m and almost one quarter [470 (22.4%)] at or above 2000 m. There were no participants residing at 500–999 m, an artefact of cluster sampling.
There was slight under‐representation of males aged 18–34 years (43.9% rather than 49.8% expected). Other sociodemographic characteristics of the sample reasonably matched those of the national population 4, 6, 14.
Age‐ and gender‐standardized migraine prevalence increased with altitude between the lowest category of <500 m and 2499 m (from 27.9% to 45.5%); thereafter, at altitude ≥2500 m, it decreased to 37.9% (Fig. 1) 6. Compared with <500 m, adjusted odds ratios for having migraine were significantly greater at all higher altitudes (Table 1), whereas the decline in prevalence from 2000–2499 m to ≥2500 m was also significant (P = 0.025; Table 2).
Figure 1.
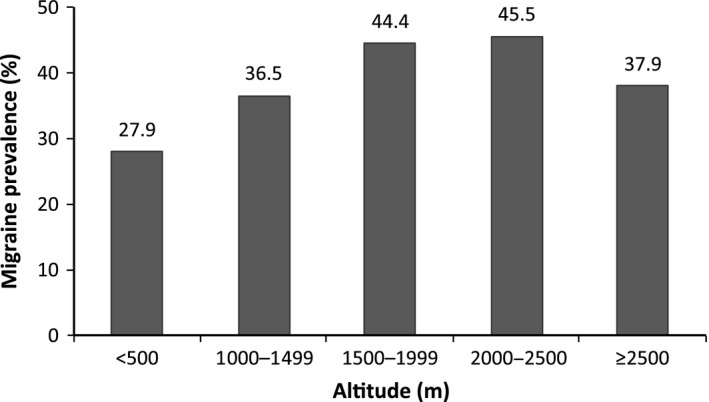
The 1‐year prevalence of migraine (age‐ and gender‐standardized) by altitude.
Table 1.
The 1‐year prevalence of migraine (age‐ and gender‐standardized) by altitude with multivariate regression analyses using the lowest altitude as reference
| Altitude (m) | n | Cases of migraine | Prevalence (%)a | Adjusted odds ratiob | 95% confidence interval | P |
|---|---|---|---|---|---|---|
| <500 | 1000 | 287 | 27.9 | Reference | ||
| 1000–1499 | 470 | 176 | 36.5 | 1.5 | 1.2–1.9 | 0.001 |
| 1500–1999 | 160 | 68 | 44.4 | 1.9 | 1.4–2.7 | <0.001 |
| 2000–2499 | 254 | 116 | 45.5 | 2.2 | 1.6–2.9 | <0.001 |
| ≥2500 | 216 | 81 | 37.9 | 1.5 | 1.1–2.1 | 0.007 |
aAge‐ and gender‐standardized against census data for the Nepali population. bAdjusted for age, gender, household consumption, habitation, systolic and diastolic blood pressure and Eysenck Personality Questionnaire Neuroticism Short Form Revised version and Hospital Anxiety and Depression Scale scores.
Table 2.
The 1‐year prevalence of migraine (age‐ and gender‐standardized) by altitude with multivariate regression analyses using 2000–2499 m as reference
| Altitude (m) | n | Cases of migraine | Prevalence (%)a | Adjusted odds ratiob | 95% confidence interval | P |
|---|---|---|---|---|---|---|
| <500 | 1000 | 287 | 27.9 | 0.5 | 0.3–0.6 | <0.001 |
| 1000–1499 | 470 | 176 | 36.5 | 0.7 | 0.5–1.1 | 0.068 |
| 1500–1999 | 160 | 68 | 44.4 | 0.9 | 0.6–1.4 | 0.62 |
| 2000–2499 | 254 | 116 | 45.5 | Reference | ||
| ≥2500 | 216 | 81 | 37.9 | 0.6 | 0.4–0.9 | 0.025 |
aAge‐ and gender‐standardized against census data for the Nepali population. bAdjusted for age, gender, household consumption, habitation, systolic and diastolic blood pressure and Eysenck Personality Questionnaire Neuroticism Short Form Revised version and Hospital Anxiety and Depression Scale scores.
Aligned with the increasing prevalence were significant enhancements of all migraine symptoms with increasing altitude. Median attack frequency more than doubled (from 1.3 to 3.0 days/month) over the range from the lowest altitude to 2000–2499 m (P < 0.001) (Table 3, Fig. 2). Above 2500 m, we observed a decline in frequency in line with the decline in prevalence, although this was not significant (Table S1). Attack duration showed similar associations. Duration data were highly skewed, but median duration again more than doubled (from 9 to 24 h) over the range <500 m to 2000–2499 (P < 0.001) (Table 3, Fig. 3). Above 2500 m, we observed a similar decline, again not significant (Table S1). Likewise, pain intensity [the proportion of participants reporting ‘bad pain’ (highest intensity)] increased from 35.5% to 56.9% between the lowest altitude and 2000–2499 m (P = 0.011), with an observed but non‐significant decline at or above 2500 m (Tables 3 and S1).
Table 3.
Frequency, duration and intensity of migraine attacks by altitude
| Altitude (m) | ||||||
|---|---|---|---|---|---|---|
| <500 (n = 287) | 1000–1499 (n = 176) | 1500–1999 (n = 68) | 2000–2499 (n = 116) | ≥2500 (n = 81) | P | |
| Frequency (days/month) | ||||||
| Median (IQR) | 1.3 (0.3–3.0) | 2.0 (0.5–3.0) | 2.0 (0.3–3.0) | 3.0 (1.0–4.0) | 2.0 (1.0–3.0) | <0.001a |
| <1 day [n (%)] | 116 (40.4) | 57 (32.4) | 23 (33.8) | 21 (18.1) | 18 (22.2) | |
| 1–2 days [n (%)] | 95 (33.1) | 52 (29.5) | 19 (28.0) | 32 (27.6) | 27 (33.3) | <0.001b |
| >2 days [n (%)] | 76 (26.5) | 67 (38.1) | 26 (38.2) | 63 (54.3) | 36 (44.5) | |
| Duration (h) | ||||||
| Median (IQR) | 9.0 (3.0–30.0) | 12.0 (4.0–48.0) | 12.0 (6.0–48.0) | 24.0 (8.2–72.0) | 18.0 (7.0–48.0) | <0.001a |
| <6 h [n (%)] | 106 (36.9) | 52 (29.5) | 14 (20.6) | 22 (19.0) | 16 (19.8) | |
| 6–18 h [n (%)] | 86 (30.0) | 48 (27.3) | 25 (36.8) | 23 (19.8) | 26 (32.1) | <0.001b |
| >18 h [n (%)] | 95 (33.1) | 76 (43.2) | 29 (42.6) | 71 (61.2) | 39 (48.1) | |
| Intensity | ||||||
| Not bad [n (%)] | 33 (11.5) | 12 (6.8) | 5 (5.2) | 6 (5.2) | 4 (4.9) | |
| Quite bad [n (%)] | 152 (53.0) | 83 (47.2) | 34 (50.0) | 44 (37.9) | 41 (50.6) | 0.011b |
| Very bad [n (%)] | 102 (35.5) | 81 (46.0) | 29 (42.6) | 66 (56.9) | 36 (44.4) | |
IQR, interquartile range. aKruskal–Wallis test; bchi‐square test.
Figure 2.

Box‐plot of migraine attack frequency by altitude. Asterisks denote ‘extreme values’; that is, values that are more than three box lengths from either end of the box. Circles denote ‘outliers, that is they are between one and a half and three box lengths from either end of the box.
Figure 3.
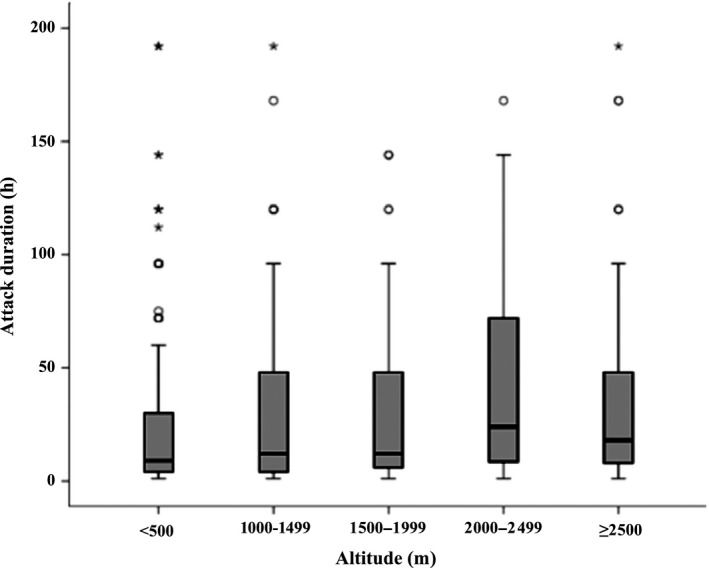
Box‐plot of migraine attack duration by altitude. For purposes of display, nine reported durations of >200 h have been removed. Asterisks denote ‘extreme values’; that is, values that are more than three box lengths from either end of the box. Circles denote ‘outliers, that is they are between one and a half and three box lengths from either end of the box.
Discussion
The study found that dwelling at high altitudes increased not only the prevalence of migraine but also all indices of symptom severity. This nationwide population‐based study is the first to show such aligned relationships. Altitude was an environmental factor that was so strongly associated with migraine that living above 1000 m led to increases in its prevalence of 31–63%. The matching effects on attack frequency, duration and headache intensity lend considerable robustness to this finding. The decrease in prevalence above 2500 m (along with declining trends in all symptom indices) is an unexpected phenomenon, for which an explanation must be sought.
The genetic and cultural diversities of Nepal do not offer an obvious explanation for these associations. The high‐altitude areas of Nepal are towards the north, in the foothills and then mountains of the Himalayas. The migraine prevalence gradient therefore increases from south to north, with a reverse trend in the very high levels of the extreme north. Nepal sits between India to the south, where the best estimate of migraine prevalence is 25.2% 15, and China to the north, where it is 9.3% 16. The estimate from India is very similar to our prevalence estimate for the Terai (lowlands) of Nepal (27.9%), which are contiguous with India. We have previously argued that the minor difference is explained as a consequence of the higher proportion of young, female adults in Nepal, combined with the exodus of large numbers of healthy people going abroad to work 6. We considered whether ethnic differences among Himalayan dwellers from most of the population in Nepal might account more plausibly than altitude for the migraine prevalence gradient. China is also ethnically diverse, but the survey in China, although covering most of the country, did not include the Himalayan areas of Tibet, which are contiguous with Nepal 16; with respect to this issue, it is not therefore very informative. In our study, in all three sampled districts lying on the border with China, migraine prevalence was not higher than the national mean of 34.7% [Mustang, 29.4%; Taplejung, 29.4%; Sindhupalanchok, 35.3% (observed prevalences, data on file)]. This speaks against different genes or lifestyle in the high Himalayas as an explanation of the associations between migraine and altitude.
More probable is a pathogenic effect of high altitude, influencing not only prevalence but also every measurable migraine characteristic. We postulate that altitude has a migraine‐aggravating effect manifesting in increased frequency, duration and intensity of attacks. The effect on prevalence is explained in this hypothesis by more perceptible attacks among people in whom they would otherwise be mild and/or infrequent, so that more of them fulfil criteria for migraine. In speculating on the mechanism and target organ of this aggravating effect, we discount high‐altitude headache as a factor. High‐altitude headache is a well‐known consequence of rapid ascent to high altitude above 2500 m, whereas the associations between altitude and migraine were strongest below 2500 m 12 and occurred in people chronically exposed to high altitude. Britze et al. 17 recently discussed hypoxic mechanisms that might be involved in migraine pathophysiology, but we are inclined to discount these as being relevant since high‐altitude dwellers are fully adapted in order to maintain adequate tissue oxygenation. Finally, cerebral blood flow appears also not to be a relevant factor. Studies have demonstrated that cerebral blood flow is unaltered, along with oxidative metabolism, after 3 weeks’ acclimatization to high altitude (5260 m) 18. An explanation therefore eludes us, but it must be relevant to the mechanism that, somewhere between 2000 and 2500 m, the relationship between increasing altitude and migraine prevalence reversed. Although physiological adaptations to moderately reduced pO2 may by some means increase migraine susceptibility, other mechanisms invoked to protect against the more severely reduced pO2 above 2000 m may allow partial reversal of the physiological changes.
Implications for public health
Globally, half a billion people dwell above 1500 m 19. Our data indicate that 16.5% (44.4% minus 27.9%) of those with migraine living at 1500–2000 m might not be affected if they lived below 500 m. Although urging caution when generalizing these results beyond Nepal, we observe nonetheless that extrapolation to the world's population aged 18–65 years (assumed to be 60% of 7 billion) suggests, conservatively, that the migraine‐provocative influence of altitude is real for at least 50 million people.
Strengths and limitations
The study had considerable strengths. We used tried and tested methods 7. We randomly selected from the whole of Nepal, with a very high participation proportion (>99%) effectively excluding participation bias. Carefully conducted face‐to‐face interviews ensured that there were no missing data. Over half of our sample were resident at or above 1000 m and nearly one quarter above 2000 m.
The principal limitation was our inability to perform a validation of the headache diagnostic questionnaire in its Nepali translation; with no headache specialists in the country, we had no means of applying a ‘gold standard’ 7. Instead we relied on the fact that the questionnaire had been validated in many other languages and countries 8, including India with its not too dissimilar culture.
Unanswered questions and suggestions for further targeted research
The fact that so many people are so strongly affected by an environmental factor, with the cause unknown, argues strongly for investment in research to understand the mechanisms. It may also be reasonable to perform clinical trials in migraine of substances and non‐pharmacological interventions that interact with putative mechanisms.
Conclusion
Migraine prevalence and indices of migraine severity are strongly associated with altitude. The mechanisms are unknown, but require investigation. A very large number of people might benefit from possible treatments that target these mechanisms.
Disclosure of conflicts of interest
The authors declare no financial or other conflicts of interest.
Supporting information
Table S1. Comparison of frequency, duration and intensity of migraine attacks between the two highest altitude groups.
Acknowledgements
This project was conducted within the Global Campaign against Headache, led by the non‐governmental organization Lifting The Burden in official relations with the World Health Organization and with the support of Dhulikhel Hospital, Kathmandu University Hospital, Dhulikhel, Kavre, Nepal. Grethe Albrektsen, professor in medical statistics at the Department of Public Health and General Practice, Norwegian University of Science and Technology, provided statistical advice.
This work was supported by Samarbeidsorganet, the Liaison Committee between the Central Norway Regional Health Authority and the Norwegian University of Science and Technology (NTNU) (grant no. 653010‐46060911).
References
- 1. Global Burden of Disease Study Collaboration . Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 386: 743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mulder EJ, Van Baal C, Gaist D, et al Genetic and environmental influences on migraine: a twin study across six countries. Twin Res 2003; 6: 422–431. [DOI] [PubMed] [Google Scholar]
- 3. Arregui A, Cabrera J, Leon‐Velarde F, Paredes S, Viscarra D, Arbaiza D. High prevalence of migraine in a high‐altitude population. Neurology 1991; 41: 1668–1669. [DOI] [PubMed] [Google Scholar]
- 4. Manandhar K, Risal A, Steiner TJ, Holen A, Koju R, Linde M. Estimating the prevalence and burden of major disorders of the brain in Nepal: methodology of a nationwide population‐based study. J Headache Pain 2014; 15: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Central Intelligence Agency . The world factbook. https://www.cia.gov/library/publications/resources/the-world-factbook/geos/np.html. 2015 (accessed 29/09/2015)
- 6. Manandhar K, Risal A, Steiner TJ, Holen A, Linde M. The prevalence of primary headache disorders in Nepal: a nationwide population‐based study. J Headache Pain 2015; 16: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stovner LJ, Al Jumah M, Birbeck GL, et al The methodology of population surveys of headache prevalence, burden and cost: principles and recommendations from the Global Campaign against Headache. J Headache Pain 2014; 15: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Steiner TJ, Gururaj G, Andree C, et al Diagnosis, prevalence estimation and burden measurement in population surveys of headache: presenting the HARDSHIP questionnaire. J Headache Pain 2014; 15: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Risal A, Manandhar K, Steiner TJ, Holen A, Koju R, Linde M. Estimating prevalence and burden of major disorders of the brain in Nepal: cultural, geographic, logistic and philosophical issues of methodology. J Headache Pain 2014; 15: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Risal A, Manandhar K, Linde M, Koju R, Steiner TJ, Holen A. Reliability and validity of a Nepali‐language version of the Hospital Anxiety and Depression Scale (HADS). Kathmandu Univ Med J (KUMJ) 2015; 13: 115–124. [DOI] [PubMed] [Google Scholar]
- 11. Manandhar K, Risal A, Linde M, Koju R, Steiner TJ, Holen A. Measuring neuroticism in Nepali: reliability and validity of the neuroticism subscale of the Eysenck personality questionnaire. Kathmandu Univ Med J (KUMJ) 2015; 13: 156–161. [DOI] [PubMed] [Google Scholar]
- 12. Headache Classification Committee of the International Headache Society . The international classification of headache disorders, 3rd edition (beta version). Cephalalgia 2013; 33: 629–808. [DOI] [PubMed] [Google Scholar]
- 13. Linde M, Dahlof C. Attitudes and burden of disease among self‐considered migraineurs – a nation‐wide population‐based survey in Sweden. Cephalalgia 2004; 24: 455–465. [DOI] [PubMed] [Google Scholar]
- 14. Government of Nepal NPCS . Population Monograph of Nepal, Vol. 1. Kathmandu, Nepal: Central Bureau of Statistics, 2014. [Google Scholar]
- 15. Kulkarni GB, Rao GN, Gururaj G, Stovner LJ, Steiner TJ. Headache disorders and public ill‐health in India: prevalence estimates in Karnataka State. J Headache Pain 2015; 16: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yu S, Liu R, Zhao G, et al The prevalence and burden of primary headaches in China: a population‐based door‐to‐door survey. Headache 2012; 52: 582–591. [DOI] [PubMed] [Google Scholar]
- 17. Britze J, Arngrim N, Schytz HW, Ashina M. Hypoxic mechanisms in primary headaches. Cephalalgia 2017; 37: 372–384. [DOI] [PubMed] [Google Scholar]
- 18. Moller K, Paulson OB, Hornbein TF, et al Unchanged cerebral blood flow and oxidative metabolism after acclimatization to high altitude. J Cereb Blood Flow Metab 2002; 22: 118–126. [DOI] [PubMed] [Google Scholar]
- 19. Cohen JE, Small C. Hypsographic demography: the distribution of human population by altitude. Proc Natl Acad Sci USA 1998; 95: 14009–14014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparison of frequency, duration and intensity of migraine attacks between the two highest altitude groups.


