Abstract
Background
Radiotherapy is the most common curative cancer therapy used for elderly patients with localized prostate cancer. However, the effectiveness of this approach has not been established. The purpose of this study is to evaluate the long-term outcomes of primary radiotherapy compared with conservative management in order to facilitate treatment decisions.
Method
This population-based study consisted of 57,749 patients with T1-T2 prostate cancer diagnosed during 1992-2007. We utilized an instrumental variable (IV) analytical approach with competing risk models to evaluate the outcomes of primary radiotherapy vs. conservative management. The IV was comprised of combined health service areas with high and low-use areas corresponding to the top and bottom tertile in radiotherapy usage rates.
Results
In patients with low/intermediate risk prostate cancer, 10-year prostate cancer-specific and overall survival was similar in high and low-radiotherapy use areas (96.1% vs. 95.4% and 56.6% vs. 56.3% respectively). In patients with high-risk disease, however, areas with high-radiotherapy use had higher 10-year cancer-specific survival (90.2% vs. 88.1%, difference 2.1%; 95% CI 0.3% – 4.0%) and 10-year overall survival (53.3% vs. 50.2%, difference 3.1%; 95% CI 1.3% – 6.3%). Results were similar irrespective of the type of radiotherapy used. To assess the robustness of our choice of IV, we repeated the IVA approach using different IVs (using the median utilization rate as the cutoff) and found the results to be similar.
Conclusions
Among men >65 years of age, the benefit of primary radiotherapy for localized disease is largely confined to patients with high-risk prostate cancer (Gleason score 7-10).
Keywords: Prostate cancer, radiotherapy, conservative management, population-based study, survival, instrumental variable analysis
Introduction
Prostate cancer is the most common non-skin cancer and the second most common cause of cancer death in men, striking approximately 1 of every 6 American men during his lifetime.1 The majority of men (over 90%) are diagnosed at localized stages (T1 or T2)2 and standard treatments include radiotherapy, surgery, or conservative management.3 For men < 65 years of age with clinically localized prostate cancer, results of a large, randomized clinical trial have demonstrated that surgery improves survival compared with conservative management.4,5 The majority of men diagnosed with localized prostate cancer, however, are over age 65. Although not specifically designed to address age effects, this same clinical trial4,5 was unable to demonstrate a survival benefit for surgery among older men.6 Although radiotherapy represents the most common form of curative treatment for elderly patients,7 long-term outcomes data following radiotherapy are sparse and a comprehensive review concluded that data are “insufficient” to support the use of radiotherapy in early stage prostate cancer.8
In the absence of data from randomized clinical trials, instrumental variable analysis (IVA) has been used to minimize biases in observational studies9-11 and produce results similar to randomized clinical trials.12 Our previous study has shown that there are substantial geographic variations in the use of prostate cancer therapies and that clinical factors play a limited role in treatment selection among elderly patients with localized prostate cancer. 13 These data suggest that IVA may be appropriate for evaluating prostate cancer therapies.
The major advantage of IVA is its ability to capture the random component of treatment choice (e.g., some patients in a geographic area will receive radiotherapy solely by chance because it is more popular or easily available in that area) and use it to balance both measured and unmeasured confounding factors. This study aims to address the long-term outcomes of primary radiotherapy vs. conservative management among patients over age 65 years old with localized prostate cancer.
Materials and Methods
Data
Data were derived from the US Surveillance, Epidemiology and End Results (SEER)14 and linked Medicare claims files. The SEER program captures 98% of all cancer cases in designated geographical regions.15 Medicare provides health insurance for people aged 65 and over in the United States. Medicare part A covers hospital, nursing home, and home health care, and part B covers physician and outpatient care. To create the SEER-Medicare linked database, each SEER registry provides information on each person diagnosed as having cancer in its area. A deterministic matching algorithm was used to link SEER data with Medicare claims files.15 To ensure confidentiality, unique identifiers are assigned. Linkage of the two databases was ∼93%.15
Participants
Inclusion criteria for the study are as follow:
Being diagnosed with localized disease (T1 or T2) at ages 66-85 years during 1992-2007 (N=192,013) in the SEER regions of Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, and Utah, Los Angeles and San Jose-Monterey, and rural Georgia. We chose the age cutoff at age 85 because patients older than 85 are substantially less likely to receive radiotherapy.
Having Medicare Part A & B coverage during the study period. Patients enrolled in Health Maintenance Organizations (HMOs) or lacking Medicare coverage within one year of cancer diagnosis (N=76,740) were excluded to ensure that there were adequate claims data to identify pre-existing co-morbidities and cancer treatments.
Exclusion criteria for the study are as follows:
Having other cancers (N=19,695) or metastatic disease within 6 months of diagnosis (N=1,882).
Having palliative radiation (N=2,550), cryotherapy (N=1,015), prostatectomy (N=16,246), primary androgen deprivation therapy (ADT) without radiotherapy (N=11,387), radioisotope treatment (N=181) within 12 months of diagnosis.
Having missing data on health service area, cancer stage, or grade (N=4,568).
Identification of Radiotherapy, Androgen Deprivation Therapy, and Conservative Management
A previously described algorithm with minor modification was used to identify use of radiotherapy,16 orchiectomy, and luteinizing hormone releasing hormone agonists.17 Primary radiotherapy was defined as therapy received within one year of diagnosis. Brachytherapy, external beam radiotherapy and combinations of the two were included in the radiation treatment group. External beam radiation therapy included three-dimensional conformal radiotherapy, intensity modulated radiation therapy (IMRT), or proton beam therapy (Table 1). Conservative management was defined as being free of surgery, radiotherapy, cryotherapy, or primary androgen deprivation therapy for at least 12 months after diagnosis.
Table 1. Characteristics of the Study Cohorta.
| Characteristics | Primary Radiation Therapy (n = 40,508) | Conservative Management (n= 17,241) |
|---|---|---|
| Age, median (IQR), y | 74 (70-77) | 76 (72-80) |
| Black race | 3,932 (9.7) | 2,052 (11.9) |
| Married at diagnosis | 29,892 (73.8) | 10,428 (60.5) |
| Urban residence | 35,138 (86.7) | 14,640 (84.9) |
| Income, median (IQR), US $ | 49,421 (38897-63682) | 46,328 (35853-60646) |
| SEER Regions | ||
| Northeast | 6,537 (16.1) | 2,121 (12.3) |
| North central | 13,486 (33.3) | 4,774 (27.7) |
| West | 17,547 (43.3) | 9,542 (55.3) |
| South | 2,938 (7.3) | 804 (4.7) |
| Cancer grade | ||
| Low/ Intermediate | 28,565 (70.5) | 14,670 (85.1) |
| High | 1,1943 (29.5) | 2,571 (14.9) |
| Clinical stage at diagnosis | ||
| T1 | 15,327 (37.8) | 8,454 (49.0) |
| T2 | 25,181 (62.2) | 8,787 (51.0) |
| PSA, median (IQR)* | 6.8 (5.0-10.2) | 6.6 (4.7-10.2) |
| Gleason Score* | ||
| <=6 | 5,129 (45.0) | 2,807 (71.2) |
| 7 | 4,506 (39.6) | 889 (22.5) |
| 8-10 | 1,759 (15.4) | 248 (6.3) |
| Comorbidity status | ||
| Charlson comorbidity score 0 | 31,465 (77.7) | 12,901 (74.8) |
| Charlson comorbidity score 1 | 6,586 (16.3) | 2,791 (16.2) |
| Charlson comorbidity score ≥2 | 2,457 (6.1) | 1,549 (8.9) |
| Year of diagnosis | ||
| 1992-1999 | 16,854 (41.6) | 9,214 (53.4) |
| 2000-2003 | 12,260 (30.3) | 4,083 (23.7) |
| 2004+ | 11,394 (28.1) | 3,944 (22.9) |
| Type of radiation therapy | ||
| External beam radiation b | 27,214 (67.2) | N/A |
| 3D Conformal Radiotherapy | 17,575 | N/A |
| Intensity-modulated radiation therapy | 6,176 | N/A |
| Protons | 240 | N/A |
| Unknown | 3,223 | N/A |
| Brachytherapy | 8,106 (20.0) | N/A |
| External beam plus brachytherapy | 5,188 (12.8) | N/A |
| ADT use in 1st year | 16,601 (41.0) | N/A |
Abbreviations: IQR, interquartile range; SD, standard deviation: PSA, prostate-specific antigen; ADT, androgen deprivation therapy; SEER, Surveillance, Epidemiology, and End Results; N/A, not applicable.
PSA and Gleason score distributions were based on cases diagnosed in 2004 or thereafter
Data are presented as No.(%) unless otherwise specified. Race was self-determined by the patients. Clinical extension information provided by SEER was used to determine cancer stage (T1, T2). PSA and Gleason score were limited to those diagnosed in 2004 or thereafter. Charlson comorbidity score was derived from Medicare claims during the year before prostate cancer diagnosis by using a validated algorithm.14 Low/intermediate grade cancer consisted of Gleason score 2-7 before 2003 and Gleason score 2-6 thereafter.
Intensity-modulated radiation therapy (IMRT) was defined as IMRT only or the combination of 3D Conformal Radiotherapy (3D CRT) and IMRT. Proton beam therapy was defined as protons only or protons combined with 3D CRT or IMRT. 3D CRT defined as 3D CRT only. Unknown included unknown type of external beam radiation therapy.
Study Endpoints and Covariates
Vital status and underlying cause of death were derived from the SEER data (∼88% agreement compared with medical records).18 Prior to 2003, the SEER database grouped Gleason scores 5-7 together and these patients were included in the low/intermediate-grade group for this study. Starting in 2003, Gleason 7-10 disease constituted the high-grade category in the SEER whereas Gleason 2-6 cancer was in the low/intermediate grade category. The Charlson score, a validated predictor of longevity in localized prostate cancer, was derived from Medicare inpatient, outpatient, and physician claims during the year prior to diagnosis using a validated algorithm.19
Instrumental Variable Analysis
To construct the instrumental variables, we first calculated the proportion of eligible patients receiving radiotherapy in each health service area (HSA), defined as one or more counties that are relatively self-contained with respect to the provision of routine hospital care.20 Each HSA with <50 eligible patients was combined with the nearest (in terms of distance between geographic centers) HSA until it reached 50 cases because lower thresholds were associated with unstable estimates. Radiotherapy utilization for low/intermediate risk and high-risk groups was calculated separately so that it was not necessary to assume that the patterns of radiotherapy utilization were the same for all risk levels within the same HSA area. We have divided the continuous predictor (radiotherapy utilization rate) into two distinct categories, as discussed in the IVA context by Angrist, Imbrens, and Rubin.21 High- and low-use areas corresponded to the top and bottom tertiles of radiotherapy utilization, and were chosen to provide sufficient differences in utilization and sample size. We also used median utilization rate as the cut off in the sensitivity analysis (Table 5).
Table 5. Adjusted Percentage Prostate Cancer-Specific and Overall Survival in High- and Low-Use Areas by Type of Instrumentsa.
| Tertile based instrumentb | Median based Instrumentc | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| High-Radiation Use | Low-Radiation Use | High vs. Low Difference (95% CI) | High-Radiation Use | Low-Radiation Use | High vs. Low Difference (95% CI) | |
|
|
|
|||||
| Adjusted Survival, % | Adjusted Survival % | Adjusted Survival, % | Adjusted Survival % | |||
| Prostate cancer-specifi survivald | c | |||||
| 10-y Low/Intermediate | 96.1 | 95.4 | 0.7 (-0.1 to 1.2) | 96.1 | 95.4 | 0.7 (-0.1 to 1.3) |
| 10-y High Risk | 90.2 | 88.1 | 2.1 (0.3 to 4.0) | 90.3 | 88.1 | 2.2 (0.4 to 4.1) |
| 10-y All localized | 94.6 | 93.5 | 1.11 (0.5 to 2.1 | 94.7 | 93.5 | 1.2 (0.6 to 2.1) |
| Overall survival | ||||||
| 10-y Low/Intermediate | 56.6 | 56.3 | 0.2 (-1.7 to 1.9) | 57.9 | 56.3 | 0.3 (-1.5 to 2.0) |
| 10-y High Risk | 53.3 | 50.2 | 3.08 (1.3 to 6.2) | 53.3 | 50.2 | 3.16 (1.3 to 6.2) |
| 10-y All localized | 55.9 | 55.7 | 1.38 (-0.15 to 2.9) | 55.7 | 54.3 | 1.44 (-0.3 to 2.9) |
Abbreviation: CI, confidence interval.
.Covariates included age, race, comorbidity status, cancer stage, income quartiles, education quartiles, urban residence, marital status, year of diagnosis, ADT usage, state buy-in status and risk group (all patients only). 95% CIs are bootstrapped. Because date of last follow-up differed for overall (December 31, 2011) and cancer-specific survival (December 31, 2009), the number of person-years of follow-up differs for each endpoint.
High- and low-use areas corresponded to the top tertile and bottom tertiles of radiotherapy utilization.
High- and low-use areas corresponded to the above median and below median of radiotherapy utilization.
In order to calculate prostate cancer-specific survival, death due to other cause was treated as a competing risk.
Statistical Analyses
Data on cancer-specific and overall mortality were available through December 31, 2009 and December 31, 2011 respectively. Survival times were modeled using Cox proportional hazards models. Covariates included age, race, zip code level income, zip code level education, SEER region (conventional Cox model only), urban/rural area, marital status, cancer grade, clinical T stage, Charlson co-morbidity score, year of diagnosis, state buy-in for Medicare (for individuals with limited income and resources), and ADT usage within the first year. The results of conventional Cox models are presented in order to compare our results with published data.
For the instrumental variable analyses, our models included age, race, education quartile, income quartile, urban residence, comorbidity status, marital status, state buy-in (poverty) status, cancer stage, use of ADT within the 1st year of diagnosis, and year of diagnosis. Cancer grade was included in the models that included all localized prostate cancer. The treatment and region variables were replaced by a single covariate indicating high- or low-radiotherapy utilization (the instrumental variable). Patients in regions with utilization rates in the middle tertile were not included in the main IV analysis. To account for variability in hazard rates among the HSAs, we included a normally distributed random effects term, known as a “frailty,” and implemented it using the “coxme” package in the R statistical package.21-23 We compared high- and low-use areas by estimating the hazard ratios and differences in 10-year survival rates for this covariate-adjusted frailty model.
We computed adjusted bootstrap confidence intervals for the 10-year fitted survival rates as follows. First, we computed, for each patient, and for each bootstrap sample, the predicted covariate-adjusted 10-year survival under two alternatives: that the patient was in a high-use area and that the patient was in a low-use area. All patients, including those in the middle tertile, could thus be included in the bootstrap analysis. The population-adjusted survival rates in high-and low-use areas were obtained by averaging these rates across the population.24 Mean and confidence intervals of the mean were then computed from 1,000 bootstrap samples.25
We computed the cumulative incidence probabilities of death due to prostate cancer by treating other causes of death as competing risks. The proportional hazards assumptions were checked using log-log plots and the Schoenfeld residuals test 24 and found to be satisfactory.
All the subgroup analyses (by cancer grade) were pre-specified. There was 80% power to detect a 15% relative difference in disease-specific hazard rate for the low-grade group. In sensitivity analyses, we used a different IV, using the median radiotherapy utilization rate as the cutoff and included all patients in the analysis (Table 5). We also limited the analysis to patients with Gleason score 8-10 cancer to assess the outcomes with and without Gleason score 7 cancer.
Results
Baseline Characteristics
There were 57,749 eligible men aged ≥66 years with localized prostate cancer diagnosed during 1992-2007 (Table 1). Median age at diagnosis was 74 years and median follow-up for overall survival was 130 months (interquartile range, 91-177 months). Concurrent or adjuvant ADT use in the first year of cancer diagnosis was common (41%) in patients receiving primary radiotherapy.
Patients treated with radiotherapy were younger and healthier than those receiving conservative management (Table 1), suggesting that conventional analytic methods would likely be subject to bias. Following the application of IVA, however, clinical characteristics (e.g., age, Charlson score, PSA, Gleason score, or use of ADT in the first year of diagnosis) were similar in areas with high- and low-radiotherapy use, suggesting that IVA was effective in reducing biases (Table 2).
Table 2. Characteristics of Men with Localized Prostate Cancer in High-and Low-Use Radiotherapy Health Service Areas.
| Cancer Grade | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Low/ Intermediate | High | All Localized | ||||
|
| ||||||
| Characteristic | High-Radiation Use (n = 14,874) | Low-Radiation Use (n = 14,507) | High-Radiation Use (n = 4,670) | Low-Radiation Use (n = 4,855) | High-Radiation Use (n = 21,895) | Low-Radiation Use (n = 19,627) |
| Radiotherapy within 12 months, No. (%) | 74.9% | 54.8% | 87.2% | 77.2% | 79.3% | 59.3% |
| Age at diagnosis, median (IQR), y | 74 (70,77) | 74 (71,78) | 74 (71,78) | 75 (71,79) | 74 (71,77) | 75 (71,78) |
| Charlson Score, % | ||||||
| 0 | 75.5% | 77.4% | 77.1% | 73.2% | 74.8% | 76.3% |
| 1 | 17.5% | 15.5% | 16.4% | 18.1% | 17.6% | 16.2% |
| 2 + | 7.1% | 7.1% | 6.5% | 8.7% | 7.5% | 7.5% |
| Clinical stage T1, No. (%) | 43.2% | 43.6% | 40.6% | 33.2% | 43.3% | 41.9% |
| PSA, meana | 8.2 | 8.5 | 12.0 | 12.0 | 10.2 | 10.2 |
| ADT use in 1st year | 22.8% | 20.0% | 47.3% | 46.9% | 28.4% | 26.9% |
PSA distributions were based on cases diagnosed in 2004 or thereafter.
Survival
There were 3,120 prostate cancer deaths and 28,733 all-cause deaths. In patients with low/intermediate disease, 10-year prostate cancer-specific was similar in high and low-use areas (96.1% [high-use] vs. 95.4% [low-use], difference 0.7; 95% CI -0.1% to 1.2%), as was 10-year overall survival (56.6% [high use] vs. 56.3% [low-use], difference 0.3%; 95% confidence interval [CI] -1.7% to 0.3%). In patients with high-risk disease, areas with high-radiotherapy use had higher 10-year cancer-specific survival (90.2% vs. 88.1%, difference 2.1%; 95% CI 0.3% to 4.0%) and 10-year overall survival (53.3% vs. 50.2%, difference 3.1%; 95% CI 1.3% to 6.3%). (Table 3 and Figures 1a and 1b). Table 4 presents hazard ratios derived from Cox models as well as IVA models, in order to contrast our data with the existing literature (which typically uses Cox models). When conventional Cox models were employed, radiotherapy was associated with 46% reduction in prostate cancer specific mortality (HR=0.54; 95% C.I. 0.49 - 0.59) and 36% in overall mortality (HR=0.64; 95% C.I 0.62 - 0.66). Outcomes were similar irrespective of the type of radiotherapy used (Figure 2).
Table 3. Adjusted Percentage Prostate Cancer-Specific and Overall Survival in High- and Low-Use Areasa.
| Cancer Grade | High-Radiation Use | Low-Radiation Use | High vs. Low Difference (95% CI) | ||
|---|---|---|---|---|---|
|
| |||||
| Deaths/ Person-Year | Adjusted Survival, % | Deaths/ Person-Year | Adjusted Survival, % | ||
| Prostate cancer-specific survivalb | |||||
| 10-y Low/ Intermediate | 496/104,798 | 96.1 | 518/100,607 | 95.4 | 0.7 (-0.1 to 1.2) |
| 10-y High | 288/24,599 | 90.2 | 371/26,227 | 88.1 | 2.1 (0.3 to 4.0) |
| 10-y All localized | 926/145,065 | 94.6 | 887/128,613 | 93.5 | 1.1 (0.5 to 2.1) |
| Overall survival | |||||
| 10-y Low/ Intermediate | 5,806/113,917 | 56.6 | 5,474/110,292 | 56.3 | 0.3 (-1.7 to 1.9) |
| 10-y High | 1,530/30,013 | 53.3 | 1,827/31,298 | 50.2 | 3.1 (1.3 to 6.3) |
| 10-y All localized | 8,356/161,003 | 55.9 | 7,368/143,601 | 55.7 | 1.4 (-0.2 to 2.9) |
Abbreviation: CI, confidence interval.
Covariates included age, race, comorbidity status, cancer stage, income quartiles, education quartiles, urban residence, marital status, year of diagnosis, SEER (Surveillance, Epidemiology, and End Results) region, ADT usage, state buy-in status and cancer grade (all patients only). SEER region was not included in the instrumental variable analysis. 95% CIs are bootstrapped.
Because date of last follow-up differed for overall (December 31, 2011) and cancer-specific survival (December 31, 2009), the number of person-years of follow-up differs for each endpoint.
In order to calculate prostate cancer-specific survival, death due to other cause was treated as a competing risk.
Analyses used 95% bootstrapped CIs. Marginal effect is the risk difference divided by the difference in radiation use (obtained from Table 2).
Figure 1a. Adjusted Cumulative Incidence of Prostate-Specific Mortality in High-Use and Low-Use Health Service Areas by Cancer Grade.
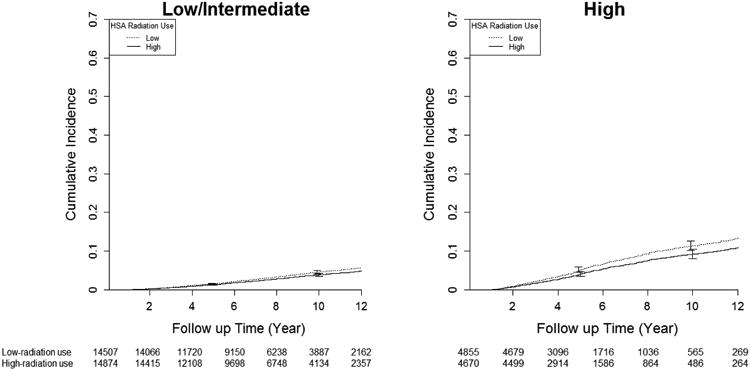
Figure 1b. 1b. Adjusted Cumulative Incidence of Overall Mortality in High-Use and Low-Use Health Service Areas by Cancer Grade.
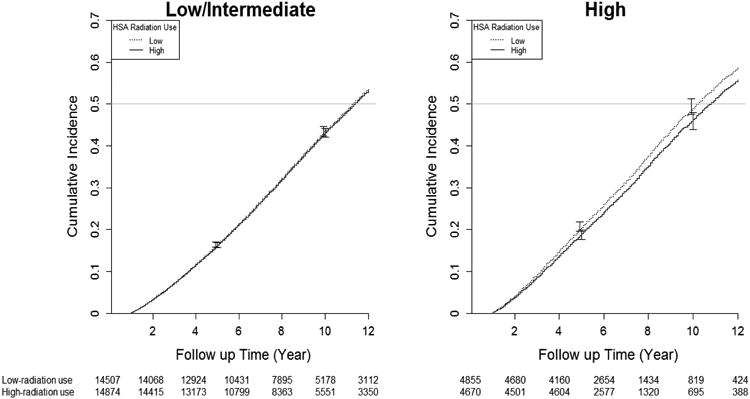
Table 4. Hazard Ratio (Radiotherapy to Conservative Management only) of Mortality According to Cancer Gradea.
| Cancer Grade | Radiotherapy | Conservative Management | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Events/ Person- Year | Rate Per 100 | Events/ Person- Year | Rate Per 100 | |||
| Conventional Cox Multivariate Results | ||||||
|
| ||||||
| Prostate cancer-specific mortality | ||||||
| Low/ Intermediate | 1,234/228,783 | 0.5 | 767/107,196 | 0.7 | 0.72(0.66-0.79) | 0.61(0.55-0.68) |
| High | 791/68,619 | 1.2 | 328/13,267 | 2.5 | 0.45(0.40-0.52) | 0.38(0.32-0.45) |
| All localized | 2,025/29,7402 | 0.7 | 1,095/120,463 | 0.9 | 0.75(0.69-0.81) | 0.54(0.49-0.59) |
| Overall mortality | ||||||
| Low/ Intermediate | 13,475/261,477 | 5.2 | 9,096/119,642 | 7.6 | 0.65(0.63-0.67) | 0.67(0.65-0.69) |
| High | 4,573/84,495 | 5.4 | 1,588/15,495 | 10.2 | 0.49(0.46-0.53) | 0.50(0.46-0.54) |
| All localized | 18,048/345,972 | 5.2 | 10,684/135,137 | 7.9 | 0.65(0.63-0.66) | 0.64(0.62-0.66) |
|
| ||||||
| Instrumental Variable Analysis Results | ||||||
|
| ||||||
| Prostate cancer-specific mortality | ||||||
| Low/ Intermediate | 627/116,190 | 0.5 | 695/111,264 | 0.6 | 0.88(0.75-1.05) | 0.84(0.73-1.03) |
| High | 326/25,858 | 1.3 | 420/27,522 | 1.5 | 0.77(0.63-0.91) | 0.79(0.65-0.93) |
| All localized | 1,139/159,643 | 0.7 | 1,116/140,719 | 0.8 | 0.76(0.56-0.85) | 0.78(0.61-0.87) |
| Overall mortality | ||||||
| Low/ Intermediate | 7,910/131,425 | 6 | 7,530/126,515 | 6 | 0.97(0.92-1.03) | 0.99(0.94-1.05) |
| High | 1,812/31,998 | 5.7 | 2,168/33,379 | 6.5 | 0.85(0.78-0.91) | 0.91(0.82-0.96) |
| All localized | 11,131/183,182 | 6.1 | 9,801/162,139 | 6 | 0.94(0.89-1.01) | 0.96(0.92-1.03) |
Abbreviation: CI, confidence interval; HR, hazard ratio.
Covariates included: age, race, comorbidity status, cancer stage, income quartiles, education quartile, urban residence, marital status, year of diagnosis, SEER (Surveillance, Epidemiology, and End Results) region, ADT usage, state buy-in status and cancer grade (all patients only). SEER region was not included in the instrumental variable analysis. The hazard ratios (radiotherapy/conservative management) by restricting the analyses to men with only Gleason scores 8-10 changed only slightly from 0.79 (95% CI 0.65-0.93) to 0.75(95% CI 0.59-0.93).
Figure 2. Adjusted Prostate Cancer Specific Survival Rate Difference at 10 year by Treatment.
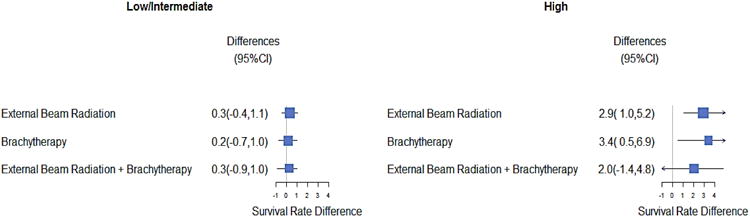
The mid-point of the box represents the point effect estimate, that is, the adjusted population average cancer-specific difference at 10yr between high and low radiation use area. The area of the box represents the sample size of each treatment group. The larger box size represents the larger sample size. The width of the line shows the 95% confidence intervals (CI) of the effect estimate of individual treatment. The 95% CI was computed from 1000 bootstrap samples. The arrow showed the lower and upper limits for clipping confidence intervals. External beam radiation therapy included 3D conformal radiotherapy (3D CRT), intensity modulated radiation therapy, proton beam therapy or unknown type therapy. The result of 3D CRT was similar to that of External Beam Radiation.
To assess the effect of grouping Gleason 7 with 8-10 in the high-risk patients, we restrictedthe analyses to men with Gleason scores 8-10 disease. The resulting hazard ratios (radiotherapy/conservative management) changed only slightly, from 0.79 (95% CI 0.65 - 0.93) to 0.75 (95% CI 0.59 - 0.93). To assess the robustness of our choice of IV, we repeated the IVA approach using different IVs (using the median utilization rate as the cutoff). The conclusion was the same when median utilization of radiotherapy was used as the cutoff for IV construction (Table 5).
Discussion
Prostate cancer treatment should rely on a thorough understanding of potential outcomes following interventions. However, to date, there are no published randomized data that adequately compare radiotherapy vs. conservative management for localized prostate cancer. Our study attempted to take a significant step towards filling this gap.
Our results based on IV analysis show that the potential survival benefit associated with radiotherapy was largely limited to patients with high grade prostate cancer (Tables 3, 4, and 5)and that the effects of different radiotherapy modalities were similar (Figure 2). These results are consistent with those of randomized trials and clinical guidelines. Radiation Therapy Oncology Group's (RTOG) trial 7706 showed that prophylactic radiation therapy to clinically orpathologically uninvolved pelvic lymph nodes does not appear to improve overall or prostatecancer-specific survival.26 Because there are no published randomized trials comparing radiotherapy vs. conservative management for T1/T2 prostate cancer, we sought to compare our results with findings of the Prostate Cancer Intervention versus Observation Trial (PIVOT)27 and the Scandinavian Prostate Cancer Group 7 (SPCG-7) Trial.28 PIVOT27 compared the outcomes of radical prostatectomy versus observation while SPCG-7 compared endocrine treatment with or without radiotherapy for T3 prostate cancer.28 Our results, based on IVA analyses, are similar to the results of these two trials, in which a survival benefit was limited to patients with high-risk features.27,28 The borderline survival benefit observed in the low/intermediate grade group in this study is also consistent with the results our earlier study,29 which showed that patients with low-grade disease have 10-year relative survivals exceeding or approaching age-matched cohorts, thus making an improvement in survival highly unlikely.29 In contrast, 10-year relative survival compared to age-matched men was only 0.6329 in patients with high-grade disease (Gleason 8-10), and effective intervention may make a clinically meaningful difference in these patients.29 Radiotherapy with adjuvant androgen deprivation therapy has been shown to improve survival for patients with high-risk prostate cancer30 and has been recommended as the initial therapy in, for example, the National Cancer Center Network (NCCN) Guideline.31
Notably, the results of our conventional Cox multivariate analyses (Table 4) for cancer-specific survival (HR 0.54 in favor of radiotherapy, 95% CI 0.49 to 0.59) was lower than those of three previously published studies32-34 included in the comprehensive Agency for Healthcare Research and Quality (AHRQ) review of radiotherapy in localized prostate cancer8 (HRs of 0.64-0.67 in favor of radiotherapy) and the study by Abdollah, et al, who used propensity-score matching analytical approach.35 Our overall survival results utilizing conventional Cox analyses (HR 0.64 in favor of radiotherapy, 95% CI 0.62-0.66) replicated those reported in the AHRQ assessment (HRs of 0.63-0.70).8 These results suggest that our findings based on conventional Cox regression might have been subject to similar biases as those in the literature.
Despite the notable finding that radiotherapy benefit is limited to high-risk disease, one potential limitation of the study was that the SEER-Medicare database did not capture PSA and detailed Gleason score information for patients diagnosed before 2004, so patients with Gleason 7 disease before 2003 were included in the low/intermediate risk group. Our inclusion of some Gleason grade 7 patients in the low/intermediate group might lead to overestimation of radiotherapy benefit in the low-risk category (although little benefit has been shown). While we could not separate out Gleason 7 among those diagnosed before 2003, limiting the analyses to men with only Gleason scores 8-10 yielded similar results, suggesting that patients with Gleason score 7 might experience a similar survival improvement as those with Gleason score 8-10.
A challenge associated with prostate cancer research is that methods of treatment often change quickly, whereas data to support the use of various therapies matures slowly due to the long natural history of the disease. For example, during the study period, various radiotherapy modalities were used, potentially making the assessment of radiotherapy efficacy a moving target.36 Nonetheless, analysis by type of radiotherapy revealed consistent results within the low/intermediate and high grade groups (Figure 2).
The primary limitation of utilizing an administrative database is the lack of detailed clinical information, which should be largely compensated for by the IVA approach. However, in spite of the ability to balance potential risk factors through IVA and to include relevant risk factors such as age, race, education, income, and marital status in the models, it is possible that residual imbalances in both measured and unmeasured confounding variables can persist, as they sometimes do even in randomized clinical trials. Therefore, other authors' confirmation of our findings would certainly provide greater confidence in our results. Finally, this study focused on men aged 65 and over and therefore the results might not be applicable to younger men. In contrast to the study's potential limitations, there were also important strengths. The statistical method employed allowed us to minimize bias, and because the study was population-based and included all patients in the relevant geographic areas rather than a select group of patients common to an institution or network, the results are more likely to be broadly applicable. Although we did exclude patients for incomplete treatment records, most clinically relevant exclusions were for patients treated with other modalities (e.g., primary androgen deprivation, cryotherapy, surgery, etc.), and the eligible population was far more extensive than is typical of clinical trials, implying that the results may be more generalizable. Also, the large sample size of 57,749 patients provided more statistical certainty than has been typical of previous studies. Finally, the IVA-based approach appears robust, illustrated by the similar results obtained when grouping HSAs by either tertiles or into two groups by median radiation utilization (Table 5).
As a consequence of cancer screening and an increasingly aged population, the number of men with localized prostate cancer will increase dramatically in the coming decades. How radiotherapy is utilized may impact the quality of life of millions of men and significantly affect patients' and society's overall healthcare costs. Complications related to sexual, urinary, and bowel function following radiotherapy can be substantial and long-lasting.37,38 Given the far-reaching impact of radiotherapy-related complications and the differences in impact on survival according to disease risk, a careful assessment of the potential risks, benefits and costs of different therapies for localized prostate cancer should be carefully reviewed before making treatment decisions.
Acknowledgments
The authors acknowledge the efforts of the Applied Research Branch, Division of Cancer Prevention and Population Science, National Cancer Institute (NCI), the Office of Information Services, and the Office of Strategic Planning, Center for Medicare and Medicaid Services (CMS); Information Management Services (IMS), Inc., and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database. This study was approved by the NCI, CMS, and Rutgers RBHS IRB. We thank Carolina Lozada and Edith Prescod for their outstanding technical and administrative assistance.
Financial Disclosures: During the past 5 years, the following authors have received financial support and maintained affiliations as follows: Dr. Grace Lu-Yao has been a consultant for Merck Research Laboratories. In addition, Dr. Siu-Long Yao has been employed by Schering-Plough, Merck, and Sun Pharmaceuticals LLC in the area of clinical research. None of these entities contributed funding, or played any role whatsoever in the design, interpretation, or drafting of our study or manuscript.
Footnotes
Disclaimers: This study utilizes the Linked SEER-Medicare Database. The project described was supported by Award number RC1CA145722 from the National Cancer Institute (NCI) and Rutgers Cancer Institute of New Jersey CCSG core grant NCI CA-72720. NCI is not involved in the design or conduct of this study. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Cancer Institute or the National Institute of Health.
References
- 1.Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, et al. SEER Cancer Statistics Review,1975-2011. National Cancer Institute; Bethesda, MD: 2014. [Google Scholar]
- 2.Shao YH, Demissie K, Shih W, Mehta AR, Stein MN, Roberts CB, et al. Contemporary Risk Profile of Prostate Cancer in the United States. J Natl Cancer Inst. 2009;101(18):1280–1283. doi: 10.1093/jnci/djp262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer. National Comprehensive Cancer Network; 2013. [DOI] [PubMed] [Google Scholar]
- 4.Bill-Axelson A, Holmberg L, Filen F, Ruutu M, Garmo H, Busch C, et al. Radical prostatectomy versus watchful waiting in localized prostate cancer: the Scandinavian prostate cancer group-4 randomized trial. J Natl Cancer Inst. 2008;100(16):1144–1154. doi: 10.1093/jnci/djn255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bill-Axelson A, Holmberg L, Garmo H, Rider JR, Taari K, Busch C, et al. Radical prostatectomy or watchful waiting in early prostate cancer. The New England journal of medicine. 2014;370(10):932–942. doi: 10.1056/NEJMoa1311593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bill-Axelson A, Holmberg L, Ruutu M, Haggman M, Andersson SO, Bratell S, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. The New England journal of medicine. 2005;352(19):1977–1984. doi: 10.1056/NEJMoa043739. [DOI] [PubMed] [Google Scholar]
- 7.Miller DC, Gruber SB, Hollenbeck BK, Montie JE, Wei JT. Incidence of Initial Local Therapy Among Men With Lower-Risk Prostate Cancer in the United States. J Natl Cancer Inst. 2006;98(16):1134–1141. doi: 10.1093/jnci/djj308. [DOI] [PubMed] [Google Scholar]
- 8.Lp S, Dvorak T, Yu W, Patel K, Obadan N, Chung M, et al. Radiation therapy for localized prostate cancer: an Update. Agency for Healthcare Research and Quality (AHRQ); Rockville, MD: 2010. [PubMed] [Google Scholar]
- 9.Stukel TA, Fisher ES, Wennberg DE, Alter DA, Gottlieb DJ, Vermeulen MJ. Analysis of Observational Studies in the Presence of Treatment Selection Bias: Effects of Invasive Cardiac Management on AMI Survival Using Propensity Score and Instrumental Variable Methods. JAMA. 2007;297(3):278–285. doi: 10.1001/jama.297.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu-Yao GL, Albertsen PC, Moore DF, Shih W, Lin Y, DiPaola RS, et al. Survival following primary androgen deprivation therapy among men with localized prostate cancer. Jama. 2008;300(2):173–181. doi: 10.1001/jama.300.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu-Yao GL, Albertsen PC, Moore DF, Shih W, Lin Y, DiPaola RS, et al. Fifteen-Year Survival Outcomes Following Primary Androgen-Deprivation Therapy for Localized Prostate Cancer. JAMA Intern Med. 2014 doi: 10.1001/jamainternmed.2014.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeliadt SB, Potosky AL, Penson DF, Etzioni R. Survival benefit associated with adjuvant androgen deprivation therapy combined with radiotherapy for high- and low-risk patients with nonmetastatic prostate cancer. Int J Radiat Oncol Biol Phys. 2006;66(2):395–402. doi: 10.1016/j.ijrobp.2006.04.048. [DOI] [PubMed] [Google Scholar]
- 13.Roberts CB, Albertsen PC, Shao YH, Moore DF, Mehta AR, Stein MN, et al. Patterns and correlates of prostate cancer treatment in older men. Am J Med. 2011;124(3):235–243. doi: 10.1016/j.amjmed.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Surveillance Epidemiology and End Results (SEER) Program Public use tape (1973-1990) National Cancer Institute, DCPC, Surveillance Program, Cancer Statistics Branch; 1993. [Google Scholar]
- 15.Warren JL, Harlan LC, Fahey A, Virnig BA, Freeman JL, Klabunde CN, et al. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40(8 Suppl):IV-55–61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- 16.Wong YN, Mitra N, Hudes G, Localio R, Schwartz JS, Wan F, et al. Survival associated with treatment vs observation of localized prostate cancer in elderly men. Jama. 2006;296(22):2683–2693. doi: 10.1001/jama.296.22.2683. [DOI] [PubMed] [Google Scholar]
- 17.Shahinian V, Kuo Y, Freeman J, Goodwin J. Risk of fracture after androgen deprivation for prostate cancer. The New England journal of medicine. 2005;352(2):154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 18.Albertsen P. When is a death from prostate cancer not a death from prostate cancer? J Natl Cancer Inst. 2000;92(8):590–591. doi: 10.1093/jnci/92.8.590. [DOI] [PubMed] [Google Scholar]
- 19.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 20.Makuc D, Haglund B, Ingram D, Kleinman J, Feldman J. In: Vital and Health Statistics -Health Service Areas for the United States. Services USDoHaH, editor. National center for Health Statistics; 1991. [PubMed] [Google Scholar]
- 21.Angrist J, Imbens G, Rubin D. Identification of causal effects using instrumental variables. Journal of the American Statistical Association. 1996;91(434):444–455. [Google Scholar]
- 22.Therneau TM, Grambach PM, Pankratz VS. Penalized survival models and frailty. J of Computational and Graphical Statistics. 2003;12:156–175. [Google Scholar]
- 23.R Development Core Team. Team R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2012. Cran.r-project.org. [Google Scholar]
- 24.Therneau TM, Grambsch PM. Modeling Survival data: extending the Cox Model. Springer; New York: 2000. [Google Scholar]
- 25.Efron B, Tibshirani RJ. An Introductionto the Bootstrap. Chapman & Hall; New York: 1993. [Google Scholar]
- 26.Asbell SO, Martz KL, Pilepich MV, Baerwald HH, Sause WT, Doggett RL, et al. Impact of surgical staging in evaluating the radiotherapeutic outcome in RTOG phase III study for A2 and B prostate carcinoma. International journal of radiation oncology, biology, physics. 1989;17(5):945–951. doi: 10.1016/0360-3016(89)90140-5. [DOI] [PubMed] [Google Scholar]
- 27.Wilt TJ, Brawer MK, Jones KM, Barry MJ, Aronson WJ, Fox S, et al. Radical Prostatectomy versus Observation for Localized Prostate Cancer. New Engl J Med. 2012;367(3):203–213. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Widmark A, Klepp O, Solberg A, Damber JE, Angelsen A, Fransson P, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet. 2009;373(9660):301–308. doi: 10.1016/S0140-6736(08)61815-2. [DOI] [PubMed] [Google Scholar]
- 29.Lu-Yao GL, Yao SL. Population-based study of long-term survival in patients with clinically localised prostate cancer [see comments] Lancet. 1997;349(9056):906–910. doi: 10.1016/S0140-6736(96)09380-4. [DOI] [PubMed] [Google Scholar]
- 30.D'Amico AV, Chen MH, Renshaw AA, Loffredo M, Kantoff PW. Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. Jama. 2008;299(3):289–295. doi: 10.1001/jama.299.3.289. [DOI] [PubMed] [Google Scholar]
- 31.NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer. National Comprehensive Cancer Network; 2015. [DOI] [PubMed] [Google Scholar]
- 32.Albertsen PC, Hanley JA, Penson DF, Barrows G, Fine J. 13-year outcomes following treatment for clinically localized prostate cancer in a population based cohort. The Journal of urology. 2007;177(3):932–936. doi: 10.1016/j.juro.2006.10.051. [DOI] [PubMed] [Google Scholar]
- 33.Zhou EH, Ellis RJ, Cherullo E, Colussi V, Xu F, Chen WD, et al. Radiotherapy and survival in prostate cancer patients: a population-based study. Int J Radiat Oncol Biol Phys. 2009;73(1):15–23. doi: 10.1016/j.ijrobp.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tewari A, Divine G, Chang P, Shemtov MM, Milowsky M, Nanus D, et al. Long-term survival in men with high grade prostate cancer: a comparison between conservative treatment, radiation therapy and radical prostatectomy--a propensity scoring approach. The Journal of urology. 2007;177(3):911–915. doi: 10.1016/j.juro.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 35.Abdollah F, Sun M, Schmitges J, Thuret R, Tian Z, Shariat SF, et al. Competing-risks mortality after radiotherapy vs. observation for localized prostate cancer: a population-based study. Int J Radiat Oncol Biol Phys. 2012;84(1):95–103. doi: 10.1016/j.ijrobp.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 36.Wilt TJ, Shamliyan T, Taylor B, MacDonald R, Tacklind J, Rutks I, et al. Comparative efffectiveness of therapies for clinically localized prostate cancer. Agency for Healthcare Research and Quality; 2008. [PubMed] [Google Scholar]
- 37.Resnick MJ, Koyama T, Fan KH, Albertsen PC, Goodman M, Hamilton AS, et al. Long-term functional outcomes after treatment for localized prostate cancer. The New England journal of medicine. 2013;368(5):436–445. doi: 10.1056/NEJMoa1209978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim S, Moore DF, Shih W, Lin Y, Li H, Shao YH, et al. Severe genitourinary toxicity following radiation therapy for prostate cancer--how long does it last? The Journal of urology. 2013;189(1):116–121. doi: 10.1016/j.juro.2012.08.091. [DOI] [PMC free article] [PubMed] [Google Scholar]


