Abstract
High-dose therapy and autologous stem cell transplantation (HDT-ASCT) with thiotepa, busulfan, cyclophosphamide (TBC) conditioning has emerged as an effective post-induction treatment strategy for patients with primary (PCNSL) or secondary central nervous system lymphoma (SCNSL), but it is associated with considerable toxicity and transplant-related mortality (TRM) in the modern era. Forty-three adult patients with chemosensitive PCNSL or SCNSL received TBC conditioned ASCT between 2006 and 2015. Twenty-eight of these patients received pharmacokinetically (PK)-targeted busulfan dosing. The median number of clinically relevant individual grade ≥3 non-hematologic toxicities per patient was 5. We found no association between pre-ASCT patient characteristics and > 5 observed grade ≥3 non-hematologic toxicities. Patients with elevated first-dose busulfan AUC levels did not experience more toxicity. Paradoxically, patients treated with >2 regimens prior to ASCT had lower first-dose busulfan AUC. With a median follow-up among survivors of 20 months, 1-year PFS and OS from the time of ASCT were 83% and 87%, respectively. While reaffirming the favorable progression-free and overall survival of TBC-conditioned ASCT for CNSL, this treatment strategy carries a large toxicity burden.
Keywords: High-dose therapy and autologous stem cell transplantation, Central nervous system lymphoma, Toxicity, Regimen-related toxicity
Introduction
Durable disease control in central nervous system lymphoma (CNSL) is elusive even for patients who attain a complete remission (CR) with induction therapy, thereby making consolidation therapy critical to overall survival (OS).1,2 High-dose chemotherapy followed by autologous stem cell transplantation (HDT-ASCT) has proven to be an effective consolidative approach in eligible patients. We have shown that HDT-ASCT in first remission for CNSL affords omission of potentially neurotoxic whole brain radiotherapy (WBRT).3,4 Patients conditioned with thiotepa, busulfan, cyclophosphamide (TBC) prior to ASCT have demonstrated favorable progression-free (PFS) and overall survival (OS) in recurrent or refractory CNSL.3,5,6 Our retrospective analysis of 17 patients with recurrent primary CNSL (PCSNL) or secondary CNSL (SCNSL) who had achieved a complete remission (CR) after salvage methotrexate (MTX)-based induction regimens proceeding to TBC conditioned ASCT demonstrated a 3-year PFS and OS of 93%.3 In this study, there were relatively few grade ≥ 3 toxicities reported, no grade 4 toxicities and no treatment-related deaths. In a phase II study conducted at our center, 26 patients with newly diagnosed PCNSL in chemo-sensitive remission after rituximab, methotrexate, procarbazine and vincristine (R-MPV) induction proceeded to first remission consolidative HDT-ASCT with TBC conditioning.7 The 2-year PFS and OS for the transplanted patients were 75% and 81%, respectively, results which are superior to a previous trial of HD-MTX/cytarabine followed by carmustine, etoposide, cytarabine, melphalan (BEAM) conditioned ASCT.8 While clearly efficacious, 3 of the patients transplanted using TBC conditioning (11.5%) died secondary to transplant-related mortality (TRM), which is higher than expected with the use of HDT-ASCT for other NHL, indicating a toxic regimen in a potentially a more susceptible population.7,9 The 3 deaths were due to infection, skin toxicity (Stevens-Johnson Syndrome) and severe colitis (possibly autologous graft-versus-host disease).
Predictable and precise dosing of busulfan, a commonly used alkylating agent in hematopoietic cell transplantation conditioning, has proven imperative in ameliorating toxicity while insuring effective myeloablation. Individualized, targeted PK-directed dosing of intravenous (IV) busulfan (both at 6 hour intervals and daily) has become more routine, yielding a more predictable area under the curve (AUC) within a desired therapeutic range.10–12 In 2012, our Adult Bone Marrow Transplant (BMT) Service at Memorial Sloan Kettering Cancer Center (MSKCC) began using daily busulfan PK levels to achieve a target AUC range with the TBC conditioning program to maintain myeloablation while reducing toxicity. Our aforementioned phase II study wherein TRM was observed in 11.5% of patients did not incorporate busulfan PK dose targeting.7
We sought to analyze potential factors contributing to TRM of consolidative TBC conditioning prior to ASCT. To that end, our primary aim was to evaluate and catalog all of the characteristic high-grade toxicities of TBC conditioning for CNSL at our institution. We hypothesized that certain baseline pre-ASCT patient characteristics would predict for incurring more grade 3–5 non-hematologic toxicities. We also aimed to evaluate the association of busulfan AUC levels with pre-ASCT patient characteristics and the development of treatment-related toxicities. We hypothesized that higher than expected busulfan AUC levels would correlate with more observed toxicity.
Materials and Methods
Eligible patients (n=43) ≥ 18 years of age with newly diagnosed or relapsed, chemosensitive PCNSL or SCNSL proceeding to consolidative TBC-conditioned HDT-ASCT between December 2006 and October 2015 were included in this MSKCC Institutional Review Board (IRB) approved retrospective chart review. All patients included were treated outside of previously reported prospective clinical trials.4,7 All grade ≥3 non-hematologic toxicities per National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) 4.0 were recorded from the initiation of TBC conditioning until 6 months post ASCT. There were 3 patients in our study who had less than 6 months of follow-up at the time of statistical analyses; however, these patients had no additional toxicities after the time of our analysis through 6 months post-transplant. Clinically relevant grade ≥ 3 non-hematologic toxicities were defined as toxicities that occurred at a frequency of ≥15% of all patients. Febrile neutropenia was not included as a clinically relevant non-hematologic toxicity for our analysis given the expected prevalence with HDT-ASCT. Individual toxicities were categorized into organ system-based toxicity groups based on CTCAE 4.0 criteria, and related toxicity groups were combined in certain cases.
Baseline patient characteristics were assessed for association with having more than the median number of clinically significant grade ≥ 3 non-hematologic toxicities using Fisher’s exact test. Differences in the median number of grade ≥ 3 non-hematologic toxicities among each baseline pre-transplant patient characteristic were assessed using the Wilcoxon rank sum test. TBC conditioning was thiotepa 250 milligrams/meter squared (mg/m2) intravenous (IV) on days -9, -8, -7; busulfan 3.2 milligrams/kilogram (mg/kg) IV on days -6, -5, -4; and cyclophosphamide 60 mg/kg IV on days -3 and -2 with autologous stem cell infusion on day 0. Per MSKCC institutional ASCT guidelines, anti-seizure prophylaxis with levetiracetam is started 24 hours prior to the first dose of busulfan and continued through 24 hours after the last dose of busulfan. Levetiracetam is given in either oral or intravenous dosing at 500 mg twice daily. For 28 patients treated with PK-targeted busulfan between 2012 and 2015, PK analysis was done after the first dose with predicted AUC reported based on 6-point kinetics. Dose adjustments per PK were made on the third busulfan dose. Target first-dose busulfan AUC was 4100–5200 umol*min/L and target total busulfan exposure was 12300–15600 umol*min/L. Per MSKCC institutional ASCT guidelines, antiviral prophylaxis with acyclovir 400 mg oral twice daily was started on admission, antibacterial prophylaxis for febrile neutropenia with ciprofloxacin 500 mg oral twice daily was started on day -2 until engraftment, and anti-fungal prophylaxis with fluconazole 400 mg daily was started on admission until engraftment. The association of pre-ASCT characteristics with busulfan AUC and total busulfan exposure was assessed using the Wilcoxon rank sum test. Progression-free (PFS) and overall survival (OS) for the entire cohort were estimated using Kaplan-Meier (KM) Method.13 PFS was defined as the date of progression of disease or death from any cause and OS was defined as date of death from any cause.
Results
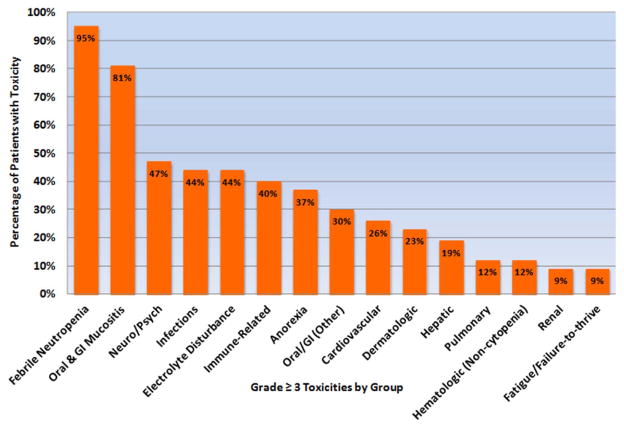
Baseline patient characteristics are detailed in Table 1.14–16 Of the16 patients that underwent TBC conditioned ASCT for SCNSL, 14 had secondary CNSL disease found at relapse, and 2 had secondary CNS disease at time of initial diagnosis. Two patients (5%) were positive for human immunodeficiency virus (HIV) prior to ASCT. The average hospital length of stay was 29 days, and 11 patients (26%) required re-hospitalization within 100 days of ASCT. All 43 patients (100%) experienced at least one grade ≥ 3 non-hematologic toxicity. Forty-one patients (95%) experienced at least one episode of febrile neutropenia in nadir; of these, 7 patients had an identified infectious source and 34 patients did not. The clinically relevant grade ≥ 3 non-hematologic toxicities (occurring in ≥ 15% of all study patients) were: oral/gastrointestinal (GI) mucositis, infections, neurologic and psychiatric (neuro/psych), metabolic – electrolyte disturbances, immune-related, metabolic – anorexia, oral and GI (other), cardiovascular, dermatologic, and hepatic. There were no cases of sinusoidal obstruction syndrome. Characteristic grade ≥ 3 non-hematologic toxicities by group and the percentage of all patients that incurred the specific toxicity are shown in Figure 1. Table 2 demonstrates all of the individual non-hematologic toxicities among all patients by toxicity group. The percentage of all patients that incurred each specific toxicity group is shown in Figure 1. The 5 most common clinically significant toxicity groups included: infections, electrolyte disturbances, oral and GI mucositis, neuro/psych, and immune-related toxicities.
Table 1.
Baseline Pre-ASCT Characteristics
| Characteristic | N (%) |
|---|---|
|
| |
| Age, median (range) | 56 (25–71) |
| • <60 | 31 (72) |
| • ≥60 | 12 (28) |
|
| |
| Gender | |
| • Male | 26 (60) |
| • Female | 17 (40) |
|
| |
| KPS, median (range) | 80 (70–90) |
| • ≥80 | 41 (95) |
| • <80 | 2 (5) |
|
| |
| HCT-CI, median (range) | 3 (0–6) |
| • >2 | 22 (51) |
| • ≤2 | 21 (49) |
|
| |
| Disease | |
| • PCNSL | 27 (63) |
| • SCNSL | 16 (37) |
|
| |
| NHL Histology | |
| • DLBCL | 36 (84) |
| • Other | 7 (16) |
|
| |
| CD34+ Dose [x106 cells/kg], median (range) | 4.64 (1.87 – 14.02) |
|
| |
| No. Prior Regimens, median (range) | 2 (1–6) |
| • ≤2 | 28 (65) |
| • >2 | 15 (35) |
|
| |
| Prior Treatment(s) | |
| • R-MPV | 30 (70) |
| • HD-MTX | 43 (100) |
| • Ara-C | 25 (58) |
| • R-CHOP-like | 15 (35) |
| • Temozolomide | 5 (12) |
| • WBRT | 9 (21) |
| • History of IO/IT Therapy | 12 (28) |
|
| |
| Status Prior to ASCT | |
| • CR/CRu | 35 (81) |
| • PR | 8 (19) |
KPS: Karnofsky performance status, HCT-CI: hematopoietic cell transplantation – comorbidity index14, PCNSL: primary central nervous system lymphoma, SCNSL: secondary central nervous system lymphoma, NHL: non-Hodgkin lymphoma, DLBCL: diffuse large B-cell lymphoma, R-MPV: rituximab/methotrexate/procarbazine/vincristine, HD-MTX: high-dose methotrexate, R-CHOP: rituximab/cyclophosphamide/doxorubicin/vincristine/prednisone, WBRT: whole-brain radiotherapy, ASCT: autologous stem cell transplantation, CR: complete response, CRu: unconfirmed complete response, PR: partial response15, IO: intra-Ommaya, IT: intra-thecal
Figure 1.
Percentage of Patients with Each Grade 3–5 Non-Hematologic Toxicity Group
Table 2.
Percentage of All Individual Toxicities by Toxicity Group
| Toxicity Group | N (%) |
|---|---|
| Electrolyte Disturbance | 44 (14) |
| Febrile Neutropenia | 43 (14) |
| Oral and GI Mucositis | 42 (14) |
| Infections | 41 (13) |
| Neuro/Psych | 31 (10) |
| Immune-Related | 21 (7) |
| Oral and GI (Other) | 17 (6) |
| Metabolic – Anorexia | 17 (6) |
| Cardiovascular | 12 (4) |
| Dermatologic | 10 (3) |
| Hepatic | 9 (3) |
| Pulmonary | 8 (3) |
| Fatigue/Failure-to-Thrive | 5 (2) |
| Hematologic (Non-Cytopenia) | 5 (2) |
| Renal | 4 (1) |
| Total | N = 309 |
Oral or GI Mucositis
Of the 30 patients (70%) who suffered from grade ≥ 3 oral mucositis, the average number of days on patient-controlled analgesia (PCA) was 10 (range 5 – 20 days). Twelve patients (28%) experienced grade 3 diarrhea. Seven patients (16%) experienced both grade ≥ 3 oral mucositis and diarrhea. Of the 16 patients who had grade ≥ 3 anorexia, 6 required total parental nutrition (TPN) for an average of 11 days.
Infections
Of all the non-febrile neutropenia infection-related toxicities (n=41), there were 11 lung infections, 5 bacteremias, 5 episodes of sepsis, and 5 other various infections. There were 7 events of viral reactivations including adenovirus (n=2), BK virus (n=1), cytomegalovirus (CMV, n=1), human herpesvirus-6 (HHV-6, n=3). There were also 8 viral organ disease events including adenovirus enterocolitis (n=2), BK cystitis (n=4), CMV colitis (n=1), and HHV-6 bone marrow infiltration (n=1). Of these viral organ diseases, one patient with chronic HIV infection had adenovirus enterocolitis, BK cystitis, and CMV colitis. There was one infection-related cause of death in patient who died of respiratory failure due to multiple lung infections.
Immune-Related
Five patients experienced an engraftment syndrome that required a short course of intravenous corticosteroids for resolution.17 Two patients were diagnosed with an immune-mediated thrombocytopenia requiring immunosuppression within the first 6 months post-transplant. There were 6 patients (14%) who had varying clinical signs and symptoms consistent with auto GVHD. Of these patients, 3 had tissue biopsy proven GVHD: one patient had grade 3 pulmonary (organizing pneumonia) and skin involvement, another developed grade 3 gastrointestinal (GI) and skin involvement, and the third had severe GI auto-GVHD that contributed to TRM. Two of the 3 remaining patients with clinically suspected auto-GVHD experienced grade 3 maculopapular rash and the third demonstrated prolonged post-transplant anorexia and chronic nausea that improved with empiric budesonide abrogating the need for endoscopic biopsy.
Electrolyte Disturbances
There were 44 total electrolyte disturbances, of which 42 (95%) were grade 3 hypocalcemia (n=7), hypokalemia (n=16), hyponatremia (n=6), hypophosphatemia (n=7), hypomagnesemia (n=1), hyperglycemia (n=4), hyperkalemia (n=1). There were two grade 4 hypocalcemia events.
Neuro/Psych
Neuro/psych toxicities (n=31) varied, but the majority were grade 3–4 acute delirium events (n=8) in the early post-ASCT setting. There were 2 new seizures: one occurred 31 days post-transplant in a patient who remains without evidence of disease (NED) and another occurred at day +90 in a patient who was found to have POD at the time of seizure. One patient was found to have multiple embolic cerebral infarcts 37 days post-transplant. The remaining neuro/psych events included: anxiety/depression (n=6), neuropathies (n=6), syncope (n=4), headache (n=3), mania (n=1). Of all 31 total individual Neuro/Psych toxicities described, 8 events occurred in 5 patients who had previously undergone WBRT prior to ASCT. The remaining toxicities occurred in patients who had not received WBRT. There was no significant difference in the frequency of Neuro/Psych toxicities between patients who received WBRT versus those who did not, 55% versus 44%, respectively (p=0.71 by Wilcoxon rank sum test).
Patient Characteristics and Toxicity
The median number of clinically significant individual grade ≥ 3 non-hematologic toxicities per patient was 5. Baseline pre-ASCT patient characteristics were not associated with incurring more than 5 grade ≥ 3 non-hematologic toxicities (Table 3). In addition, there were no statistically significant differences in the median number of grade 3–5 toxicities within each baseline pre-ASCT variable assessed (Table 3).
Table 3.
Association of Pre-ASCT Characteristics and Grade ≥ 3 Toxicities
| Pre-ASCT Characteristic | Median number of Toxicities | p-value | ≤ 5 Toxicities N (%) |
> 5 Toxicities N (%) |
p-value |
|---|---|---|---|---|---|
|
| |||||
| Age | 0.45 | 0.49 | |||
| • <60 | 5 | 20 (77) | 11 (65) | ||
| • ≥60 | 6 | 6 (23) | 6 (35) | ||
|
| |||||
| Gender | 0.72 | 0.53 | |||
| • Male | 6 | 17 (65) | 9 (53) | ||
| • Female | 5 | 9 (35) | 8 (47) | ||
|
| |||||
| KPS | 0.52 | 0.99 | |||
| • ≥80 | 5 | 25 (96) | 16 (94) | ||
| • <80 | 10 | 1 (4) | 1 (6) | ||
|
| |||||
| HCT-CI | 0.57 | 0.99 | |||
| • >2 | 5 | 13 (50) | 9 (53) | ||
| • ≤2 | 5 | 13 (50) | 8 (47) | ||
|
| |||||
| Disease | 0.69 | 0.75 | |||
| • PCNSL | 5 | 17 (65) | 10 (59) | ||
| • SCNSL | 6 | 9 (35) | 7 (41) | ||
|
| |||||
| NHL Histology | 0.78 | 0.41 | |||
| • DLBCL | 5 | 23 (88) | 13 (76) | ||
| • Other | 6 | 3 (12) | 4 (24) | ||
|
| |||||
| CD34+ Dose [x106 cells/kg], median | 0.82 | 0.99 | |||
| • ≤4.6 | 5 | 13 (50) | 8 (47) | ||
| • >4.6 | 5 | 13 (50) | 9 (53) | ||
|
| |||||
| No. Prior Regimens | 0.18 | 0.21 | |||
| • ≤2 | 5 | 19 (73) | 9 (53) | ||
| • >2 | 6 | 7 (27) | 8 (47) | ||
|
| |||||
| WBRT | 0.34 | 0.99 | |||
| • Yes | 4 | 6 (23) | 3 (18) | ||
| • No | 5 | 20 (77) | 14 (82) | ||
|
| |||||
| Status Prior to ASCT | 0.10 | 0.23 | |||
| • CR/CRu | 5 | 23 (88) | 12 (71) | ||
| • PR | 8 | 3 (12) | 5 (29) | ||
|
| |||||
| History of IO/IT Therapy | 0.25 | 0.49 | |||
| • Yes | 6 | 6 (23) | 6 (35) | ||
| • No | 5 | 20 (77) | 11 (65) | ||
KPS: Karnofsky performance status, HCT-CI: hematopoietic cell transplantation – comorbidity index14, PCNSL: primary central nervous system lymphoma, SCNSL: secondary central nervous system lymphoma, NHL: non-Hodgkin lymphoma, DLBCL: diffuse large B-cell lymphoma, WBRT: whole-brain radiotherapy, ASCT: autologous stem cell transplantation, CR: complete response, CRu: unconfirmed complete response, PR: partial response15, IO: intra-Ommaya, IT: intra-thecal
PK-Targeted Busulfan
Twenty-eight patients in our cohort (65%) received TBC conditioning with PK-targeted busulfan. Among these patients, median first dose busulfan AUC and median total busulfan exposure were 5595 umol*min/L (range 3268 – 7464 umol*min/L) and 15116 umol*min/L (range 11236 – 19240 umol*min/L), respectively. Six of 28 patients (21%) were within the therapeutic range for first dose busulfan AUC. Three patients (11%) required a dose increase, and 19 patients (68%) required a dose decrease based on predicted AUC after first dose PK analysis. Patients who received >2 regimens prior to transplant had lower initial busulfan AUC (p=0.02), though had a poorer 1-year OS than patients who received ≤ 2 prior regimens, 95% and 72%, respectively (p=0.02). Baseline pre-ASCT patient characteristics including age, HCT-CI, and number of prior regimens were not associated with higher than expected busulfan AUC levels. In addition, first-dose busulfan AUC and total busulfan exposure were not correlated with incurring greater than the median number (5) of ≥ grade 3 non-hematologic toxicities. There was no difference in requirement for dose reduction based on baseline pre-ASCT patient characteristics. Of the patients treated with PK-targeted busulfan, those with greater than the median busulfan AUC level had a median of 4.5 toxicities, while patients with less than the median AUC had a median of 6 toxicities. Moreover, there was no significant difference in toxicity between those who received or did not receive PK-targeted busulfan.
Outcome
With a median follow-up among survivors of 20 months, 1-year PFS and OS from the time of ASCT was 83% and 87%, respectively (Figures 2 and 3). During the study period assessed, 7 patients had progression of disease (POD), and of these, 5 patients experienced POD within the first 12 months of transplant. Of the 2 POD beyond 12 months: one occurred at 4.4 years post-transplant, and one patient who was lost to follow-up was thought to have relapsed shortly before dying 5.1 years after transplant. Of all 7 POD events, 6 occurred in patients with diffuse large B-cell lymphoma (DLBCL) histology (3 PCNSL and 3 SCNSL), and 1 occurred in a SCNSL patient with DLBCL with anaplastic features. There were a total of 8 deaths during the follow-up period of which 4 were secondary to POD. Three of these 4 patients had SCNSL, with isolated CNS relapse in 2 of these patients. Three patients died secondary to TRM (7%) at 2 months (respiratory failure due to multiple lung infections), 6 months (auto-GVHD) and 7.2 years (metastatic spindle cell sarcoma) post HDT-ASCT.
Figure 2.
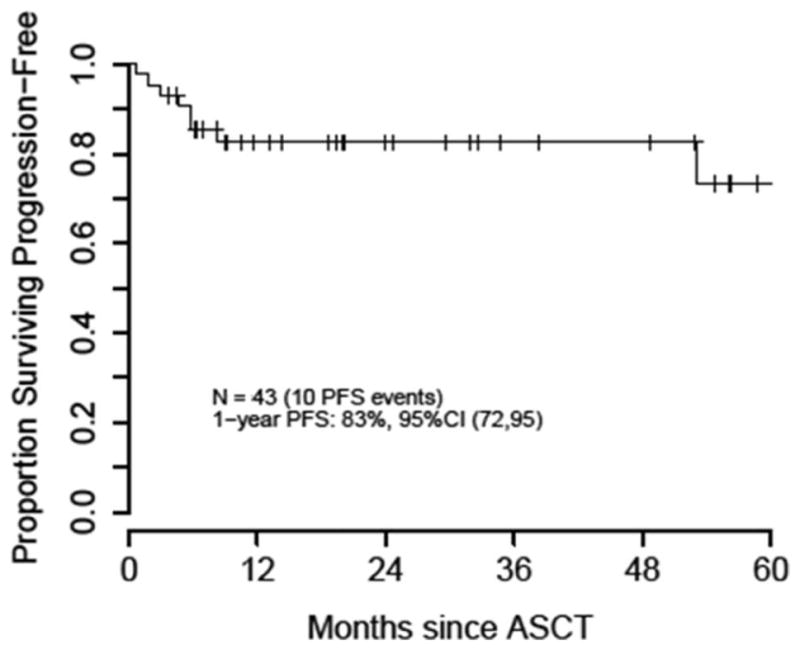
Progression-Free Survival after TBC-Conditioned ASCT
Figure 3.
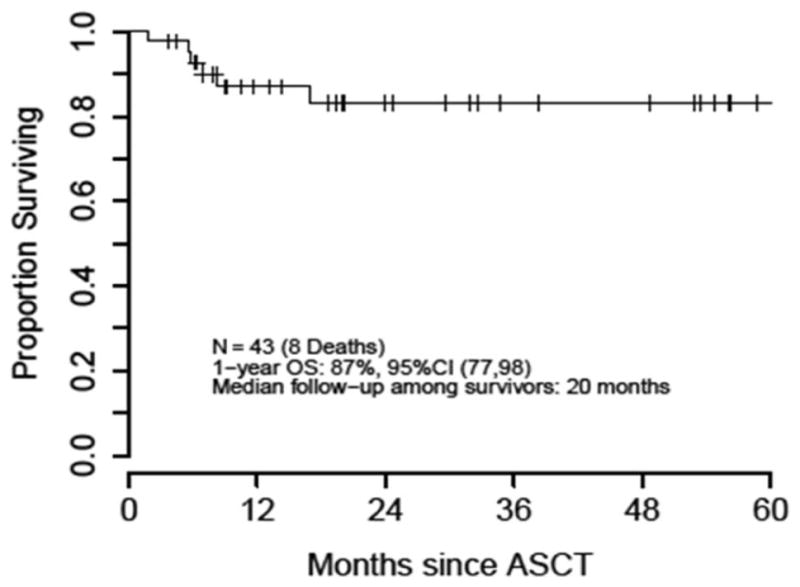
Overall Survival after TBC-Conditioned ASCT
Discussion
This is the most comprehensive analysis of toxicity associated with TBC conditioned HDT-ASCT for CNSL, and this is the first reported study of PK-targeted busulfan and its association with patient characteristics and toxicity in patients with CNSL. While clearly an effective consolidative therapeutic modality, TBC-conditioned ASCT for CNSL is associated with a large non-hematologic toxicity burden. Three patients (7%) died of treatment-related mortality (TRM), appearing potentially greater than the expected contemporary rate for other NHL patients undergoing HDT-ASCT independent of age or comorbidity.9
A recent comprehensive retrospective registry analysis of thiotepa-based conditioned ASCT in Hodgkin lymphoma and NHL showed comparable disease control and toxicity as compared to standard BEAM-conditioned ASCT.18 While many of the grade ≥ 3 non-hematologic toxicities we noted are common after HDT-ASCT, our analysis demonstrates that TBC conditioning is associated with significantly more mucosal toxicity, infections and febrile neutropenia as compared to BEAM or thiotepa, etoposide, cytarabine, melphalan (TEAM).19 Although their data analysis did not specify all subtypes of infection, the rates of viremia, viral organ disease, and immune-related toxicities we observed appear higher than expected after ASCT. Moreover, significantly higher rates of engraftment syndrome have been reported in patients undergoing HDT-ASCT with melphalan conditioning for multiple myeloma as compared to BEAM conditioning for NHL and Hodgkin lymphoma.20 While our retrospective analysis can only raise concerns, the considerable mucosal injury of the TBC preparative regimen elicits a profound inflammatory stimulus that may provide the first signal toward autoimmunity similar to myeloablative conditioning and allogeneic hematopoietic cell transplantation.21–23 This may explain the unexpected cases of engraftment syndrome and tissue biopsy proven auto-GVHD, which are generally considered rare in the ASCT setting and likely represent a spectrum of auto-inflammatory post-ASCT complications.17,24–26
Our study has several limitations inherent to a retrospective analysis in a relatively small patient cohort. As such, we were unable to show that baseline pre-ASCT patient characteristics were associated with incurring more toxicity. In addition, pre-ASCT patient characteristics were not associated with higher than expected first dose busulfan AUC levels in patients treated with PK-targeted busulfan. We chose to study the correlation of busulfan AUC with toxicities in order to find a potential association that could be targeted in a prospective study. While PK-targeted dosing of busulfan resulted in higher than expected initial AUC levels out of the target range in the majority of patients, the strategy appeared to normalize the total busulfan exposure within our acceptable target range. We found that patients having received >2 regimens prior to ASCT had lower first-dose busulfan AUC, though had a poorer 1-year OS than patients who received ≤ 2 prior regimens. By contrast, PFS, OS and number of toxicities were similar between patients who had a first-dose busulfan AUC greater than the median AUC compared to those that did not. While this was evaluated in a limited retrospective cohort, our results suggest that busulfan AUC and total exposure are not solely responsible for toxicity and outcome, highlighting the inherent differences in chemotherapy pharmacokinetics and responses among patients with the same diagnosis and the difficulty in mitigating toxicity with myeloablative conditioning regimens.27
Our results again reaffirmed the favorable and durable PFS and OS with this treatment program.3,5,7,28 Notably, 12 of our patients (28%) were ≥ 60 years-old at the time of transplant, demonstrating that consolidation therapy with TBC-conditioned ASCT remains a viable option for patients of more advanced age with adequate performance status. Despite a heavily pre-treated and heterogeneous patient cohort, there were few POD events. In contrast to WBRT, which has been associated with chronic neurocognitive changes, late neurotoxicity was uncommon with TBC, as only 1 patient (2%) developed chronic mild cognitive impairment post-ASCT. Importantly, this patient had received WBRT prior to HDT-ASCT. It must be noted that unlike our previous prospective study, neuropsychological evaluations were not performed in patients included in this study, which may underestimate the rate of more subtle cognitive impairment among transplanted patients as a result of HDT-ASCT.7,29,30
The lack of large, prospective randomized phase III clinical trials comparing different conditioning regimens and the variability of transplant strategies for this disease among institutions make it difficult to suggest the definitive superiority of one conditioning regimen over another. Given the consistently favorable PFS and OS results we and others have published with TBC conditioning, we feel it is imperative to reduce the unfavorable toxicity profile of this effective regimen. Our analysis has identified areas of investigation to potentially mitigate the significant burden of toxicity with targeted interventions. We are planning a prospective single-arm phase II study whose primary endpoint will be a composite event-free survival (EFS) which will include the most common grade ≥ 3 toxicities as events. We plan on prospectively evaluating busulfan, thiotepa and cyclophosphamide PKs on the study. Secondarily, we plan to perform comprehensive neurotoxicity assessments to prospectively evaluate for possible subtle late neurocognitive dysfunction, and post-ASCT immune reconstitution in order to better elucidate characteristic factors affecting viral immunity and immune-related toxicities.
Highlights.
While effective, TBC-conditioned ASCT for CNSL is associated with suboptimal TRM.
Patient characteristics and busulfan AUC levels did not correlate with increased toxicity.
We identified excessive mucosal toxicity, which will be targeted in future studies.
Acknowledgments
Sources of stipend for Michael Scordo, M.D. include the Mortimer J. Lacher Fellowship Fund and institutional funding. This research was funded in part through the NIH/NCI Cancer Center Support Grant (CCSG – Core Grant) P30 CA008748.
Footnotes
Conflicts of Interest: Matthew J. Matasar, M.D. receives research support from Genentech, Inc. There are no other relevant conflicts of interests in relation to the work described.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Michael Scordo, Department of Medicine, Adult Bone Marrow Transplant (BMT) Service, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, Box 8, New York, NY 10065.
Valkal Bhatt, Department of Medicine, Adult BMT Service, Memorial Sloan Kettering Cancer Center.
Meier Hsu, Department of Biostatistics and Epidemiology, Memorial Sloan Kettering Cancer Center.
Antonio M. Omuro, Department of Neurology, Memorial Sloan Kettering Cancer Center, Department of Neurology, Weill Cornell Medical College.
Matthew J. Matasar, Department of Medicine, Lymphoma Service, Adult BMT Service, Memorial Sloan Kettering Cancer Center Department of Medicine, Weill Cornell Medical College.
Lisa M. DeAngelis, Department of Neurology, Memorial Sloan Kettering Cancer Center, Department of Neurology, Weill Cornell Medical College.
Parastoo B. Dahi, Department of Medicine, Adult BMT Service, Memorial Sloan Kettering Cancer Center, Department of Medicine, Weill Cornell Medical College.
Craig H. Moskowitz, Department of Medicine, Lymphoma Service, Adult BMT Service, Memorial Sloan Kettering Cancer Center, Department of Medicine, Weill Cornell Medical College.
Sergio A Giralt, Department of Medicine, Adult BMT Service, Memorial Sloan Kettering Cancer Center, Department of Medicine, Weill Cornell Medical College.
Craig S. Sauter, Department of Medicine, Adult BMT Service, Memorial Sloan Kettering Cancer Center, Department of Medicine, Weill Cornell Medical College.
References
- 1.Abrey LE. Primary central nervous system lymphoma. Curr Opin Neurol. 2009;22:675–680. doi: 10.1097/WCO.0b013e328332533b. [DOI] [PubMed] [Google Scholar]
- 2.Graber JJ, Omuro A. Pharmacotherapy for primary CNS lymphoma: Progress beyond methotrexate? CNS Drugs. 2011;25(6):447–457. doi: 10.2165/11589030-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Welch MR, Sauter CS, Matasar MJ, et al. Autologous stem cell transplant in recurrent or refractory primary or secondary central nervous system lymphoma using thiotepa, busulfan and cyclophosphamide. Leuk Lymphoma. 2015;56(2):361–367. doi: 10.3109/10428194.2014.916800. [DOI] [PubMed] [Google Scholar]
- 4.Omuro AMP, Correa D, Moskowitz C, et al. Rituximab, methotrexate (MTX), procarbazine, and vincristine (R-MPV) followed by consolidation high-dose chemotherapy (HDC) and autologous stem-cell transplant (ASCT) for newly diagnosed primary CNS lymphoma (PCNSL) J Clin Oncol. 2012;(1) http://meeting.ascopubs.org/cgi/content/abstract/30/15_suppl/2008?sid=e5c17e3a-9639-4a55-9c10-ab95c079e5e9.
- 5.Soussain C, Hoang-Xuan K, Taillandier L, et al. Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: Société Française de Greffe de Moëlle Osseuse-Thérapie Cellulaire. J Clin Oncol. 2008;26(15):2512–2518. doi: 10.1200/JCO.2007.13.5533. [DOI] [PubMed] [Google Scholar]
- 6.Cote GM, Hochberg EP, Muzikansky A, et al. Autologous Stem Cell Transplantation with Thiotepa, Busulfan, and Cyclophosphamide (TBC) Conditioning in Patients with CNS Involvement by Non-Hodgkin Lymphoma. Biol Blood Marrow Transplant. 2012;18(1):76–83. doi: 10.1016/j.bbmt.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Omuro A, Correa DD, DeAngelis LM, et al. R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood. 2015;125(9):1403–1410. doi: 10.1182/blood-2014-10-604561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abrey LE, Moskowitz CH, Mason WP, et al. Intensive methotrexate and cytarabine followed by high-dose chemotherapy with autologous stem-cell rescue in patients with newly diagnosed primary CNS lymphoma: An intent-to-treat analysis. J Clin Oncol. 2003;21(22):4151–4156. doi: 10.1200/JCO.2003.05.024. [DOI] [PubMed] [Google Scholar]
- 9.Dahi PB, Tamari R, Devlin SM, et al. Favorable outcomes in elderly patients undergoing high-dose therapy and autologous stem cell transplantation for non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2014;20(12):2004–2009. doi: 10.1016/j.bbmt.2014.08.019. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=prem&NEWS=N&AN=25 175794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez HF, Tran HT, Albrecht F, Lennon S, Caldera H, Goodman MS. Evaluation of safety and pharmacokinetics of administering intravenous busulfan in a twice-daily or daily schedule to patients with advanced hematologic malignant disease undergoing stem cell transplantation. Biol Blood Marrow Transplant. 2002;8(9):486–492. doi: 10.1053/bbmt.2002.v8.pm12374453. http://dx.doi.org/10.1053/bbmt.2002.v8.pm12374453. [DOI] [PubMed] [Google Scholar]
- 11.Madden T, de Lima M, Thapar N, et al. Pharmacokinetics of Once-Daily IV Busulfan as Part of Pretransplantation Preparative Regimens: A Comparison with an Every 6-Hour Dosing Schedule. Biol Blood Marrow Transplant. 2007;13(1):56–64. doi: 10.1016/j.bbmt.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H, Graiser M, Hutcherson DA, et al. Pharmacokinetic-Directed High-Dose Busulfan Combined with Cyclophosphamide and Etoposide Results in Predictable Drug Levels and Durable Long-Term Survival in Lymphoma Patients Undergoing Autologous Stem Cell Transplantation. Biol Blood Marrow Transplant. 2012;18(8):1287–1294. doi: 10.1016/j.bbmt.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Efron B. Logistic Regression, Survival Analysis, and the Kaplan-Meier Curve. J Am Stat Assoc. 1988;83(402):414–425. doi: 10.1080/01621459.1988.10478612. [DOI] [Google Scholar]
- 14.Sorror ML. How I assess comorbidities before hematopoietic cell transplantation. Blood. 2013;121(15):2854–2863. doi: 10.1182/blood-2012-09-455063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abrey LE, Batchelor TT, Ferreri AJM, et al. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 2005;23(22):5034–5043. doi: 10.1200/JCO.2005.13.524. [DOI] [PubMed] [Google Scholar]
- 16.Cheson BBD, Horning SJ, Coiffier B, et al. Report of an International Workshop to Standardize Response Criteria for Non-Hodgkin ’ s Lymphomas. J Clin Oncol. 2011;17(4):1244–1253. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 17.Cornell RF, Hari P, Drobyski WR. Engraftment Syndrome after Autologous Stem Cell Transplantation: An Update Unifying the Definition and Management Approach. Biol Blood Marrow Transplant. 2015;21(12):2061–2068. doi: 10.1016/j.bbmt.2015.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sellner L, Boumendil A, Finel H, et al. Thiotepa-based high-dose therapy for autologous stem cell transplantation in lymphoma: a retrospective study from the EBMT. Bone Marrow Transplant. 2015;2014(October 2014):1–7. doi: 10.1038/bmt.2015.273. [DOI] [PubMed] [Google Scholar]
- 19.Chaudhry HM, Bruce AJ, Wolf RC, et al. The Incidence and Severity of Oral Mucositis among Allogeneic Hematopoietic Stem Cell Transplantation Patients: A Systematic Review. Biol Blood Marrow Transplant. 2015 doi: 10.1016/j.bbmt.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Cornell RF, Hari P, Zhang M-J, et al. Divergent effects of novel immunomodulatory agents and cyclophosphamide on the risk of engraftment syndrome after autologous peripheral blood stem cell transplantation for multiple myeloma. Biol Blood Marrow Transplant. 2013;19(9):1368–1373. doi: 10.1016/j.bbmt.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 21.Bilgrami S, Aslanzadeh J, Feingold JM, et al. Cytomegalovirus viremia, viruria and disease after autologous peripheral blood stem cell transplantation: no need for surveillance. Bone Marrow Transpl. 1999;24(1):69–73. doi: 10.1038/sj.bmt.1701827. [DOI] [PubMed] [Google Scholar]
- 22.Fassas A, Bola??os-Meade J, Buddharaju LN, et al. Cytomegalovirus infection and non-neutropenic fever after autologous stem cell transplantation: High rates of reactivation in patients with multiple myeloma and lymphoma. Br J Haematol. 2001;112(1):237–241. doi: 10.1046/j.1365-2141.2001.02487.x. [DOI] [PubMed] [Google Scholar]
- 23.Ljungman P, Hakki M, Boeckh M. Cytomegalovirus in hematopoietic stem cell transplant recipients. Infect Dis Clin North Am. 2010;24(2):319–337. doi: 10.1016/j.idc.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Drobyski WR, Hari P, Keever-Taylor C, Komorowski R, Grossman W. Severe autologous GVHD after hematopoietic progenitor cell transplantation for multiple myeloma. Bone Marrow Transplant. 2009;43(May 2008):169–177. doi: 10.1038/bmt.2008.295. [DOI] [PubMed] [Google Scholar]
- 25.Mielcarek M, Martin PJ, Leisenring W, et al. Graft-versus-host disease after nonmyeloablative versus conventional hematopoietic stem cell transplantation. Blood. 2003;102(2):756–762. doi: 10.1182/blood-2002-08-2628. [DOI] [PubMed] [Google Scholar]
- 26.Zenz T, Ritgen M, Dreger P, et al. Autologous graft-versus-host disease-like syndrome after an alemtuzumab-containing conditioning regimen and autologous stem cell transplantation for chronic lymphocytic leukemia. Blood. 2006;108(6):2127–2130. doi: 10.1182/blood-2006-04-007898. [DOI] [PubMed] [Google Scholar]
- 27.Petros WP, Hopkins PJ, Spruill S, et al. Associations between drug metabolism genotype, chemotherapy pharmacokinetics, and overall survival in patients with breast cancer. J Clin Oncol. 2005;23(25):6117–6125. doi: 10.1200/JCO.2005.06.075. [DOI] [PubMed] [Google Scholar]
- 28.Soussain C, Suzan F, Hoang-Xuan K, et al. Results of intensive chemotherapy followed by hematopoietic stem-cell rescue in 22 patients with refractory or recurrent primary CNS lymphoma or intraocular lymphoma. J Clin Oncol. 2001;19(3):742–749. doi: 10.1200/JCO.2001.19.3.742. [DOI] [PubMed] [Google Scholar]
- 29.Syrjala KL, Artherholt SB, Kurland BF, et al. Prospective neurocognitive function over 5 years after allogeneic hematopoietic cell transplantation for cancer survivors compared with matched controls at 5 years. J Clin Oncol. 2011;29(17):2397–2404. doi: 10.1200/JCO.2010.33.9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah AJ, Epport K, Azen C, et al. Progressive declines in neurocognitive function among survivors of hematopoietic stem cell transplantation for pediatric hematologic malignancies. J Pediatr Hematol Oncol. 2008;30(6):411–418. doi: 10.1097/MPH.0b013e318168e750. [DOI] [PubMed] [Google Scholar]