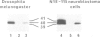Abstract
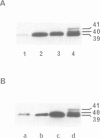
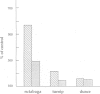
G proteins couple receptors for extracellular signals to several intracellular effector systems and play a key role in signalling transduction mechanisms. In particulate preparations of Drosophila melanogaster heads, only one substrate for pertussis toxin at 39-40 kd was detected. This substrate, which showed only one isoform when analysed by isoelectric focusing, was recognized by immunoblotting and immunoprecipitation techniques using a polyclonal antibody against the alpha subunit of the Go protein purified from bovine brain and can be thus considered as a Go-like protein. Antibodies obtained against a carboxy-terminal sequence of the alpha subunit of Go (but not of Gi1 or Gi2) and against an internal sequence shared by all the alpha subunits, were also able to cross-react with the alpha subunit of this protein in insects. We have also studied the Go-like protein in several D.melanogaster mutants, primarily in memory and learning mutants. In these mutants there was a sex-dependent enhancement in pertussis toxin-catalysed ADP-ribosylation with respect to the wild-type. This increase could be attributed in part to an increase in the alpha subunit of the Go-like protein, as revealed by immunoblotting with anti-Go alpha polyclonal antibody. This report constitutes the first evidence for the participation of a Go protein in learning and memory.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Audigier Y., Pantaloni C., Bigay J., Deterre P., Bockaert J., Homburger V. Tissue expression and phylogenetic appearance of the beta and gamma subunits of GTP binding proteins. FEBS Lett. 1985 Sep 9;189(1):1–7. doi: 10.1016/0014-5793(85)80830-9. [DOI] [PubMed] [Google Scholar]
- Bentrop J., Paulsen R. Light-modulated ADP-ribosylation, protein phosphorylation and protein binding in isolated fly photoreceptor membranes. Eur J Biochem. 1986 Nov 17;161(1):61–67. doi: 10.1111/j.1432-1033.1986.tb10124.x. [DOI] [PubMed] [Google Scholar]
- Brabet P., Dumuis A., Sebben M., Pantaloni C., Bockaert J., Homburger V. Immunocytochemical localization of the guanine nucleotide-binding protein Go in primary cultures of neuronal and glial cells. J Neurosci. 1988 Feb;8(2):701–708. doi: 10.1523/JNEUROSCI.08-02-00701.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codina J., Hildebrandt J., Iyengar R., Birnbaumer L., Sekura R. D., Manclark C. R. Pertussis toxin substrate, the putative Ni component of adenylyl cyclases, is an alpha beta heterodimer regulated by guanine nucleotide and magnesium. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4276–4280. doi: 10.1073/pnas.80.14.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y. Neurogenetic dissection of learning and short-term memory in Drosophila. Annu Rev Neurosci. 1988;11:537–563. doi: 10.1146/annurev.ne.11.030188.002541. [DOI] [PubMed] [Google Scholar]
- Ewald D. A., Sternweis P. C., Miller R. J. Guanine nucleotide-binding protein Go-induced coupling of neuropeptide Y receptors to Ca2+ channels in sensory neurons. Proc Natl Acad Sci U S A. 1988 May;85(10):3633–3637. doi: 10.1073/pnas.85.10.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrion J., Brabet P., Nguyen Than Dao B., Homburger V., Dumuis A., Sebben M., Rouot B., Bockaert J. Ultrastructural localization of the GTP-binding protein Go in neurons. Cell Signal. 1989;1(1):107–123. doi: 10.1016/0898-6568(89)90025-9. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Harris-Warrick R. M., Hammond C., Paupardin-Tritsch D., Homburger V., Rouot B., Bockaert J., Gerschenfeld H. M. An alpha 40 subunit of a GTP-binding protein immunologically related to Go mediates a dopamine-induced decrease of Ca2+ current in snail neurons. Neuron. 1988 Mar;1(1):27–32. doi: 10.1016/0896-6273(88)90206-1. [DOI] [PubMed] [Google Scholar]
- Hartley D. A., Preiss A., Artavanis-Tsakonas S. A deduced gene product from the Drosophila neurogenic locus, enhancer of split, shows homology to mammalian G-protein beta subunit. Cell. 1988 Dec 2;55(5):785–795. doi: 10.1016/0092-8674(88)90134-1. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Rosenthal W., Trautwein W., Schultz G. The GTP-binding protein, Go, regulates neuronal calcium channels. 1987 Jan 29-Feb 4Nature. 325(6103):445–447. doi: 10.1038/325445a0. [DOI] [PubMed] [Google Scholar]
- Higashijima T., Ferguson K. M., Sternweis P. C., Smigel M. D., Gilman A. G. Effects of Mg2+ and the beta gamma-subunit complex on the interactions of guanine nucleotides with G proteins. J Biol Chem. 1987 Jan 15;262(2):762–766. [PubMed] [Google Scholar]
- Hildebrandt J. D., Sekura R. D., Codina J., Iyengar R., Manclark C. R., Birnbaumer L. Stimulation and inhibition of adenylyl cyclases mediated by distinct regulatory proteins. Nature. 1983 Apr 21;302(5910):706–709. doi: 10.1038/302706a0. [DOI] [PubMed] [Google Scholar]
- Homburger V., Brabet P., Audigier Y., Pantaloni C., Bockaert J., Rouot B. Immunological localization of the GTP-binding protein Go in different tissues of vertebrates and invertebrates. Mol Pharmacol. 1987 Apr;31(4):313–319. [PubMed] [Google Scholar]
- Hopkins R. S., Stamnes M. A., Simon M. I., Hurley J. B. Cholera toxin and pertussis toxin substrates and endogenous ADP-ribosyltransferase activity in Drosophila melanogaster. Biochim Biophys Acta. 1988 Jul 29;970(3):355–362. doi: 10.1016/0167-4889(88)90135-8. [DOI] [PubMed] [Google Scholar]
- Hurley J. B. Molecular properties of the cGMP cascade of vertebrate photoreceptors. Annu Rev Physiol. 1987;49:793–812. doi: 10.1146/annurev.ph.49.030187.004045. [DOI] [PubMed] [Google Scholar]
- Ito I., Okada D., Sugiyama H. Pertussis toxin suppresses long-term potentiation of hippocampal mossy fiber synapses. Neurosci Lett. 1988 Jul 19;90(1-2):181–185. doi: 10.1016/0304-3940(88)90808-7. [DOI] [PubMed] [Google Scholar]
- Itoh H., Kozasa T., Nagata S., Nakamura S., Katada T., Ui M., Iwai S., Ohtsuka E., Kawasaki H., Suzuki K. Molecular cloning and sequence determination of cDNAs for alpha subunits of the guanine nucleotide-binding proteins Gs, Gi, and Go from rat brain. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3776–3780. doi: 10.1073/pnas.83.11.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar R., Rich K. A., Herberg J. T., Premont R. T., Codina J. Glucagon receptor-mediated activation of Gs is accompanied by subunit dissociation. J Biol Chem. 1988 Oct 25;263(30):15348–15353. [PubMed] [Google Scholar]
- Jelsema C. L., Axelrod J. Stimulation of phospholipase A2 activity in bovine rod outer segments by the beta gamma subunits of transducin and its inhibition by the alpha subunit. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3623–3627. doi: 10.1073/pnas.84.11.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. T., Reed R. R. Molecular cloning of five GTP-binding protein cDNA species from rat olfactory neuroepithelium. J Biol Chem. 1987 Oct 15;262(29):14241–14249. [PubMed] [Google Scholar]
- Kim D., Lewis D. L., Graziadei L., Neer E. J., Bar-Sagi D., Clapham D. E. G-protein beta gamma-subunits activate the cardiac muscarinic K+-channel via phospholipase A2. Nature. 1989 Feb 9;337(6207):557–560. doi: 10.1038/337557a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lochrie M. A., Simon M. I. G protein multiplicity in eukaryotic signal transduction systems. Biochemistry. 1988 Jul 12;27(14):4957–4965. doi: 10.1021/bi00414a001. [DOI] [PubMed] [Google Scholar]
- Lucchesi J. C., Manning J. E. Gene dosage compensation in Drosophila melanogaster. Adv Genet. 1987;24:371–429. doi: 10.1016/s0065-2660(08)60013-9. [DOI] [PubMed] [Google Scholar]
- Mattera R., Codina J., Sekura R. D., Birnbaumer L. Guanosine 5'-O-(3-thiotriphosphate) reduces ADP-ribosylation of the inhibitory guanine nucleotide-binding regulatory protein of adenylyl cyclase (Ni) by pertussis toxin without causing dissociation of the subunits of Ni. Evidence of existence of heterotrimeric pt+ and pt- conformations of Ni. J Biol Chem. 1987 Aug 15;262(23):11247–11251. [PubMed] [Google Scholar]
- Mattera R., Graziano M. P., Yatani A., Zhou Z., Graf R., Codina J., Birnbaumer L., Gilman A. G., Brown A. M. Splice variants of the alpha subunit of the G protein Gs activate both adenylyl cyclase and calcium channels. Science. 1989 Feb 10;243(4892):804–807. doi: 10.1126/science.2536957. [DOI] [PubMed] [Google Scholar]
- Mitschulat H. Dynamic properties of the Ca2+/calmodulin-dependent protein kinase in Drosophila: identification of a synapsin I-like protein. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5988–5992. doi: 10.1073/pnas.86.15.5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neer E. J., Clapham D. E. Roles of G protein subunits in transmembrane signalling. Nature. 1988 May 12;333(6169):129–134. doi: 10.1038/333129a0. [DOI] [PubMed] [Google Scholar]
- Neer E. J., Lok J. M., Wolf L. G. Purification and properties of the inhibitory guanine nucleotide regulatory unit of brain adenylate cyclase. J Biol Chem. 1984 Nov 25;259(22):14222–14229. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Olate J., Jorquera H., Purcell P., Codina J., Birnbaumer L., Allende J. E. Molecular cloning and sequence determination of a cDNA coding for the alpha-subunit of a Go-type protein of Xenopus laevis oocytes. FEBS Lett. 1989 Feb 13;244(1):188–192. doi: 10.1016/0014-5793(89)81190-1. [DOI] [PubMed] [Google Scholar]
- Orgad S., Llamazares S., Dudai Y., Ferrús A. The Drosophila Mutant tetanic Interacts with a Gene Complex Including the Structural Locus of K+ Channels and Shows Altered Dephosphorylation and Learning. Eur J Neurosci. 1989 Jul;1(4):367–373. doi: 10.1111/j.1460-9568.1989.tb00801.x. [DOI] [PubMed] [Google Scholar]
- Otey C. A., Kalnoski M. H., Lessard J. L., Bulinski J. C. Immunolocalization of the gamma isoform of nonmuscle actin in cultured cells. J Cell Biol. 1986 May;102(5):1726–1737. doi: 10.1083/jcb.102.5.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Slepak V. Z., Pronin A. N., Shlensky A. B., Levina N. B., Voeikov V. L., Lipkin V. M. Primary structure of bovine cerebellum GTP-binding protein G39 and its effect on the adenylate cyclase system. FEBS Lett. 1987 Dec 21;226(1):91–95. doi: 10.1016/0014-5793(87)80557-4. [DOI] [PubMed] [Google Scholar]
- Provost N. M., Somers D. E., Hurley J. B. A Drosophila melanogaster G protein alpha subunit gene is expressed primarily in embryos and pupae. J Biol Chem. 1988 Aug 25;263(24):12070–12076. [PubMed] [Google Scholar]
- Quan F., Wolfgang W. J., Forte M. A. The Drosophila gene coding for the alpha subunit of a stimulatory G protein is preferentially expressed in the nervous system. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4321–4325. doi: 10.1073/pnas.86.11.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouot B., Carrette J., Lafontan M., Lan Tran P., Fehrentz J. A., Bockaert J., Toutant M. The adipocyte Go alpha-immunoreactive polypeptide is different from the alpha subunit of the brain Go protein. Biochem J. 1989 May 15;260(1):307–310. doi: 10.1042/bj2600307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G. M. Drosophila melanogaster as an experimental organism. Science. 1988 Jun 10;240(4858):1453–1459. doi: 10.1126/science.3131880. [DOI] [PubMed] [Google Scholar]
- Schwartz J. H., Greenberg S. M. Molecular mechanisms for memory: second-messenger induced modifications of protein kinases in nerve cells. Annu Rev Neurosci. 1987;10:459–476. doi: 10.1146/annurev.ne.10.030187.002331. [DOI] [PubMed] [Google Scholar]
- Selinger Z., Minke B. Inositol lipid cascade of vision studied in mutant flies. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 1):333–341. doi: 10.1101/sqb.1988.053.01.040. [DOI] [PubMed] [Google Scholar]
- Sternweis P. C., Robishaw J. D. Isolation of two proteins with high affinity for guanine nucleotides from membranes of bovine brain. J Biol Chem. 1984 Nov 25;259(22):13806–13813. [PubMed] [Google Scholar]
- Thambi N. C., Quan F., Wolfgang W. J., Spiegel A., Forte M. Immunological and molecular characterization of Go alpha-like proteins in the Drosophila central nervous system. J Biol Chem. 1989 Nov 5;264(31):18552–18560. [PubMed] [Google Scholar]
- VanDongen A. M., Codina J., Olate J., Mattera R., Joho R., Birnbaumer L., Brown A. M. Newly identified brain potassium channels gated by the guanine nucleotide binding protein Go. Science. 1988 Dec 9;242(4884):1433–1437. doi: 10.1126/science.3144040. [DOI] [PubMed] [Google Scholar]
- Watkins D. C., Northup J. K., Malbon C. C. Regulation of G-proteins in differentiation. Altered ratio of alpha- to beta-subunits in 3T3-L1 cells. J Biol Chem. 1987 Aug 5;262(22):10651–10657. [PubMed] [Google Scholar]
- Yarfitz S., Provost N. M., Hurley J. B. Cloning of a Drosophila melanogaster guanine nucleotide regulatory protein beta-subunit gene and characterization of its expression during development. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7134–7138. doi: 10.1073/pnas.85.19.7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J., Shortridge R. D., Bloomquist B. T., Schneuwly S., Perdew M. H., Pak W. L. Molecular characterization of Drosophila gene encoding G0 alpha subunit homolog. J Biol Chem. 1989 Nov 5;264(31):18536–18543. [PubMed] [Google Scholar]
- de Sousa S. M., Hoveland L. L., Yarfitz S., Hurley J. B. The Drosophila Go alpha-like G protein gene produces multiple transcripts and is expressed in the nervous system and in ovaries. J Biol Chem. 1989 Nov 5;264(31):18544–18551. [PubMed] [Google Scholar]