Summary
Background
Previous studies have reached conflicting conclusions regarding the efficacy of mesalazine in the prevention of recurrent diverticulitis.
Aim
To investigate the efficacy and safety of mesalazine granules in the prevention of recurrence of diverticulitis after acute uncomplicated diverticulitis.
Methods
Two phase 3, randomised, placebo‐controlled, double‐blind multicentre trials (SAG‐37 and SAG‐51) investigated mesalazine granules in patients with prior episodes (<6 months) of uncomplicated left‐sided diverticulitis. Patients were randomised to receive either 3 g mesalazine once daily or placebo (SAG‐37, n=345) or to receive either 1.5 g mesalazine once daily, 3 g once daily or placebo for 96 weeks (SAG‐51, n=330). The primary endpoint was the proportion of recurrence‐free patients during 48 weeks (SAG‐37 and SAG‐51) or 96 weeks (SAG‐51) of treatment.
Results
Mesalazine did not increase the proportion of recurrence‐free patients over 48 or 96 weeks compared to placebo. In SAG‐37, the proportion of recurrence‐free patients during 48 weeks was 67.9% with mesalazine and 74.4% with placebo (P=.226). In SAG‐51, the proportion of recurrence‐free patients over 48 weeks was 46.0% with 1.5 g mesalazine, 52.0% with 3 g mesalazine and 58.0% with placebo (P=.860 for 3 g mesalazine vs placebo) and over 96 weeks 6.9%, 9.8% and 23.1% respectively (P=.980 for 3 g mesalazine vs placebo). Patients with only one diverticulitis episode in the year prior to study entry had a lower recurrence risk compared to >1 episode. Safety data revealed no new adverse events.
Conclusion
Mesalazine was not superior to placebo in preventing recurrence of diverticulitis.
1. INTRODUCTION
Diverticula are herniations of the intestinal mucosa through the bowel wall. Diverticula are often asymptomatic, then termed diverticulosis, but can be associated with abdominal pain, bloating and changes in bowel habits, in which case they are referred to in a more general way as diverticular disease (DD).1, 2 These symptoms can represent symptomatic uncomplicated diverticular disease (SUDD), in which the abdominal symptoms are attributed to diverticula, but in the absence of significant inflammation.1, 2 SUDD is a pathogenetically ill‐defined entity with a heterogeneous disease pattern, often difficult to differentiate from irritable bowel syndrome.3 In contrast to SUDD, acute diverticulitis is defined by active inflammation of diverticula. Diverticulitis can be either uncomplicated or complicated. Uncomplicated diverticulitis shows colonic wall thickening and peridiverticulitis by cross‐sectional imaging, while complicated diverticulitis is associated with abscesses or fistula or obstruction or perforation. Once an acute episode of uncomplicated diverticulitis has resolved, 13% to 36% of patients experience a recurrent episode of acute diverticulitis.4 Considering patients with complicated diverticulitis, the majority of these patients present with complicated diverticulitis as their first episode and 47% have recurrent diverticulitis.5 Perforation, the most dangerous complication, happens nearly always during the first attack of diverticulitis.5 Complicated diverticulitis was only described in 5% of the patients after an initial uncomplicated episode in a 8 year follow‐up,6 but about 13% of patients with prior episodes required hospital admissions for the treatment of the recurrent episode.7 Therefore, recurrent episodes of acute diverticulitis not only dramatically decrease the patient quality of life, but also substantially increase the burden on health care resources.2 In addition, as the risk of recurrence seems to increase with each episode,8 an efficacious prevention strategy is desirable. However, largely due to a lack of blinded, randomised, controlled trials, recommendations for the preventive treatment of recurrent acute diverticulitis are seldom based on adequate scientific evidence and are often inconsistent.2
As the medical management of inflammatory bowel disease such as ulcerative colitis has classically been based on aminosalicylates including mesalazine, a number of studies have also evaluated the role of mesalazine (also known as mesalamine or 5‐ASA [5‐aminosalicylic acid]) in the management of DD. Indeed, benefits of mesalazine for the treatment of SUDD have been reported.9, 10, 11, 12, 13, 14, 15 Some randomised controlled trials have also found beneficial effects of mesalazine in reducing symptoms of patients with a history of acute uncomplicated diverticulitis, but showed no impact on the recurrence rate.16, 17 Furthermore, two recent placebo‐controlled trials investigating the efficacy of mesalazine for the prevention of acute diverticulitis recurrence demonstrated negative results.18 Although these studies had clear diagnostic criteria on recurrence, they have been criticised for ignoring the course of global symptoms throughout the study period.19 Therefore, to resolve these inconsistent conclusions, further well‐designed studies are urgently needed.20
Here, we report the findings of two multicentre, international, randomised, double‐blind studies undertaken to determine whether mesalazine is superior to placebo in preventing diverticulitis recurrence after acute uncomplicated diverticulitis, and to evaluate whether there are subgroups of patients who may respond to preventive anti‐inflammatory treatment.
2. METHODS
2.1. Study design and conduct
Two phase 3, multicentre, randomised, double‐blind, placebo‐controlled studies (SAG‐37 and SAG‐51) were conducted at specialised gastroenterology centres (NCT00695643, NCT01038739). The SAG‐37 study was performed from 2008 to 2011 at 57 centres in 11 countries; SAG‐51 was undertaken from 2010 to 2013 at 74 centres in nine countries. Both studies used an adaptive, multistage group sequential design. The SAG‐51 study was stopped due to futility after a planned interim analysis, following a recommendation from the Independent Data Monitoring Committee, with immediate withdrawal of treatment and premature study termination in all patients who had not yet completed the study.
The study protocols were approved by the institutional review board at each centre and written informed consent was obtained from all participants.
2.2. Patients
Patients were eligible for the study if (1) they were between 30 and 80 years old (SAG‐51) or 40‐80 years old (SAG‐37), (2) they had a prior diagnosis of left‐sided uncomplicated acute diverticulitis confirmed by ultrasonography or computed tomography (CT) with at least one diverticulum in the left colon (In SAG‐51, abdominal CT was performed to depict the thickness of the colonic wall as well as signs of inflammation around the diverticula. Scans were analysed by central reading. In SAG‐37, assessment of colonic wall thickening by ultrasonography or CT was required.), (3) the prior episode of left‐sided uncomplicated diverticulitis was within the preceding 6 months and has been brought to clinical remission with antibiotics and/or dietary modification, documented by medical records, (4) they had ≥3 of the following symptoms at the start of the most recent episode of diverticulitis: left lower quadrant pain, fever, altered bowel habit (diarrhoea, constipation, passage of mucus, or urgency) and systemic signs (nausea, lethargy), (5) C‐reactive protein (CRP) exceeded the upper limit of normal (ULN) or leucocytosis (>10 000/mm3) at the start of the most recent episode.
Patients with chronic inflammatory bowel disease (eg, Crohn's disease or ulcerative colitis) were excluded. Additional exclusion criteria included complicated diverticulitis (diverticulitis with associated abscess, fistula, obstruction or perforation), right‐sided diverticulitis, previous colonic surgery, symptomatic organic disease of the GI tract, active colorectal cancer or history of colorectal cancer, active malignancy other than colorectal cancer or treatment with anticancer drugs during the previous 5 years, haemorrhagic diathesis, active peptic ulcer disease, local intestinal infection, asthma without careful medical monitoring, abnormal hepatic function or liver cirrhosis, abnormal renal function, severe co‐morbidity and/or immobility and known intolerance/hypersensitivity/resistance to study drug or drugs of similar chemical structure. Patients who had received mesalazine‐containing drugs, glucocorticosteroids, opioid analgesics, laxatives, antidiarrhoeals, immunosuppressants or nonsteroidal anti‐inflammatory drugs after the most recent episode were also excluded.
2.3. Intervention
Randomisation was performed by means of a computer‐generated randomisation list, using randomly permuted blocks. Product assignment to each patient was undertaken according to this list. In SAG‐37, patients were randomly assigned in a 1:1 ratio to receive mesalazine 3.0 g once daily (Salofalk granules, Dr. Falk Pharma GmbH, Freiburg, Germany) or placebo over a period of 48 weeks. In SAG‐51, patients were randomised to mesalazine 1.5 g once daily (Salofalk granules, arm A), mesalazine 3.0 g once daily (Salofalk granules, arm B) or placebo (arm C) for 96 weeks. The appearance and size of the placebo treatments were indistinguishable from the active mesalazine treatments and both patients and investigator were unaware of the treatment assignment.
2.4. Evaluation
The SAG‐37 trial included six study visits, and SAG‐51 included 12 visits. At baseline and the final visit, demographic data and total symptom score of Patient Health Questionnaire 15 (PHQ‐15)21 were assessed and body weight was recorded. Laboratory monitoring at all visits included CRP, leucocyte count and stool examination. Patients in whom faecal culture was positive for pathogenic microorganisms were withdrawn from the study. Total colonoscopy or sigmoidoscopy and biopsy were performed a minimum of 6 weeks after the acute index episode of diverticulitis, but were to be postponed if the episode was followed by another acute attack.
2.5. Study endpoints
The primary endpoint was the proportion of patients who remained free of diverticulitis recurrence over 48 weeks (SAG‐37 and SAG‐51) or 96 weeks (SAG‐51). Recurrence of acute diverticulitis was based on the same diagnostic criteria as the prior episodes being defined as CRP >ULN or leucocytosis and presence of diverticulitis‐like clinical signs plus typical findings on CT scan or ultrasonography (see above, same criteria for CT and ultrasonography used as for inclusion). As defined in the statistical analysis plan, the primary endpoint was analysed in subpopulations of patients stratified according to the number of diverticulitis episodes in the year prior to study entry (1 or >1) and CRP at study entry (>ULN or ≤ULN). Secondary endpoints for both studies included the time to recurrence; changes of erythrocyte sedimentation rate (ESR), CRP level and leucocytosis; occurrence of diverticulitis‐associated fever; percentage of days with left lower quadrant pain; percentage of days with solid stools, soft or solid stools, diarrhoea (>3 stools per day) or watery stools; average number of stools per week; use of spasmolytics; use of analgesics; and total symptom score of PHQ‐15 (regardless of missing answers). Adverse events were recorded throughout the whole study period.
2.6. Statistical analysis
The safety population included all randomised patients who received ≥1 dose of study medication. Frequency of adverse events was stratified by treatment (mesalazine vs placebo). The intent‐to‐treat (ITT) population included all randomised patients who received ≥1 dose of study medication and who had a documented attack of diverticulitis within 6 months prior to study entry. The per protocol (PP) population included all ITT patients who met all eligibility criteria and had no major protocol deviations. To avoid that patients with termination due to stopping of the study would be analysed as patients with recurrence of diverticulitis, two modified versions of both the ITT and PP analysis set were defined. These modified versions of the ITT and PP analysis set were subsets of the ITT and PP analysis set excluding patients who terminated the study due to stopping of the study before week 48 and week 96, respectively (Figure 1).
Figure 1.
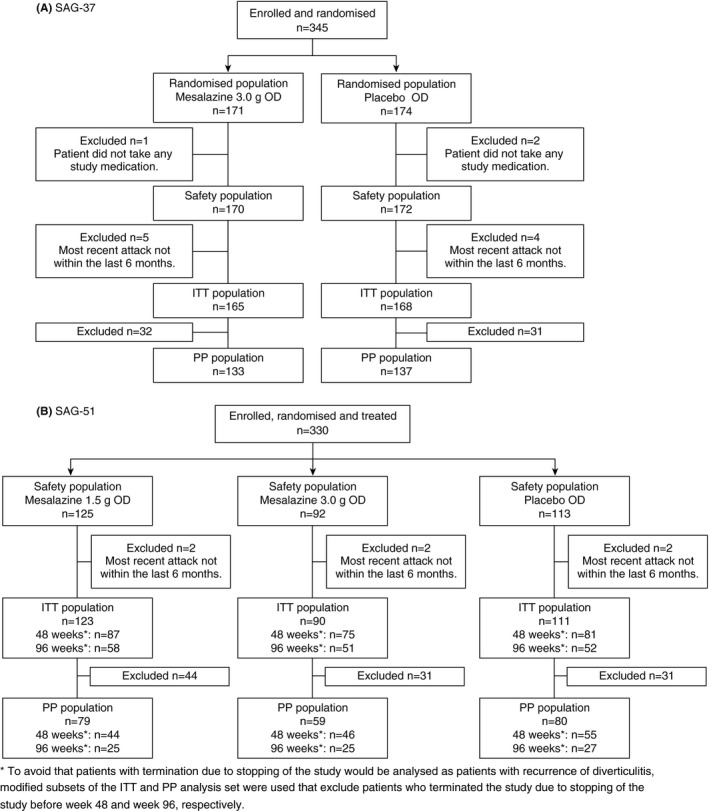
Patient disposition (ITT and PP population)
The adaptive designs of the studies, planned interim analyses and the stopping rules are described in the Data S1. In brief, the planned interim analysis of study SAG‐37, based on 133 ITT patients, was inconclusive and the Independent Data Monitoring Committee recommended an additional interim analysis after recruitment of another 100 patients based on a recalculated sample size. The second interim analysis (233 ITT patients) again proved inconclusive and recruitment was stopped, at which point 342 patients had been recruited. Having the results of SAG‐37 as well as negative results published in the same indication for another mesalazine formulation (press release, Shire, March 30, 2012) the interim analysis for SAG‐51 was brought forward and a rule for stopping the study due to futility (nonbinding) was introduced. Interim analysis was performed based on 180 ITT patients, and superiority of the mesalazine groups vs placebo was not shown, leading to the study being stopped in accordance with the pre‐defined study discontinuation rules.
Confirmatory hypothesis testing at the interim analyses and the final analyses was based on the inverse normal method of combining P‐values from each stage, using the normal approximation test for comparing two rates. To estimate the treatment effect, differences between the proportions of recurrence‐free patients were calculated with the corresponding two‐sided 97.5% repeated confidence interval (CI) values. Fisher's exact test, two‐sided, was used to calculate an unadjusted P‐value for the overall comparison of proportions of recurrence‐free patients between treatment groups. The unadjusted P‐value does not take into account the recursive design and has to be interpreted in the exploratory sense. To analyse the time to recurrence, a time‐to‐event analysis was performed for the event “recurrence of diverticulitis” including calculation of hazard ratio and corresponding 95% confidence interval if appropriate. All other analyses were exploratory. The statistical design, the sample size calculation and statistical analysis were performed with ADDPLAN 6, licensed by ADDPLAN GmbH, an ICON company, Cologne, Germany, and using the software package SAS version 9.1 or higher (SAS Institute, Cary, NC, USA).
3. RESULTS
3.1. Patient population
In total, 345 patients were randomised in the SAG‐37 trial (171 mesalazine 3.0 g once daily, 174 placebo). Three patients did not receive any study medication such that the safety population comprised 342 patients (170 mesalazine 3.0 g once daily, 172 placebo) (Figure 1A). Of these, 247 (72%) completed the 48‐week study. Lack of efficacy was the most frequent reason for discontinuation (Figure S1A). The most recent episode of diverticulitis was >6 months prior to study entry in nine patients in SAG‐37, so the ITT population included 333 patients (165 mesalazine 3.0 g once daily, 168 placebo) (Figure 1A). In the SAG‐51 study, 330 were randomised and treated and formed the safety population (125 mesalazine 1.5 g once daily [arm A], 92 mesalazine 3.0 g once daily [arm B], 113 placebo [arm C]) (Figure 1B, Figure S1B). In six patients, the most recent episode of diverticulitis was >6 months earlier, so the ITT population included 324 patients (123, 90 and 111 patients respectively). To avoid that patients with termination due to stopping of the study would be analysed as patients with recurrence of diverticulitis, modified subsets of the ITT and PP analysis set were defined that excluded patients who terminated the study due to stopping of the study before week 48 and week 96 respectively. In SAG‐51, 243 patients completed the week 48 of the study or withdrew early for reasons other than the early study cessation, which occurred before week 48, and 161 patients completed the week 96 visit or withdrew early for reasons other than the early stopping of the study. The proportion of patients who discontinued prematurely in the SAG‐37 trial was 31.2% in the mesalazine group vs 24.4% in the placebo group; in SAG‐51 the proportions were 97.6%, 94.6%, 89.4% in arms A, B and C, respectively (primary reason: study cessation after interim analysis).
The demographics and clinical characteristics of the study groups were well balanced across study arms in both trials (Table S1). The number of previous episodes of diverticulitis and CRP levels were comparable. The mean (SD) time since the start of the most recent episode of diverticulitis was 84 (42) days in the mesalazine arm and 90 (53) days in the placebo arm in SAG‐37 (overall median 75 days, range 3‐334 days). In SAG‐51, the mean (SD) time since the start of the most recent episode was 93 (44) days in the 1.5 g mesalazine arm, 87 (44) in the 3.0 g mesalazine arm and 88 (45) days in the placebo arm (overall median 78 days, range 5‐223 days).
3.2. Efficacy: primary endpoint
In SAG‐37, the primary endpoint of freedom from diverticulitis recurrence over 48 weeks was similar for the patients in the mesalazine 3.0 g group and the placebo group (67.9% vs 74.4%; P=.226; ITT population) (Table 1; Figure 2A). Recurrence by week 48 was documented in 18.8% (31/165) and 11.9% (20/168) of patients in the mesalazine and placebo groups, respectively (Table S2), with recurrence‐free withdrawal in 13.3% and 13.7% of patients. In the per protocol population, the proportions of recurrence‐free patients were 78.9% (105/133) and 89.8% (123/137) (P=.018), respectively.
Table 1.
Primary endpoint and subpopulation analysis (ITT population)
| SAG‐37 | SAG‐51 | ||||
|---|---|---|---|---|---|
| Mesalazine 3.0 g n=165 | Placebo n=168 | Mesalazine 1.5 g n=87a/58b | Mesalazine 3.0 g n=75a/51b | Placebo n=81a/52b | |
| Primary endpoint | |||||
| Percentage of patients free of diverticulitis recurrence | |||||
| Over 48 weeks (n/N) | 67.9 (112/165)c | 74.4 (125/168) | 46.0 (40/87) | 52.0 (39/75)d | 58.0 (47/81) |
| Over 96 weeks (n/N) | NA | NA | 6.9 (4/58) | 9.8 (5/51)a | 23.1 (12/52) |
| Subpopulation analysis | |||||
| Recurrence‐free over 48 weeks | |||||
| Number of diverticulitis episodes <1 year before study entry | |||||
| 1, n/N (%) | 61/92 (66.3) | 71/91 (78.0) | 26/47 (55.3) | 21/38 (55.3) | 20/33 (60.6) |
| >1, n/N (%) | 51/73 (69.9) | 54/76 (71.1) | 14/40 (35.0) | 18/37 (48.6) | 27/48 (56.3) |
| CRP at study entry | |||||
| CRP >ULN, n/N (%) | 39/63 (61.9) | 52/70 (74.3) | 6/23 (26.1) | 10/20 (50.0) | 15/26 (57.7) |
| CRP ≤ULN, n/N (%) | 73/102 (71.6) | 71/96 (74.0) | 33/63 (52.4) | 29/55 (52.7) | 32/55 (58.2) |
NA, not applicable.
Patients with completion of week 48 or study termination for another reason than “stop of the whole study” before week 48.
Patients with completion of week 96 or study termination for another reason than “stop of the whole study” before week 96.
Fisher's exact test: 48 week mesalazine 3.0 g vs Placebo (SAG‐37): P=.226.
Fisher's exact test: 48 week mesalazine 3.0 g vs Placebo (SAG‐51): P=.520.
Fisher's exact test: 96 week mesalazine 3.0 g vs Placebo (SAG‐51): P=.110.
Figure 2.
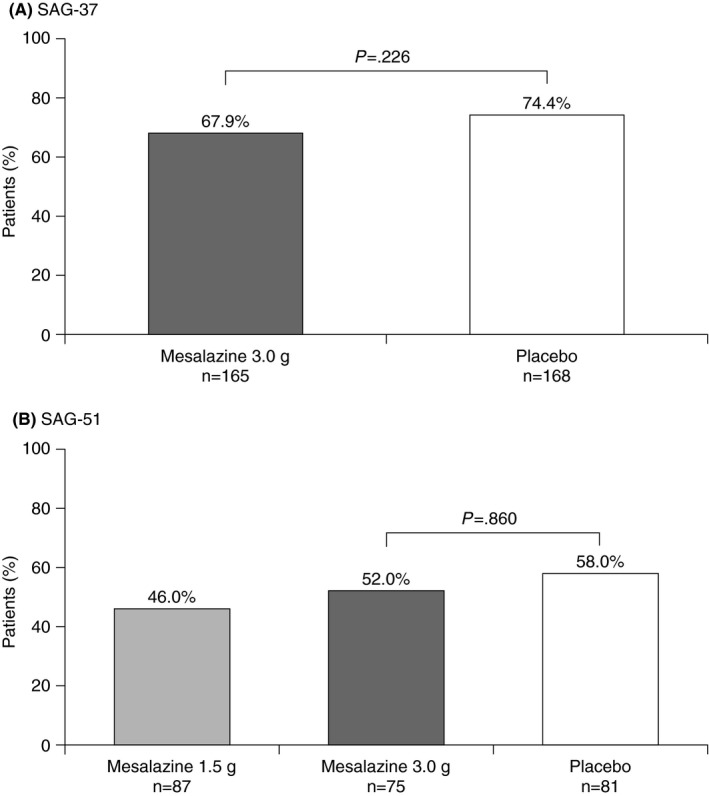
Proportion of patients free of diverticulitis recurrence over 48 weeks (SAG‐37: ITT population; SAG‐51: modified ITT population)
In SAG‐51, 46.0% of patients treated with mesalazine 1.5 g, 52.0% of patients given mesalazine 3.0 g and 58.0% of placebo‐treated patients remained free from diverticulitis recurrence over the first 48 weeks (P=.860 for mesalazine 3.0 g vs placebo; modified ITT) (Table 1; Figure 2B). Recurrence was observed in 17.2% (15/87), 20.0% (15/75), and 21.0% (17/81) of patients, respectively (Table S2), with recurrence‐free withdrawal in 35.6%, 25.3% and 18.5% (recurrence was not evaluable in five patients). Of the 161 patients who were followed to week 96, the proportion who remained recurrence‐free throughout the 96‐week treatment was 6.9% in the mesalazine 1.5 g group, 9.8% in the mesalazine 3.0 g group, and 23.1% in the placebo arm (P=.980 for mesalazine 3.0 g vs placebo; modified ITT; Table 1). Recurrence was observed in 27.6% (16/58), 33.3% (17/51), and 38.5% (20/52) of patients, respectively.
3.3. Secondary efficacy endpoints
SAG‐37 showed no significant difference in the time to diverticulitis recurrence between treatment groups (Table S2): mean (SD) 136 (100) days in the mesalazine group (n=31) vs 141 (103) days in the placebo group (n=20); hazard ratio (95% CI) 0.60 (0.34, 1.05) (log‐rank P=.069). Similar results were obtained in the SAG‐51 trial, where the mean (SD) time to recurrence was 116 (134) days in the mesalazine 1.5 g once daily group (n=16), 191 (125) days in the mesalazine 3.0 g once daily group (n=17), and 147 (162) days in the placebo group (n=20); hazard ratio (95% CI) 0.74 (0.38, 1.43) (log‐rank P=.369) for mesalazine 1.5 g once daily vs placebo and 1.02 (0.53, 1.94) (log‐rank P=.957) for mesalazine 3.0 g once daily vs placebo (Table S2).
Furthermore, there were no relevant differences nor any positive effects of mesalazine in either the SAG‐37 or SAG‐51 study regarding other efficacy endpoints, including the results of ESR, CRP and leucocytosis at baseline and week 48, recurrence‐associated fever or leucocytosis, the mean percentage of days with any left lower quadrant pain or stools of different consistency, mean number of stools per week, and use of spasmolytics or analgesics (Table S2). Consistently, the assessment of symptom severity with the Patient Health Questionnaire 15 (PHQ‐15) did not show any difference between the mesalazine treatment and placebo groups on symptom severity (Table S2).
3.4. Subpopulation analyses
The proportion of patients in SAG‐37 and SAG‐51 with one, two, three or more than three episodes of diverticulitis in the year prior to study entry was similar between treatment groups (Table S1). Treatment with mesalazine showed no advantage for diverticulitis recurrence vs placebo in the subpopulations with one or more than one prior episode in either study (Table 1). Similarly, no treatment effect was observed when patients were stratified according to CRP at study entry (Table 1).
3.5. Recurrence of diverticulitis
Overall, 71.2% (237/333) of patients in SAG‐37 and 51.9% (126/243) of patients in SAG‐51 remained free from diverticulitis recurrence by week 48. In SAG‐51, 13.0% (21/161) of patients were recurrence‐free until week 96. The mean recurrence rate of all treatment arms of both studies was 17.0% (98/576) for 48 weeks and 32.9% (53/161) for 96 weeks. A pooled post hoc subgroup analysis of the combined placebo arms from the two studies showed the risk of recurrence was lower in patients with only one diverticulitis episode in the year prior to study entry compared to >1 episode, with a nonsignificant trend to higher risk for recurrence in patients aged less than 60 years vs ≥60 years (Table 2). Other factors, including the number of diverticula at baseline, showed no association with risk for recurrence (Table 2). Increased CRP (>ULN) during the most recent diverticulitis episode was predictive for recurrence, but since this was an inclusion criterion, the virtual absence of a comparator group (CRP ≤ULN) makes the analysis unreliable. Consistent with reported cases of CT‐diagnosed acute diverticulitis,22 less than 50% of the patients with recurrent diverticulitis showed recurrence‐associated fever (26%; 25/98) and/or leucocytosis (41%; 40/98).
Table 2.
Post hoc subgroup analysis of the combined placebo arms from SAG‐37 (n=145) and SAG‐51 (n=66) for risk factors for diverticulitis recurrence by week 48; recurrence‐free withdrawals (n=68) excluded
| Risk factor | No recurrence N=172 | Recurrence N=39 | Odds ratio | 95% CI | P‐value |
|---|---|---|---|---|---|
| Number of previous diverticulitis episodes, n (%) | |||||
| 1 | 91 (52.9) | 12 (30.8) | 0.40 | 0.19‐0.83 | .014 |
| >1 | 81 (47.1) | 27 (69.2) | |||
| CRP at most recent episode, n (%) | |||||
| >ULN | 136 (79.1) | 31 (79.5) | 6.58 | 1.05‐41.07 | .044 |
| ≤ULN | 2 (1.2) | 3 (7.7) | |||
| Age at baseline, n (%) | |||||
| <60 | 94 (54.7) | 28 (71.8) | 2.11 | 0.99‐4.51 | .054 |
| ≥60 | 78 (45.3) | 11 (28.2) | |||
| Gender, n (%) | |||||
| Male | 73 (42.4) | 21 (53.8) | 0.63 | 0.31‐1.27 | .198 |
| Female | 99 (57.6) | 18 (46.2) | |||
| Body mass index at baseline, kg/m2, n (%) | |||||
| <30 | 120 (69.8) | 24 (61.5) | 0.69 | 0.34‐1.43 | .320 |
| ≥30 | 52 (30.2) | 15 (38.5) | |||
| Numbers of diverticula at baseline, n (%) | |||||
| ≤5 | 19 (11.0) | 3 (7.7) | 0.65 | 0.18‐2.39 | .519 |
| >5 | 95 (55.2) | 23 (59.0) | |||
| Concomitant treatment with psyllium, n (%) | |||||
| Yes | 13 (7.6) | 2 (5.1) | 0.66 | 0.14‐3.06 | .597 |
| No | 159 (92.4) | 37 (94.9) | |||
| Time since most recent attack of uncomplicated diverticulitis, n (%) | |||||
| ≤6 weeks | 23 (13.4) | 6 (15.4) | 1.15a | 0.41‐3.26a | .752a |
| >6 weeks and ≤12 weeks | 73 (42.4) | 16 (41.0) | 0.97a | 0.46‐2.06a | .786a |
| >12 weeks | 75 (43.6) | 17 (43.6) | |||
Bold P‐values: statistically significant (P <.05)
vs >12 weeks.
3.6. Safety
Overall, 85% (327/387) of participants on mesalazine and 79% (225/285) of participants on placebo reported adverse events irrespective of causality, predominantly infections and gastrointestinal disorders (Table S3). 14% (55/387) of patients on mesalazine and 10% (29/285) of patients on placebo experienced serious adverse events. Two serious adverse events (agranulocytosis, colectomy due to diverticulitis) were considered by the investigator to be related to intake of mesalazine. Adverse events led to discontinuation of the study drug in 25% (97/387) of the mesalazine group and 18% (51/285) of the placebo group of which the majority were cases of diverticulitis (71 on mesalazine, 38 on placebo).
4. DISCUSSION
These two randomised, double‐blind SAG‐37 and SAG‐51 trials showed no benefit of mesalazine in reducing the risk of recurrent acute diverticulitis. The primary endpoint, the proportion of recurrence‐free patients during 48 or 96 weeks of treatment, was comparable for patients treated with mesalazine (1.5 g/d or 3.0 g/d) or placebo. In addition, there was no significant difference between the mesalazine and placebo groups with respect to the time to recurrence, any of the other secondary outcomes related to diverticulitis‐associated symptoms or the markers for inflammation or infection.
These findings concur with those of two recent large, placebo‐controlled, double‐blind, randomised trials (PREVENT 1 and 2), which failed to show an effect of mesalazine (1.2, 2.4 or 4.8 g/d) in preventing diverticulitis recurrence vs placebo.18 In PREVENT 1 and 2, the recurrence‐free rate varied between 53% and 69% in the mesalazine treatment groups, compared to 65% and 69% in the placebo arms. The presence of abdominal pain was also similar between study arms. The authors concluded that mesalazine should not be used for prevention of recurrent diverticulitis. These trials were criticised to only use CT data as the diagnostic tool for recurrent diverticulitis, while ignoring global symptoms.19 In contrast, we included in our primary endpoint for diagnosing prior and recurrent episodes next to imaging criteria (CT or ultrasonography) also diverticulitis‐like clinical symptoms and increased inflammation markers. However, also the inclusion of more global symptoms in our study led to the same conclusion, ie, mesalazine does not prevent recurrent acute diverticulitis.
Previously, several randomised trials concluded that mesalazine is effective in the treatment of SUDD.9, 10, 11, 12, 13, 14, 15 However, SUDD and acute diverticulitis are different entities. SUDD, a controversial diagnosis, also sometimes confusingly referred to as symptomatic diverticulosis, is defined only by abdominal symptoms and not by the presence of objective signs of inflammation such as increased CRP,3, 23 or typical findings by cross‐sectional imaging. In contrast, acute diverticulitis has clear diagnostic criteria based on clinical symptoms and objective confirmation of active inflammation. Furthermore, differential diagnosis between SUDD and IBS is particularly very difficult and recent reports suggesting some benefits of mesalazine in treating subgroups of IBS patients may further complicate the interpretation of these studies.24, 25 Nevertheless, treatment with mesalazine reduced pain associated with SUDD better than placebo11 and appeared to be better than placebo for maintaining remission of SUDD.13 In the light of these results, some studies also looked at the role of mesalazine in preventing recurrence of acute diverticulitis.16, 17 In the study by Parente et al., 800 mg mesalazine was given twice daily for 10 days every month and compared to placebo.16 Both the first episode of acute diverticulitis (<12 months) as well as recurrence were diagnosed similarly to our study with the presence of abdominal pain, associated with leucocytosis and/or fever and confirmation by CT and/or ultrasonography. Consistent with our data, mesalazine did not reduce the risk of recurrence but induced significant improvement of patients’ physical conditions and significantly lowered the additional consumption of other gastrointestinal drugs. While we could confirm that the rate of recurrence was not affected by mesalazine treatment, we could not detect any effect in patient's health assessment (PHQ‐15). Furthermore, as we did not see any difference in the consumption of analgesics or spasmolytics between the different treatment arms, we could not confirm a reduction in pain in acute diverticulitis by mesalazine as it has been reported for the treatment of SUDD.11
Stollman et al.17 treated in the DIVA study patients with clinical diagnosis of acute diverticulitis confirmed by CT with mesalazine for 12 weeks and followed them for 9 months. Although not statistically significant for the prevention of diverticulitis recurrence, the DIVA study observed a decrease in mean global symptom score for mesalazine compared to placebo. However, in contrast to our study or the PREVENT trials, the recurrent episode was not assessed by CT or ultrasonography. Whether mesalazine may effect global symptoms is subject to broader discussions,19 but our trials did not show any effect of mesalazine on symptoms compared to placebo. Nevertheless, it should be noted that the DIVA and our trials used different approaches to record symptoms, with the DIVA trial applying a more rigorous global symptom score, but with evaluation after 12 weeks of treatment only.
Post hoc subpopulation analyses in placebo‐treated patients showed that more than one prior episode of diverticulitis, compared to a single episode, was associated with a higher risk for recurrence. The degree of CRP elevation during the previous episode of diverticulitis showed no association with risk for recurrence, but this is not unexpected given that the median time since the last episode was 75‐78 days, and fewer than 40% of patients in either study presented with elevated CRP at baseline. Overall recurrence‐free rates seen in both trials were different (71.2% and 51.9%). A reason for the higher rate in SAG‐37 may be that relapses were diagnosed by ultrasound or CT, while in SAG‐51 imaging by CT was mandatory; the latter could result in earlier admission to hospital. In addition, the inflammation of diverticula and the peri‐colonic area was only in SAG‐51 explicitly assessed. In SAG‐37, however, the assessment of only colonic wall thickening without explicit assessing of inflammatory signs might have also overestimated the incidence of true acute diverticulitis and thus, attributed to the higher reported recurrence rate. Overestimation as well as underestimation for the diagnosis of diverticulitis has been demonstrated using CT.26, 27
Comparison of our safety data for mesalazine in the prevention of recurrent acute diverticulitis with the cumulative experience in inflammatory bowel disease did not raise any new safety concern for the patient population under investigation. Frequency of drug‐related GI disorders was identical in both treatment arms. Mesalazine was tolerated well in patients who had suffered from left‐sided uncomplicated acute diverticulitis with a very low rate of drug‐related serious adverse events. However, one patient showed serious agranulocytosis with sore throat as a typical initial symptom, which is a well‐known and very rare side effect of mesalazine. The patient withdrew mesalazine and was treated with filgrastim upon hospital admission. The patient fully recovered and the agranulocytosis was resolved. Diverticulitis as the most common safety reason for drug interruption clearly reflects the negative results of efficacy in both trials. However, taking into account the very rare, but serious side effect of agranulocytosis reported in one patient treated with mesalazine, our study emphasises the importance to prescribe even drugs with a very good safety profile such as mesalazine only for indications in which efficacy is proven.
Both trials as well as PREVENT 1 and 2 provide prospective data on true recurrence rates presenting evidence that recurrence is a rather frequent event within 1‐2 years after a previous attack. These data are useful for planning of future clinical trials evaluating whether other therapies prevent recurrent acute diverticulitis. We observed recurrence rates of 17% and 33% during 48 and 96 weeks of treatment respectively. The PREVENT trials generated similar recurrence rates of 27% each for patients who completed the study to week 104,18 a value comparable to our observation.
In conclusion, our data show that mesalazine was not superior to placebo to prevent recurrence of acute diverticulitis. Whether the anti‐inflammatory effects of mesalazine are too weak to prevent recurrence or whether the pathophysiology of acute diverticulitis cannot be compared to chronic inflammatory conditions such as ulcerative colitis remains highly speculative.
AUTHORSHIP
Guarantor of the article: Kruis.
Author contributions: All authors contributed to study design, analysis, drafting and finalisation of the manuscript, in particular: Greinwald, Kruis, Mohrbacher, Dilger, Spiller developed study concept and design; Kruis, Kardalinos, Eisenbach, Lukas, Vich, Bunganic, Pokrotnieks, Derova, Kondrackiene, Safadi, Tuculanu, Tulassay, Banai, Curtin, Dorofeyev, Zakko, Ferreira, Björck, Diez Alonso, Mäkelä recruited study patients; Greinwald, Kruis, Mohrbacher, Dilger, Spiller involved in generation, collection assembly, analysis and interpretation of data; Greinwald, Kruis, Mohrbacher, Dilger drafted the manuscript; all authors critically revised the manuscript for important intellectual content.
All authors have approved the final version of the article, including the authorship list.
Supporting information
ACKNOWLEDGEMENTS
Members of the independent data monitoring committee: SAG‐37 ‐ Professor Walter Lehmacher (statistician), Professor Volker Groß (gastroenterologist), Professor Andreas Tromm (gastroenterologist) SAG‐51 ‐ Martin Krauss (statistician), Professor Volker Groß (gastroenterologist), Professor Tilo Andus (gastroenterologist). Central reading centre: Professor Mathias Langer, Professor Elmar Kotter, Dr. Dieter Burger (University of Freiburg, Medical Centre, Department of Radiology). Statistical analysis: We thank Andrea Kreter for the statistical analysis of the data. Medical writing: Editorial assistance, comprising revision and finalisation of the draft manuscript prepared by the authors, was provided by Caroline Dunstall, freelance writer, and Dr. Michael Stieß, employee Dr. Falk Pharma GmbH. Grant support: Dr. Falk Pharma GmbH, Freiburg, Germany.
Declaration of personal interests: Kruis and Spiller received lecture fees and travel costs by Dr. Falk Pharma GmbH. Dilger, Greinwald and Mohrbacher are employees of Dr. Falk Pharma GmbH. All other authors have nothing to disclose.
Appendix 1.
Affiliations of the Authors
1.
Wolfgang Kruis, MD, Medical Department, Evangelisches Krankenhaus Kalk, Cologne, Germany; Vassilios Kardalinos, MD, Gastroenterologist in private practice, Stuhr, Germany; Thomas Eisenbach, MD, Gastroenterologist in private practice, Leverkusen, Germany; Milan Lukas, MD, IBD Clinical and Research Centre, ISCARE a.s., Praha, Czech Republic; Tomáš Vich, MD, Gastroenterologická ambulance, Nemocnice Hranice a.s., Hranice, Czech Republic; Ivan Bunganic, MD, Department of Gastroenterology, Gastro I. s.r.o., Presov, Slovakia; Juris Pokrotnieks, MD, Pauls Stradins Clinical University Hospital, Riga, Latvia; Jelena Derova, MD, Department of Gastroenterology, Latvian Maritime Medical Centre, Riga, Latvia; Jurate Kondrackiene, MD, Department of Gastroenterology, Medical Academy, Lithuanian University of Health Sciences, Kaunas, Lithuania; Rifaat Safadi, MD, Holy Family Hospital, Nazareth, Israel; Daniel Tuculanu, MD, Medical Center Tuculanu, Timisoara, Romania; Zsolt Tulassay, MD, Department of Gastroenterology, Semmelweis University Medical School Budapest, Budapest, Hungary; János Banai, MD, Department of Gastroenterology, Military Hospital, Budapest, Hungary; Austin Curtin, MD, Lismore Base Hospital, Lismore, NSW, Australia; Andrey E. Dorofeyev, MD, Center of Donetsk State Medical University, Donetsk, Ukraine; Salam F. Zakko, MD, Connecticut Gastroenterology Institute at Bristol Hospital, Bristol, CT, United States; Nelson Ferreira, MD, Washington County Hospital, Hagerstown, MD, United States; Stellan Björck, MD, Sahlgren's Hospital Surgical Clinic, Gothenburg, Sweden; Manuel M. Diez Alonso, MD, Hospital Principe de Asturias, Alcalá de Henares, Spain; Jyrki Mäkelä, MD, Oulu University Hospital, Oulu, Finland; Nicholas J. Talley, MD, University of Newcastle, Callaghan, NSW, Australia; Karin Dilger, MD, Drug Safety, Dr. Falk Pharma GmbH, Freiburg, Germany; Roland Greinwald, PhD, Research & Development, Dr. Falk Pharma GmbH, Freiburg, Germany; Ralf Mohrbacher, MSc, Research & Development, Dr. Falk Pharma GmbH, Freiburg, Germany; Robin Spiller, MD, Nottingham Digestive Diseases Centre Biomedical Research Unit, University of Nottingham, Nottingham, United Kingdom.
Kruis W, Kardalinos V, Eisenbach T, et al. Randomised clinical trial: mesalazine versus placebo in the prevention of diverticulitis recurrence. Aliment Pharmacol Ther. 2017;46:282–291. https://doi.org/10.1111/apt.14152
Funding information
This study was funded in full by Dr. Falk Pharma GmbH. Writing support was provided by Caroline Dunstall, freelance writer, funded by Dr. Falk Pharma GmbH, and Dr. Michael Stieß, employee Dr. Falk Pharma GmbH.
The Handling Editor for this article was Dr Colin Howden, and it was accepted for publication after full peer‐review.
For affiliations of the authors, see Appendix 1
REFERENCES
- 1. Tursi A, Papa A, Danese S. Review article: the pathophysiology and medical management of diverticulosis and diverticular disease of the colon. Aliment Pharmacol Ther. 2015;42:664‐684. [DOI] [PubMed] [Google Scholar]
- 2. Pfützer RH, Kruis W. Management of diverticular disease. Nat Rev Gastroenterol Hepatol. 2015;12:629‐638. [DOI] [PubMed] [Google Scholar]
- 3. Spiller R. Is it diverticular disease or is it irritable bowel syndrome? Dig Dis. 2012;30:64‐69. [DOI] [PubMed] [Google Scholar]
- 4. Humes DJ, Spiller RC. Review article: the pathogenesis and management of acute colonic diverticulitis. Aliment Pharmacol Ther. 2014;39:359‐370. [DOI] [PubMed] [Google Scholar]
- 5. Chapman J, Davies M, Wolff B, et al. Complicated diverticulitis: is it time to rethink the rules? Ann Surg 2005;242:576‐581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eglinton T, Nguyen T, Raniga S, Dixon L, Dobbs B, Frizelle FA. Patterns of recurrence in patients with acute diverticulitis. Br J Surg. 2010;97:952‐957. [DOI] [PubMed] [Google Scholar]
- 7. Broderick‐Villa G, Burchette RJ, Collins JC, Abbas MA, Haigh PI. Hospitalization for acute diverticulitis does not mandate routine elective colectomy. Arch Surg. 2005;140:576‐581. [DOI] [PubMed] [Google Scholar]
- 8. Hupfeld L, Burcharth J, Pommergaard HC, Rosenberg J. Risk factors for recurrence after acute colonic diverticulitis: a systematic review. Int J Colorectal Dis. 2017;32:611‐622. [DOI] [PubMed] [Google Scholar]
- 9. Comparato G, Fanigliulo L, Cavallaro LG, et al. Prevention of complications and symptomatic recurrences in diverticular disease with mesalazine: a 12‐month follow‐up. Dig Dis Sci. 2007;52:2934‐2941. [DOI] [PubMed] [Google Scholar]
- 10. Di Mario F, Aragona G, Leandro G, et al. Efficacy of mesalazine in the treatment of symptomatic diverticular disease. Dig Dis Sci. 2005;50:581‐586. [DOI] [PubMed] [Google Scholar]
- 11. Kruis W, Meier E, Schumacher M, et al. Randomised clinical trial: mesalazine (Salofalk granules) for uncomplicated diverticular disease of the colon–a placebo‐controlled study. Aliment Pharmacol Ther. 2013;37:680‐690. [DOI] [PubMed] [Google Scholar]
- 12. Trespi E, Colla C, Panizza P, et al. Therapeutic and prophylactic role of mesalazine (5‐ASA) in symptomatic diverticular disease of the colon. Minerva Gastroenterol Dietol. 1999;245‐252. [PubMed] [Google Scholar]
- 13. Tursi A, Brandimarte G, Elisei W, et al. Randomised clinical trial: mesalazine and/or probiotics in maintaining remission of symptomatic uncomplicated diverticular disease–a double‐blind, randomised, placebo‐controlled study. Aliment Pharmacol Ther. 2013;38:741‐751. [DOI] [PubMed] [Google Scholar]
- 14. Tursi A, Brandimarte G, Giorgetti GM, Elisei W. Mesalazine and/or Lactobacillus casei in preventing recurrence of symptomatic uncomplicated diverticular disease of the colon: a prospective, randomized, open‐label study. J Clin Gastroenterol. 2006;40:312‐316. [DOI] [PubMed] [Google Scholar]
- 15. Tursi A, Brandimarte G, Giorgetti GM, Elisei W. Continuous versus cyclic mesalazine therapy for patients affected by recurrent symptomatic uncomplicated diverticular disease of the colon. Dig Dis Sci. 2007;52:671‐674. [DOI] [PubMed] [Google Scholar]
- 16. Parente F, Bargiggia S, Prada A, et al. Intermittent treatment with mesalazine in the prevention of diverticulitis recurrence: a randomised multicentre pilot double‐blind placebo‐controlled study of 24‐month duration. Int J Colorectal Dis. 2013;28:1423‐1431. [DOI] [PubMed] [Google Scholar]
- 17. Stollman N, Magowan S, Shanahan F, Quigley EM, Group DI . A randomized controlled study of mesalamine after acute diverticulitis: results of the DIVA trial. J Clin Gastroenterol 2013;47:621‐629. [DOI] [PubMed] [Google Scholar]
- 18. Raskin JB, Kamm MA, Jamal MM, et al. Mesalamine did not prevent recurrent diverticulitis in phase 3 controlled trials. Gastroenterology. 2014;147:793‐802. [DOI] [PubMed] [Google Scholar]
- 19. Floch MH. Preventing diverticulitis: mesalamine may still be indicated in the decision. Gastroenterology. 2015;148:856. [DOI] [PubMed] [Google Scholar]
- 20. Centor RM. Acute uncomplicated diverticulitis: what to do until we have better data. Ann Intern Med. 2016;164:120‐121. [DOI] [PubMed] [Google Scholar]
- 21. Kroenke K, Spitzer RL, Williams JB. The PHQ‐15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. 2002;64:258‐266. [DOI] [PubMed] [Google Scholar]
- 22. Longstreth GF, Iyer RL, Chu LH, et al. Acute diverticulitis: demographic, clinical and laboratory features associated with computed tomography findings in 741 patients. Aliment Pharmacol Ther. 2012;36:886‐894. [DOI] [PubMed] [Google Scholar]
- 23. Stollman NH, Raskin JB. Diagnosis and management of diverticular disease of the colon in adults. Ad Hoc Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol. 1999;94:3110‐3121. [DOI] [PubMed] [Google Scholar]
- 24. Barbara G, Cremon C, Annese V, et al. Randomised controlled trial of mesalazine in IBS. Gut. 2016;65:82‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lam C, Tan W, Leighton M, et al. A mechanistic multicentre, parallel group, randomised placebo‐controlled trial of mesalazine for the treatment of IBS with diarrhoea (IBS‐D). Gut. 2016;65:91‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ritz JP, Lehmann KS, Loddenkemper C, Frericks B, Buhr HJ, Holmer C. Preoperative CT staging in sigmoid diverticulitis–does it correlate with intraoperative and histological findings? Langenbecks Arch Surg. 2010;395:1009‐1015. [DOI] [PubMed] [Google Scholar]
- 27. Gielens MP, Mulder IM, van der Harst E, et al. Preoperative staging of perforated diverticulitis by computed tomography scanning. Tech Coloproctol. 2012;16:363‐368. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


