Abstract
Background
Recognizing the factors associated with falling in Parkinson’s disease (PD) would improve identification of at-risk individuals.
Objective
To examine frequency of falling and baseline characteristics associated with falling in PD using the National Institute of Neurological Disorders and Stroke (NINDS) Exploratory Trials in PD Long-term Study-1 (NET-PD LS-1) dataset.
Methods
The LS-1 database included 1741 early treated PD subjects (median 4 year follow-up). Baseline characteristics were tested for a univariate association with post-baseline falling during the trial. Significant variables were included in a multivariable logistic regression model. A separate analysis using a negative binomial model investigated baseline factors on fall rate.
Results
728 subjects (42%) fell during the trial, including at baseline. A baseline history of falls was the factor most associated with post-baseline falling. Men had lower odds of post-baseline falling compared to women, but for men, the probability of a post-baseline fall increased with age such that after age 70, men and women had similar odds of falling. Other baseline factors associated with a post-baseline fall and increased fall rate included the Unified PD Rating Scale (UPDRS) Activities of Daily Living (ADL) score, total functional capacity (TFC), baseline ambulatory capacity score and dopamine agonist monotherapy.
Conclusion
Falls are common in early treated PD. The biggest risk factor for falls in PD remains a history of falling. Measures of functional ability (UPDRS ADL, TFC) and ambulatory capacity are novel clinical risk factors needing further study. A significant age by sex interaction may help to explain why age has been an inconsistent risk factor for falls in PD.
Keywords: Parkinson’s disease, Falls, NET-PD
1. Introduction
Falls are common in Parkinson’s disease (PD)[1–4], and both falls and postural instability negatively affect health-related quality of life in PD[5–7]. Persons with PD are about 3 times as likely to sustain a fall as their peers[8], and up to 50% of falls in PD result in injury[1–3,9,10]. Falls and fall-related injuries remain one of the top causes of increased health services utilization and costs for those with PD[11,12], and preventing and treating balance problems was recently identified as the top PD research priority by 1000 participants in the United Kingdom who understood the issues of living with PD[13].
The most consistently reported risk factor for falls in PD is a prior history of falls[3,4,6,14]. However, a meta-analysis of six prospective studies revealed that 21% of fallers had no history of falling[3], making it difficult to consistently identify at-risk individuals. Other factors associated with falls in PD include age, disease/motor severity measures, gait/axial impairments, self-rated disability, impaired cognition, fear of falling, and poor health-related quality of life, though these have been reported inconsistently[3,4,6,14–18]. Most research on falls in PD has been conducted in older cohorts with a longer disease duration (>5 years)[18], when freezing and postural instability are more prominent. Existing data on falls and falls risk is limited in early PD cohorts (<5 years disease duration). Voss et al. analyzed two randomized, clinical trials of early, untreated PD patients and found that lower PD-related quality of life, age and prior history of falls were associated with falls[6]. A recent study followed 91 newly diagnosed, falls-naïve PD patients for 3 years and found that slower gait, shorter stance time and Hoehn-Yahr score of III were the most significant predictors of a first fall[17]. A better understanding of clinical risk factors that predict falls in early PD, other than a prior history of falls, would improve identification of those at risk of falling and guide interventions to prevent further falls.
We analyzed the National Institute of Neurological Disorders and Stroke (NINDS) Exploratory Trials in Parkinson’s Disease (NET-PD) Long-term Study-1 (LS-1) data set[19], a large cohort of early (within 5 years of diagnosis), treated PD subjects. Because there are limited data on factors associated with falls in an early PD population but numerous factors correlated with falls in more advanced populations, we explored many possible associations. Our main aims were to determine the frequency of falling and to examine characteristics that separate fallers from non-fallers.
2. Methods
2.1 Subjects and Assessments
The NET-PD LS-1 study enrolled 1741 participants in a large, randomized, multicenter, placebo-controlled trial of creatine as a potential disease modifying agent for PD[19]. All participants were within 5 years of PD diagnosis and on dopaminergic medication at least 3 months and not more than 2 years. Exclusion criteria included “any significant features suggestive of a diagnosis of atypical parkinsonism.” The study was terminated early due to an interim analysis that found no significant benefit of creatine over placebo, so participants were followed for 3 to 6 years (median 4 years). All subjects participating in LS-1 provided written informed consent. The institutional review boards of the 45 participating sites approved the study. This analysis is based on the final LS-1 database, which was locked on May 5, 2014. Consistent with the primary trial findings, treatment with creatine was not related to post-baseline falling (p=0.78), thus, data were pooled across treatment assignment in LS-1 (either creatine or placebo).
Falling was defined as the presence of any of the following: the Unified PD Rating Scale (UPDRS) item 13 (Falling) greater than 0; UPDRS item 14 (Freezing) equal to 4 (“frequent falls from freezing”); or a report of an adverse event of “fall” or injury related to “fall”. The UPDRS item 13 (Falling) has 5 grades of severity: 0 – no falling, 1- rare falling, 2 – occasional, less than one per day, 3 – falls an average of once daily, and 4 – falls more than once daily. UPDRS items 13 and 14 ask only about falling or freezing that occurred “during the past week”. Adverse events were solicited through an open-ended question such as “What unusual symptoms or medical problems have you experienced since the last visit?” There was one subject who was identified as having fallen based on UPDRS item 14 equal to 4, that would not otherwise have been counted based on UPDRS item 13.
The UPDRS was assessed at baseline and at months 3, 12, 24, 36, 48, 60 and 72, although not all subjects were expected to have assessments beyond 36 months. All other baseline assessments demographic characteristics used as variables in this analysis are listed in supplementary eTable 1.
2.2 Statistical Methods
Because the precise fall time was often unknown and the ascertainment of falls on the UPDRS was only annually, a survival analysis approach to model the time to fall was not conducted. A heat map of the pattern of falls over time (supplementary eFigure 1) demonstrates the intermittent nature of falls in this study (i.e. a participant who falls at one assessment is not guaranteed to fall at the next assessment).
All analyses were conducted in SAS version 9.3. Baseline demographic and clinical characteristics above were independently tested for an association with the probability of ever falling post-baseline. For binary or categorical variables, the univariate association was tested with a chi-square test. For continuous baseline characteristics, an unadjusted logistic regression was used. Given the large sample size, the statistical significance was set at alpha of 0.00125 for the univariate comparisons (Bonferroni adjustment 0.05/40 univariate comparisons). Variables significant in the univariate analyses were included in a multivariable logistic regression model of the probability of ever falling. For variables that measured the same domain (motor, ADL, quality of life, cognition, dopaminergic therapy, function), the multivariable regression was conducted repeatedly by including only one of the measures from that domain to ensure that there were no redundancies or collinearities. Preliminary exploratory graphics suggested an interaction by sex and age group, so an interaction term for age and sex was considered. As a sensitivity analysis, automated selection procedures (stepwise p=0.01 for entry and p< 0.1 to stay) was also used to select the final model.
To examine the baseline covariates associated with the rate of post-baseline falls over the long-term follow-up, a negative binomial regression model was used. Since the amount of follow-up varied widely among participants, the rate of falls, rather than the total number of falls was modelled by including an offset (log of days of follow- up). Our dataset showed an excess number of ‘zero’ counts (58% of participants experienced no post-baseline falls). A zero-inflated Poisson (ZIP) regression model was considered, but the negative binomial regression model was used because it outperformed the ZIP model (lower Akaike information criterion (AIC); SAS procedure PROC GENMOD (distribution=negbin link=log)). A similar approach to model building was used to select the final negative binomial model. Because of the multiple models fit during the model building process, an alpha level of 0.001 was set for interpreting the significance of covariates in the in the final reported models.
3. Results
Including baseline assessment, 728 (42%) of the 1,741 subjects reported a fall at any point during the trial, including the baseline assessment. Of these 728 subjects, 353 (48%) reported falls at only 1 visit, 157 (22%) reported falls at 2 visits, and 218 (30%) reported falls at three or more visits. 609 (35%) participants experienced falls based on the UPDRS items. 322 (18%) participants reported an adverse event related to falling, 119 (7%) of whom were not captured from the UPDRS questions. At baseline, 132 (8%) participants reported a fall.
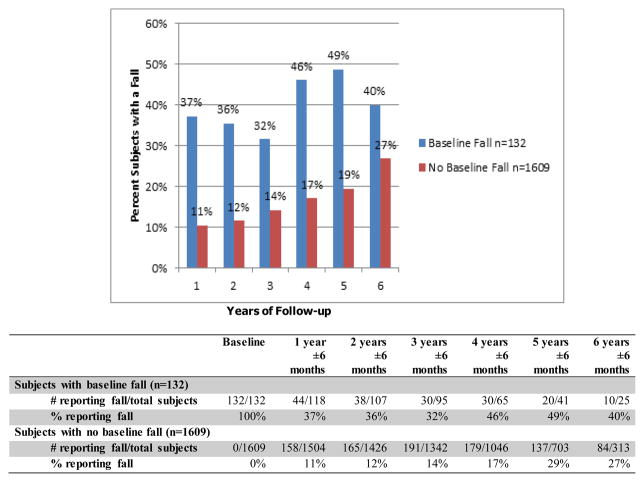
Figure 1 shows the percentage of subjects with falls at each yearly assessment, based on whether or not they reported a fall at baseline. In the group that reported a baseline fall, the proportion of subjects falling in subsequent years ranged from 32–49%. In the group that did not report a baseline fall, the percentage of subjects who fell over time increased from 11% at year 1 to 27% at year 6.
Figure 1.
Percentage of Falls Over Follow-up Time
For our analysis, only subjects who reported fall(s) after the baseline visit were classified as fallers. The 37 (2.1%) subjects who reported a fall at the baseline visit only were included in the non-fallers group, leaving 691 (40%) participants in the fallers group. Table 1 shows the baseline characteristics for post-baseline fallers versus non-fallers.
Table 1.
Baseline Characteristics of Post-baseline Fallers versus Non-Fallers (n=1741)
| Subjects Reporting a Post-Baseline Fall | ||||||
|---|---|---|---|---|---|---|
| Baseline Characteristics | Yes (N=691) | No** (N=1050) | Unadjusted Odds Ratio (95% CI) | Univariate p- value | ||
| Mean | SD | Mean | SD | |||
| Age in years | 63.4 | 9.8 | 60.7 | 9.4 | 1.16 (1.10,1.22) 5 year change |
<.0001 |
| Years since PD Diagnosis | 1.6 | 1.1 | 1.5 | 1.1 | 1.03 (0.94,1.13) | 0.51 |
| Years since PD symptom Onset | 3.3 | 2.1 | 3.3 | 2.3 | 0.99 (.95, 1.04) | 0.72 |
| UPDRS total | 29.21 | 12.26 | 24.27 | 10.31 | 1.22 (1.16, 1.27) 5 point change |
<.0001 |
| UPDRS motor | 19.49 | 8.92 | 16.62 | 7.78 | 1.23 (1.16, 1.30) 5 point change |
<.0001 |
| UPDRS ADL | 8.20 | 4.31 | 6.48 | 3.54 | 1.12 (1.09, 1.15) | <.0001 |
| UPDRS mental | 1.52 | 1.40 | 1.18 | 1.34 | 1.20 (1.12, 1.28) | <.0001 |
| Average Tremor score | 0.43 | 0.33 | 0.43 | 0.30 | 1.00 (0.74, 1.36) | 0.98 |
| Average PIGD score | 0.43 | 0.36 | 0.28 | 0.25 | 5.58 (3.93, 7.91) | <.0001 |
| Ambulatory Capacity | 2.17 | 1.79 | 1.40 | 1.27 | 1.41 (1.32, 1.51) | <.0001 |
| Total Functional Capacity (TFC)* | 11.7 | 1.6 | 12.3 | 1.2 | 0.74 (0.69, 0.80) | <.0001 |
| EQ-5D total | 0.78 | 0.19 | 0.84 | 0.17 | 0.19 (0.11, 0.33) | <.0001 |
| SCOPA-Cog* | 29.48 | 5.56 | 30.79 | 5.14 | 0.96 (0.94, 0.97) | <.0001 |
| SDMT Total | 42.43 | 12.13 | 45.77 | 11.26 | 0.98 (0.97, 0.98) | <.0001 |
| Total Daily LED (mg) | 410 | 273 | 366 | 222 | 1.08 (1.04, 1.12) 100mg change |
0.0002 |
| % | Freq | % | Freq | Unadjusted Odds Ratio | p-value | |
| Male | 59% | 410 | 68% | 713 | 0.69 (0.57, 0.84) | 0.0003 |
| Non-Hispanic white | 89% | 616 | 91% | 955 | 0.82 (0.59, 1.12) | 0.21 |
| More than 12 years of Education | 81% | 563 | 83% | 872 | 0.90 (0.70, 1.15) | 0.40 |
| Orthostatic hypotension | 29% | 198 | 22% | 232 | 1.42 (1.14, 1.77) | 0.002 |
| Modified Rankin score | 2.19 (1.78, 2.69) | <.0001 | ||||
| 0 | 1% | 4 | 2% | 19 | ||
| 1 | 69% | 479 | 82% | 865 | ||
| 2 | 27% | 186 | 15% | 159 | ||
| 3 | 3% | 22 | 1% | 7 | ||
| UPDRS item 13 > 0 | 14% | 95 | 4% | 37 | 4.36 (2.95, 6.47) | <.0001 |
| UPDRS item 14 > 0 | 18% | 122 | 10% | 103 | 1.97 (1.49, 2.61) | <.0001 |
| UPDRS item 15 > 0 | 77% | 530 | 67% | 706 | 1.60 (1.29, 1.99) | <.0001 |
| UPDRS item 29 > 0 | 48% | 337 | 34% | 359 | 1.84 (1.51, 2.24) | <.0001 |
| UPDRS item 30 > 0 | 28% | 194 | 16% | 166 | 2.08 (1.65, 2.63) | <.0001 |
| Polypharmacy | 83% | 572 | 79% | 834 | 1.24 (0.97, 1.59) | 0.08 |
| Beck Depression Inventory >17 | 6% | 39 | 4% | 44 | 1.37 (0.88, 2.13) | 0.16 |
| PDQ-39 item 9 “Fear of Falling” > 0 | 30% | 204 | 14% | 147 | 2.57 (2.03, 3.27) | <.0001 |
| Amantadine | 7% | 49 | 9% | 92 | 0.79 (0.55, 1.14) | 0.21 |
| Antipsychotic | 0% | 1 | 0% | 2 | -- | 1 |
| Sedatives | 14% | 99 | 16% | 168 | 0.88 (0.67, 1.15) | 0.34 |
| Narcotics | 4% | 28 | 3% | 33 | 1.30 (0.78, 2.17) | 0.31 |
| Antidepressants | 26% | 182 | 18% | 186 | 1.66 (1.32, 2.09) | <.0001 |
| SSRI only | 20% | 138 | 12% | 130 | 1.77 (1.36, 2.29) | <.0001 |
| Anticholinergics | 10% | 71 | 8% | 84 | 1.32 (0.95, 1.83) | 0.10 |
| Vitamin B12 | 8% | 55 | 6% | 67 | 1.27 (0.88, 1.84) | 0.21 |
| Vitamin D | 18% | 122 | 15% | 160 | 1.19 (0.92, 1.54) | 0.18 |
| Dopaminergic Therapy Type | 99% | 684 | 99% | 1043 | 0.77 (0.26, 2.29) | 0.63 |
| Levodopa alone, n=671 | 44% | 301 | 35% | 370 | 1.75 (1.41, 2.18) | <.0001 |
| Levodopa+DA, n=322 | 22% | 149 | 16% | 173 | 1.85(1.42, 2.43) | |
| Dopamine agonist alone, n=741 (reference group) | 34% | 235 | 48% | 506 | -- | |
| Living in Local Community | 99% | 684 | 99% | 1043 | 0.77 (0.26, 2.29) | 0.63 |
| Hospitalization in 3 months prior to baseline | 5% | 31 | 4% | 42 | 1.13 (0.70, 1.81) | 0.61 |
Higher scores represent “better” clinical status.
Non-faller includes a subset of 37 (2.1%) patients who reported a fall at baseline, but no post baseline fall. Odds Ratios are given for a 1-unit change unless otherwise stated.
Univariate test p-values are Chi-square test for binary or categorical variables or unadjusted logistic regression of the probability of falling for continuous variables. Significance level is 0.00125 for univariate tests. UPDRS=Unified Parkinson Disease Rating Scale; PIGD=Postural Instability Gait Disorder; PDQ -39= Parkinson’s disease Questionnaire; EQ-5D= EuroQOL 5 Dimensional Questionnaire; SCOPA-Cog=Scales for Outcomes in Parkinsons-Cognition; SDMT=Symbol Digit Modalities Test; LED= levodopa equivalence dose; BDI=Beck Depression Inventory; Sedatives= Benzodiazepines + Zolpidem; SSRI= selective serotonin reuptake; DA=dopamine agonist.
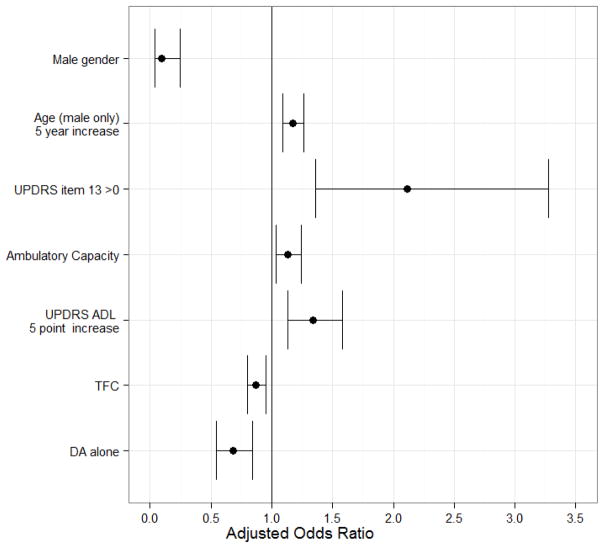
The multivariable logistic regression model investigating baseline characteristics on the probability of a post-baseline fall included 1730 subjects (11 subjects had some missing baseline data and were not included in the model). The adjusted odds ratios and confidence intervals are provided in Figure 2. The following baseline covariates were statistically significant: UPDRS item 13 (falling)>0 at baseline (p=0.0008), male sex (p<0.0001), age by sex interaction (p<0.0001), UPDRS ADL (p=0.0006), and the use of dopamine agonist alone (p=0.001). The TFC (p=0.0016) and ambulatory capacity (p=0.0063) were not statistically significant at the 0.001 level, but were retained in the final model because of other model selection criteria (AIC was smaller than the model without them). Females were more likely to fall compared to males (OR=10.75, 95% CI = [4.11, 28.14]). However, there was a significant interaction between sex and age. For females, there was no effect of age (p=0.77). For males, the probability of a post-baseline fall increased with age (ever 5 years, odds increase by 1.2 compared to males 5 years younger, p<0.0001).
Figure 2. Adjusted Odds Ratio of A Post-baseline Fall with 95% Confidence Intervals, multiple logistic model.
The model is logit(probability of ever falling)=0.931 + 0.7487*UPDRSFalling -2.3748*Male + 0.0317*Male*Age + 0.1259*AmbulCapacity + 0.0582*UPDRSADL-0.1365*TFC - 0.3876*DAA (N=1730, 683 subjects ever fall).
The adjusted odds ratios and confidence intervals are OR=2.11, 95% CI [1.36,3.28] for patients reporting baseline UPDRS item 13 > 0 (UPDRS Falling); OR=0.09, 95% CI [0.04,0.24] for Males versus Females; OR=1.17, 95% CI [1.09,1.26] per 5 year increase in age for males; OR=1.13, 95% CI [1.04,1.24] per 1-unit increase in ambulatory capacity; OR=1.34, 95% CI [1.13,1.58] per 5-unit increase in UPDRS ADL score (UPDRSADL); OR=0.87, 95% CI [0.80,0.95] per 1-unit increase in baseline TFC; OR=0.68, 95% CI [0.55,0.84] if on dopamine agonist alone at baseline (DAA); all else held constant. For TFC higher scores represent “better” clinical status at baseline. For all else, higher scores are “worse”.
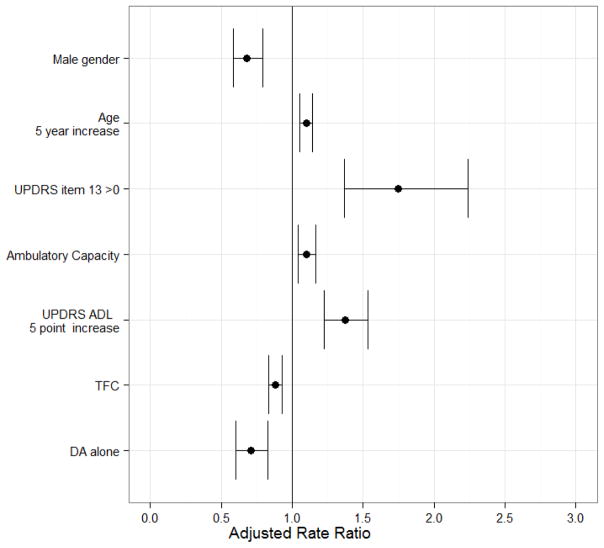
In the negative binomial regression model (Figure 3), the following baseline covariates were significantly associated with the post-baseline fall rate: UPDRS item 13 (falling) > 0 at baseline (p<0.0001), male sex (p<0.0001), age (p<0.0001), ambulatory capacity (p=0.001), UPDRS ADL (p<0.0001), the use of dopamine agonist alone (p<0.0001), and TFC (p<0.0001). Unlike the logistic model, the interaction of age and gender was not significant after adjusting for the main effects of age and sex. Therefore, the effect of age on the probability of ever falling affects only men, while age increases the rate of falling similarly for both men and women after adjusting for all else.
Figure 3. Adjusted Fall Rate Ratio (RR) and 95% Confidence Intervals, negative binomial model.
Adjusted Odds Ratios estimated from negative binomial regression model of the rate of post-baseline falls (N=1730). Adjusted rate ratios are RR=1.75, 95% CI [1.37, 2.24] for patients reporting baseline UPDRS item 13 > 0 (versus those who did not); RR=0.68, 95% CI [0.59, 0.79] for males; RR=1.10, 95% CI [1.05, 1.14] per 5-year increase in age; RR=1.10, 95% CI [1.04,1.16] per 1-point increase in ambulatory capacity; RR=1.37, 95% CI [1.23,1.53] per 5-point increase in UPDRS ADL; RR=0.88, 95% CI [0.83,0.93] per 1-point increase in TFC; RR=0.71, 95% CI [0.60, 0.83] for use of dopamine agonist alone at baseline; all else being held constant. For TFC higher scores represent “better” clinical status at baseline. For all else, higher scores are “worse”.
4. Discussion
We analyzed a large, early PD clinical trial cohort (>1700 subjects, mean~1.5 years since diagnosis) being treated with dopaminergic therapy and found that over a median follow-up of 4 years, falls were common, occurring in 42% of participants. In a recent systematic review of 22 PD studies with prospective fall collection data, the proportion of subjects who fell ranged from 35–90%, with a mean of 60.5%[18]. Compared to the NET-PD LS-1 cohort, the majority of the studies included in this review enrolled older PD subjects with longer disease duration. A higher percentage of these subjects would therefore be expected to experience falls. Our cohort was in the “honeymoon” period, where PD symptoms typically respond well to medication and falls are thought to be less prominent. Voss et al. analyzed two randomized, clinical trials of early, untreated PD patients and reported a fall frequency of 23%[6]. Our results, combined with the findings from Voss et al., suggest that falls occur frequently in PD, even early in the disease course.
In this study, the baseline factor most significantly associated with falling (OR=2.11) was a fall at baseline (as reported on the UPDRS item 13 greater than zero). This is consistent with what has been reported in the PD fall literature [3,6,8,14], but is of limited clinical utility. Identification of new clinical risk factors associated with falls that could be modified or targeted for intervention is an unmet need in PD. Lord et al. [17] found that gait parameters such as speed and stance time could predict a first fall in PD patients. Because the NET-PD LS-1 study did not specifically assess gait parameters, we could not confirm these findings. Instead, we discovered several novel factors that increased the probability of falling in PD.
We found a unique interaction between age and sex on the probability of a post-baseline fall in our multiple logistic regression model. In general, men were much less likely to experience a post-baseline fall (OR=0.09) compared to women, regardless of age. For men, however, the probability of a post-baseline fall increases with age so that by age 70, men and women have similar odds of experiencing a fall. Age is consistently associated with falling in the general population [20]. While age was also a predictor of falls in the Voss et al. analysis of the two NET-PD futility trials (Futility Study 1 [FS-1] and Futility Study 2 [FS-TOO])[6], age has been an inconsistent predictor of falls in the PD population [3]. This interaction between age and sex in PD may provide an explanation for the inconsistency of the reported association or lack thereof between age and probability of falling in PD. In our negative binomial model, which looked at baseline covariates on falling rate, there was no interaction between age and sex. Instead, increasing age was associated with a higher rate of falling, regardless of sex, though men had lower fall rates compared with women. This suggests that age and sex may contribute differently to the probability of ever falling (predicting the next fall) vs. fall frequency (identifying recurrent fallers).
Worsened ability to perform daily functions at the start of the trial, as assessed by the UPDRS ADL score and the total functional capacity (TFC) score, was associated with both an increased probability of a post-baseline fall and a more frequent fall rate in this study. The TFC, used more commonly in studies of Huntington’s disease, assesses one’s capacity to handle job, financial, daily living and domestic responsibilities. Higher TFC scores indicate better status. Higher (worse) UPDRS ADL score has previously been found to be associated with falls in PD[15,21], but to our knowledge, this is the first report of the TFC being associated with fall rate in either PD or Huntington’s disease. Further study would be helpful to explore whether these might useful clinical scales to predict fall risk in PD. Given that general functional abilities depend on motor function, it is surprising that motor scores are not a predictor of fall probability or fall rate. General functional ability scales may capture something that motor scales do not.
The ambulatory capacity score is a relatively new construct that has been validated against other objective and self-reported measures of gait and balance in PD[22]. It is calculated as the sum of UPDRS items 13 (falling), 14 (freezing), 15 (walking), 29 (gait), and 30 (postural stability). Each 1-point increase in the baseline ambulatory capacity score in our analysis increased the odds of experiencing a post-baseline fall and increased fall rate. Given that the ambulatory capacity score itself includes UPDRS item 13 (falling) it is not surprising that it was associated with falls. However, ambulatory capacity remained in both the multiple logistic and negative binomial models after adjusting for UPDRS item 13 (falling), suggesting that items 14, 15, 29 and 30 may be the individual UPDRS items most associated with falling.
Finally, our data show that being on a dopamine agonist alone (compared to either levodopa alone or a combination of levodopa with a dopamine agonist) is associated with decreased odds of a post-baseline fall and with a lower fall rate. This contrasts with the findings of Allcock et al.[23], who found that dopamine agonist use was associated with increased falling frequency in PD. However, their study population was older with a longer disease duration. In general, dopamine agonist monotherapy in PD is used for patients who are younger and in earlier stages of disease. Most patients enrolled on a dopamine agonist alone in LS-1 later went on to either levodopa alone or combination therapy with levodopa and an agonist. It is possible that patients with more severe symptoms were preferentially placed initially on levodopa rather than an agonist. We cannot completely rule out a protective effect of dopamine agonists on falls, but dopamine agonist monotherapy in our analysis is more likely to be a marker of disease severity. Increased LED dose and a higher (worse) UPDRS motor score at baseline were both associated with an increased probability of falling post-baseline in univariate comparisons, but the use of an agonist alone was a stronger predictor in the multiple logistic and negative binomial models.
The biggest strength of this study was the large cohort size. The main limitation was our method of ascertainment, which was sporadic and likely underestimated the total number of falls. The UPDRS captures falls only within the past week, and since most study visits were a year apart, we likely captured only severe falls as an adverse event. However, this limitation only strengthens our conclusion that falls occur frequently in early PD.
5. Conclusions
Falls are common in early, treated PD, affecting approximately 40% of subjects in our analysis. The biggest risk factor influencing the probability of falling as well as fall rate remains a history of falling, even after adjusting for other characteristics. The strength of the association we observed is likely underestimated since we did not formally assess “history of falls” beyond UPDRS items at baseline or adverse effect reporting once a year. New findings in this study may explain previous inconsistencies about the contribution of age and how it intertwines with sex: women of any age appear to be at a high risk of falling, while for men, the probability of falling increases with age. Fall screening or fall prevention measures could thus be more effective if directed towards women of any age or men over the age of 70. In this exploratory study, we also identified two new measures (TFC and ambulatory capacity) associated with falling in PD and add to the evidence associating the UPDRS ADL score with falls. These measures could potentially be used clinically to identify PD patients at risk of future falls, though further research will need to validate our findings and establish cut-off scores.
Supplementary Material
Highlights.
Using the NET-PD LS-1 dataset, we examined factors associated with falling in PD
728 subjects (42%) fell during the trial, including those reporting falls at baseline.
The biggest risk factor for falls in PD remains a history of falling
Compared to women, men were less likely to fall until after age 70, when men and women had similar odds of falling
The UPDRS Activities of Daily Living subscale, total functional capacity (TFC) and ambulatory capacity are novel clinical risk factors needing further study.
Acknowledgments
The NET-PD LS-1 Trial Investigators were supported, in part, by grants U01NS043127, U01NS043128, and U10NS44415-44555 from the National Institute of Neurologic Disorders and Stroke.
Funding/Support: The NET-PD LS-1 Trial Investigators were supported, in part, by grants U01NS043127, U01NS043128, and U10NS44415-44555 from the National Institute of Neurologic Disorders and Stroke.
Author Contributions
Conception and design of the study: Chou, Elm, Aminoff, Liang, Hauser, Sudarsky, Voss, Juncos, Boyd, Bodis-Wollner, Morgan, Wills, Parashos; Acquisition of data: All authors; Analysis of data: Elm; Interpretation of data: All authors
Drafting the article: Chou, Elm, Parashos; Revising it critically for important intellectual content: All authors
Final approval of the version to be submitted: All authors
Declaration of Interest
Dr. Aminoff has received royalties from publishing Neurology & General Medicine (Elsevier, 1989–2015), Electrodiagnosis in Clinical Neurology (Elsevier, 1980–2015), Clinical Neurology (McGraw-Hill, 1989–2015), chapters in Cecil Textbook of Medicine (W.B. Saunders; 2004, 2008, 2012), Harrison’s Principles of Internal Medicine (McGraw-Hill, 1994–2015), Handbook of Clinical Neurology (Elsevier; 2003–2015), The Netter Collection: Nervous System (Elsevier, 2013), Current Medical Diagnosis & Treatment (McGraw-Hill, 1985–2015) and Oxford University Press (Brown-Sequard); has received honoraria for lectures or educational activities not funded by industry; serves as editor-in-chief, Neurology section, Uptodate, for which he receives royalties; receives support from the National Parkinson Foundation.
Dr. Bodis-Wollner has received research support from the Michael J. Fox Foundation. He has received royalties from publishing the following books: Dopaminergic Mechanisms in Vision, Non-motor dysfunction in Parkinson Disease (2nd edition), and Visual Evoked potentials.
Dr. Boyd receives research support from AbbVie, Auspex, Biotie, NIH, MJ Fox Foundation, Cure Huntington Disease Initiative, and the Frederick C. Binter Center for Parkinson’s Disease and Movement Disorders; and serves as a consultant for AbbVie, Auspex, and Lundbeck.
Dr. Chou receives research support from NIH grant NS091856-01 and the Michael J. Fox Foundation, participates as a site-PI in clinical trials sponsored by the Parkinson Study Group (STEADY-PDIII and SURE-PD3), receives royalties from UpToDate, Springer Publishing and Demos Health and serves as a consultant for Accordant, Advanced Medical, and Cynapsus Therapeutics.
Dr. Christine receives research support from the Parkinson Study Group to support work in a clinical study (STEADY-PDIII) as well as from the Michael J. Fox Foundation to support participation in a clinical trial and clinical research.
Dr. Elm receives research support from the NIH and Remedy Pharmaceuticals, and has served as a consultant for Remedy Pharmaceuticals and Teva.
Dr. Fang receives support from NIH (grant NS044555), participates as a site-PI in clinical trials sponsored by the Parkinson Study Group (SURE-PD3), and receives consulting support from GLG, Inc.
Dr. Hauser has received honoraria or payments for consulting, advisory services or speaking services over the past 12 months from AbbVie, Acroda, Auspex, AstraZeneca, Biotie Therapeutics, Impax Laboratories, Neurocrine, Indus, Ipsen Biopharmaceuticals, HealthLogix, Lundbeck, Merck/MSD, Orion, USWorldMeds, Pfizer, Sarepta, ProPhase, Teva, Cowen and Company, Inventive, Cynapsus, Michael J. Fox Foundation. Dr. Hauser has received royalties in the past 12 months from the University of South Florida and is supported in part by a Center of Excellence Grant from the National Parkinson’s Foundation. Dr. Hauser’s Institution has received research support over the past 12 months from AstraZeneca, Biotie Therapeutics, Chelsea, Civitas, Cynapsus, Impax Laboratories, Kyoway Hakko Kirn Pharma, the Michael J. Fox Foundation for Parkinson’s Research, NINDs, The Parkinson Study Group, Teve Neuroscience, Pharma2B and Revance Therapeutics.
Dr. Juncos received research support from the NIH-NINDS 5U10NS044464-07; NICHHD R01HD02990910A2, Chelsea Therapeutics, and the American Parkinson Disease Emory Center of Research Excellence in Parkinson Disease
Dr. Lee receives research support from the Michael J. Fox Foundation, participates as a site-PI in clinical trials sponsored by the Parkinson Study Group (STEADY-PDIII).
Dr. Liang is employed by and owns equity in Neurocrine Biosciences, Inc.
Dr. Mari receives research support from the NIH (U01NS082133), the National Parkinson Foundation, and the Michael J. Fox Foundation, participates as a site-PI in clinical trials sponsored by Avid Radiopharmaceuticals (AV-133), Quintiles (LCIG) and Parkinson Study Group (STEADY-PDIII), and serves as a consultant for NAVIDEA.
Dr. Morgan has received honoraria for speaking for Impax and Teva. He has served as a consultant for Abbvie, Acorda, Cynapsus, Impax, Lundbeck, National Parkinson Foundation, Teva, UCB. He has also received compensation for serving as an expert witness in various neurological legal cases. He has served as a site PI or sub-I for clinical trials with Abbvie, Acadia, Acorda, Biotie, CHDI, Cynapsus, Impax, Kyowa, Lundbeck, NIH, NPF, PSG, and Serina.
Dr. Parashos received research support from the NIH, Park Nicollet Foundation, National Parkinson Foundation, Astellas Pharma US, IMPAX Pharmaceuticals, Adamas Pharmaceutical, Accorda Therapeutics, honoraria from the Parkinson’s Disease Foundation, royalties from Oxford University Press, and is a shareholder of St. Jude Medical, and the Minneapolis Clinic of Neurology, Ltd.
Dr. Simon receives grant support from the NIH (NINDS; 1R01NS086352; 1P50NS094733), the National Parkinson Foundation (NPF) and the Westin Brain Institute. Dr. Simon participates in clinical trials sponsored by Edison Pharmaceuticals and by Lysosomal Therapeutics. Dr. Simon receives honoraria for grant review committee service from the NIH, Michael J. Fox Foundation and the Westin Brain Institute, and the serves as a consultant for Lysosomal Therapeutics.
Dr. Sudarsky has no financial disclosures to report.
Dr. Umeh has received research support from the Parkinson’s Research Fund, Brigham and Women’s Hospital.
Dr. Voss has no financial disclosures to report. She is an employee of Merck (salary and stock options).
Dr. Wielinski has no financial disclosures to report.
Dr. Wills has received research support from the NIH, the MDA, ALSA, has consulting agreements with Accordant, a CVS/Caremark disease management company and has participated in clinical trials funded by Schering-Plough/Merck, Pfizer and Civitas/Acorda therapeutics.
Footnotes
Financial Disclosure/COI: The authors report no other financial disclosures related to the content of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ashburn A, Stack E, Pickering RM, Ward CD. A community-dwelling sample of people with Parkinson’s disease: characteristics of fallers and non-fallers. Age Ageing. 2001 Jan;30(1):47–52. doi: 10.1093/ageing/30.1.47. [DOI] [PubMed] [Google Scholar]
- 2.Balash Y, Peretz C, Leibovich G, Herman T, Hausdorff JM, Giladi N. Falls in outpatients with Parkinson’s disease: frequency, impact and identifying factors. J Neurol. 2005 Nov;252(11):1310–5. doi: 10.1007/s00415-005-0855-3. [DOI] [PubMed] [Google Scholar]
- 3.Pickering RM, Grimbergen YA, Rigney U, Ashburn A, Mazibrada G, Wood B, et al. A meta-analysis of six prospective studies of falling in Parkinson’s disease. Mov Disord. 2007 Oct 15;22(13):1892–900. doi: 10.1002/mds.21598. [DOI] [PubMed] [Google Scholar]
- 4.Wood BH, Bilclough JA, Bowron A, Walker RW. Incidence and prediction of falls in Parkinson’s disease: a prospective multidisciplinary study. J Neurol Neurosurg Psychiatry. 2002 Jun;72(6):721–5. doi: 10.1136/jnnp.72.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marras C, McDermott MP, Rochon PA, Tanner CM, Naglie G, Lang AE, et al. Predictors of deterioration in health-related quality of life in Parkinson’s disease: results from the DATATOP trial. Mov Disord. 2008 Apr 15;23(5):653–9. doi: 10.1002/mds.21853. quiz 776. [DOI] [PubMed] [Google Scholar]
- 6.Voss TS, Elm JJ, Wielinski CL, Aminoff MJ, Bandyopadhyay D, Chou KL, et al. Fall frequency and risk assessment in early Parkinson’s disease. Parkinsonism Relat Disord. 2012 Aug;18(7):837–41. doi: 10.1016/j.parkreldis.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahman S, Griffin HJ, Quinn NP, Jahanshahi M. Quality of life in Parkinson’s disease: the relative importance of the symptoms. Mov Disord. 2008 Jul 30;23(10):1428–34. doi: 10.1002/mds.21667. [DOI] [PubMed] [Google Scholar]
- 8.Deandrea S, Lucenteforte E, Bravi F, Foschi R, La Vecchia C, Negri E. Risk factors for falls in community-dwelling older people: a systematic review and meta-analysis. Epidemiology. 2010 Sep;21(5):658–68. doi: 10.1097/EDE.0b013e3181e89905. [DOI] [PubMed] [Google Scholar]
- 9.Gray P, Hildebrand K. Fall risk factors in Parkinson’s disease. J Neurosci Nurs. 2000 Aug;32(4):222–8. doi: 10.1097/01376517-200008000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Wielinski CL, Erickson-Davis C, Wichmann R, Walde-Douglas M, Parashos SA. Falls and injuries resulting from falls among patients with Parkinson’s disease and other parkinsonian syndromes. Mov Disord. 2005 Apr;20(4):410–5. doi: 10.1002/mds.20347. [DOI] [PubMed] [Google Scholar]
- 11.Temlett JA, Thompson PD. Reasons for admission to hospital for Parkinson’s disease. Intern Med J. 2006 Aug;36(8):524–6. doi: 10.1111/j.1445-5994.2006.01123.x. [DOI] [PubMed] [Google Scholar]
- 12.Vossius C, Nilsen OB, Larsen JP. Parkinson’s disease and hospital admissions: frequencies, diagnoses and costs. Acta Neurol Scand. 2010 Jan;121(1):38–43. doi: 10.1111/j.1600-0404.2009.01239.x. [DOI] [PubMed] [Google Scholar]
- 13.Deane KH, Flaherty H, Daley DJ, Pascoe R, Penhale B, Clarke CE, et al. Priority setting partnership to identify the top 10 research priorities for the management of Parkinson’s disease. BMJ Open. 2014;4(12):e006434. doi: 10.1136/bmjopen-2014-006434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bloem BR, Grimbergen YA, Cramer M, Willemsen M, Zwinderman AH. Prospective assessment of falls in Parkinson’s disease. J Neurol. 2001 Nov;248(11):950–8. doi: 10.1007/s004150170047. [DOI] [PubMed] [Google Scholar]
- 15.Almeida LR, Sherrington C, Allen NE, Paul SS, Valenca GT, Oliveira-Filho J, et al. Disability is an Independent Predictor of Falls and Recurrent Falls in People with Parkinson’s Disease Without a History of Falls: A One-Year Prospective Study. J Parkinsons Dis. 2015;5(4):855–64. doi: 10.3233/JPD-150651. [DOI] [PubMed] [Google Scholar]
- 16.Hiorth YH, Larsen JP, Lode K, Pedersen KF. Natural history of falls in a population-based cohort of patients with Parkinson’s disease: an 8-year prospective study. Parkinsonism Relat Disord. 2014 Oct;20(10):1059–64. doi: 10.1016/j.parkreldis.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 17.Lord S, Galna B, Yarnall AJ, Coleman S, Burn D, Rochester L. Predicting first fall in newly diagnosed Parkinson’s disease: Insights from a fall-naive cohort. Mov Disord. 2016 Dec;31(12):1829–36. doi: 10.1002/mds.26742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen NE, Schwarzel AK, Canning CG. Recurrent falls in Parkinson’s disease: a systematic review. Parkinsons Dis. 2013;2013:906274. doi: 10.1155/2013/906274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Writing Group for the NETiPDI. Kieburtz K, Tilley BC, Elm JJ, Babcock D, Hauser R, et al. Effect of creatine monohydrate on clinical progression in patients with Parkinson disease: a randomized clinical trial. JAMA. 2015 Feb 10;313(6):584–93. doi: 10.1001/jama.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Todd C, Skelton D. What are the main risk factors for falls among older people and what are the most effective interventions to prevent these falls? WHO Regional Office for Europe Health Evidence Network; 2004. [Google Scholar]
- 21.Parashos SA, Wielinski CL, Nance MA, Erickson-Davis C, Lenarz S. Association Between the UPDRS and Falls and Near Falls in Parkinson’s Disease. Mov Disord. 2012;27(Supp 1):S522. [Google Scholar]
- 22.Parashos SA, Elm J, Boyd JT, Chou KL, Dai L, Mari Z, et al. Validation of an Ambulatory Capacity Measure in Parkinson Disease: A Construct Derived from the Unified Parkinson’s Disease Rating Scale. J Parkinsons Dis. 2014 Oct 13; doi: 10.3233/JPD-140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allcock LM, Rowan EN, Steen IN, Wesnes K, Kenny RA, Burn DJ. Impaired attention predicts falling in Parkinson’s disease. Parkinsonism Relat Disord. 2009 Feb;15(2):110–5. doi: 10.1016/j.parkreldis.2008.03.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.