Abstract
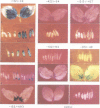
We have fused various DNA sequences located upstream of the Drosophila melanogaster s36 chorion gene TATA box to a heterologous basal promoter and reporter gene (hsp70/lacZ). The expression of these constructs, following P-element-mediated germline transformation, was examined in 144 independent lines by histological staining of dissected ovaries for beta-galactosidase activity. A short 84 bp segment of the proximal 5' flanking DNA was sufficient to confer a wild-type gene expression pattern, including temporal specificity for early choriogenic follicles. Surprisingly, initial expression was very localized at the anterior and posterior poles of the follicle. The downstream half of that DNA segment permitted expression at both poles, but especially at the anterior tip, while the upstream half only favored expression in the posterior pole; these results suggested the existence of multiple, spatially specific cis-regulatory elements. When the proximal 84 bp segment was placed 1.5 kb upstream of the basal promoter, beta-galactosidase activity was observed in an altered spatial pattern, indicating that the cis-regulatory element(s) that favor expression in the posterior half of the follicle are position independent, while the element(s) that favor expression elsewhere in the follicle are position sensitive. A distal regulatory segment containing redundant DNA element(s) specific for expression in the anterior pole was identified much further upstream of s36. Thus, the expression of this chorion gene throughout the follicular epithelium is actually composite, occurring in distinct spatial domains under the control of corresponding DNA elements.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berleth T., Burri M., Thoma G., Bopp D., Richstein S., Frigerio G., Noll M., Nüsslein-Volhard C. The role of localization of bicoid RNA in organizing the anterior pattern of the Drosophila embryo. EMBO J. 1988 Jun;7(6):1749–1756. doi: 10.1002/j.1460-2075.1988.tb03004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Delidakis C., Kafatos F. C. Amplification of a chorion gene cluster in Drosophila is subject to multiple cis-regulatory elements and to long-range position effects. J Mol Biol. 1987 Sep 5;197(1):11–26. doi: 10.1016/0022-2836(87)90605-x. [DOI] [PubMed] [Google Scholar]
- Driever W., Thoma G., Nüsslein-Volhard C. Determination of spatial domains of zygotic gene expression in the Drosophila embryo by the affinity of binding sites for the bicoid morphogen. Nature. 1989 Aug 3;340(6232):363–367. doi: 10.1038/340363a0. [DOI] [PubMed] [Google Scholar]
- Fasano L., Kerridge S. Monitoring positional information during oogenesis in adult Drosophila. Development. 1988 Oct;104(2):245–253. doi: 10.1242/dev.104.Supplement.245. [DOI] [PubMed] [Google Scholar]
- Fischer J. A., Maniatis T. Drosophila Adh: a promoter element expands the tissue specificity of an enhancer. Cell. 1988 May 6;53(3):451–461. doi: 10.1016/0092-8674(88)90165-1. [DOI] [PubMed] [Google Scholar]
- Galanopoulos V. K., Orr W., Szabad J., Kafatos F. C. Genetic analysis of chorion formation in Drosophila melanogaster: I. The effects of one somatic-specific and seven germ-line-specific mutations. Dev Genet. 1989;10(2):87–97. doi: 10.1002/dvg.1020100204. [DOI] [PubMed] [Google Scholar]
- Garabedian M. J., Shepherd B. M., Wensink P. C. A tissue-specific transcription enhancer from the Drosophila yolk protein 1 gene. Cell. 1986 Jun 20;45(6):859–867. doi: 10.1016/0092-8674(86)90560-x. [DOI] [PubMed] [Google Scholar]
- Griffin-Shea R., Thireos G., Kafatos F. C. Organization of a cluster of four chorion genes in Drosophila and its relationship to developmental expression and amplification. Dev Biol. 1982 Jun;91(2):325–336. doi: 10.1016/0012-1606(82)90039-2. [DOI] [PubMed] [Google Scholar]
- Haenlin M., Roos C., Cassab A., Mohier E. Oocyte-specific transcription of fs(1)K10: a Drosophila gene affecting dorsal-ventral developmental polarity. EMBO J. 1987 Mar;6(3):801–807. doi: 10.1002/j.1460-2075.1987.tb04822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiromi Y., Gehring W. J. Regulation and function of the Drosophila segmentation gene fushi tarazu. Cell. 1987 Sep 11;50(6):963–974. doi: 10.1016/0092-8674(87)90523-x. [DOI] [PubMed] [Google Scholar]
- Hiromi Y., Kuroiwa A., Gehring W. J. Control elements of the Drosophila segmentation gene fushi tarazu. Cell. 1985 Dec;43(3 Pt 2):603–613. doi: 10.1016/0092-8674(85)90232-6. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Mitsialis S. A., Spoerel N., Mariani B., Lingappa J. R., Delidakis C. Studies on the developmentally regulated expression and amplification of insect chorion genes. Cold Spring Harb Symp Quant Biol. 1985;50:537–547. doi: 10.1101/sqb.1985.050.01.066. [DOI] [PubMed] [Google Scholar]
- Kalfayan L., Levine J., Orr-Weaver T., Parks S., Wakimoto B., de Cicco D., Spradling A. Localization of sequences regulating Drosophila chorion gene amplification and expression. Cold Spring Harb Symp Quant Biol. 1985;50:527–535. doi: 10.1101/sqb.1985.050.01.065. [DOI] [PubMed] [Google Scholar]
- Lehmann R., Nüsslein-Volhard C. Abdominal segmentation, pole cell formation, and embryonic polarity require the localized activity of oskar, a maternal gene in Drosophila. Cell. 1986 Oct 10;47(1):141–152. doi: 10.1016/0092-8674(86)90375-2. [DOI] [PubMed] [Google Scholar]
- Logan S. K., Garabedian M. J., Wensink P. C. DNA regions that regulate the ovarian transcriptional specificity of Drosophila yolk protein genes. Genes Dev. 1989 Sep;3(9):1453–1461. doi: 10.1101/gad.3.9.1453. [DOI] [PubMed] [Google Scholar]
- Margaritis L. H., Kafatos F. C., Petri W. H. The eggshell of Drosophila melanogaster. I. Fine structure of the layers and regions of the wild-type eggshell. J Cell Sci. 1980 Jun;43:1–35. doi: 10.1242/jcs.43.1.1. [DOI] [PubMed] [Google Scholar]
- Mariani B. D., Lingappa J. R., Kafatos F. C. Temporal regulation in development: negative and positive cis regulators dictate the precise timing of expression of a Drosophila chorion gene. Proc Natl Acad Sci U S A. 1988 May;85(9):3029–3033. doi: 10.1073/pnas.85.9.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsialis S. A., Spoerel N., Leviten M., Kafatos F. C. A short 5'-flanking DNA region is sufficient for developmentally correct expression of moth chorion genes in Drosophila. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7987–7991. doi: 10.1073/pnas.84.22.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nüsslein-Volhard C., Frohnhöfer H. G., Lehmann R. Determination of anteroposterior polarity in Drosophila. Science. 1987 Dec 18;238(4834):1675–1681. doi: 10.1126/science.3686007. [DOI] [PubMed] [Google Scholar]
- O'Kane C. J., Gehring W. J. Detection in situ of genomic regulatory elements in Drosophila. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9123–9127. doi: 10.1073/pnas.84.24.9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr W., Komitopoulou K., Kafatos F. C. Mutants suppressing in trans chorion gene amplification in Drosophila. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3773–3777. doi: 10.1073/pnas.81.12.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks S., Wakimoto B., Spradling A. Replication and expression of an X-linked cluster of Drosophila chorion genes. Dev Biol. 1986 Sep;117(1):294–305. doi: 10.1016/0012-1606(86)90372-6. [DOI] [PubMed] [Google Scholar]
- Petri W. H., Wyman A. R., Kafatos F. C. Specific protein synthesis in cellular differentiation. III. The eggshell proteins of Drosophila melanogaster and their program of synthesis. Dev Biol. 1976 Mar;49(1):185–199. doi: 10.1016/0012-1606(76)90266-9. [DOI] [PubMed] [Google Scholar]
- Price J. V., Clifford R. J., Schüpbach T. The maternal ventralizing locus torpedo is allelic to faint little ball, an embryonic lethal, and encodes the Drosophila EGF receptor homolog. Cell. 1989 Mar 24;56(6):1085–1092. doi: 10.1016/0092-8674(89)90641-7. [DOI] [PubMed] [Google Scholar]
- Raghavan K. V., Crosby M. A., Mathers P. H., Meyerowitz E. M. Sequences sufficient for correct regulation of Sgs-3 lie close to or within the gene. EMBO J. 1986 Dec 1;5(12):3321–3326. doi: 10.1002/j.1460-2075.1986.tb04646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano C. P., Bienz-Tadmor B., Mariani B. D., Kafatos F. C. Both early and late Drosophila chorion gene promoters confer correct temporal, tissue and sex specificity on a reporter Adh gene. EMBO J. 1988 Mar;7(3):783–790. doi: 10.1002/j.1460-2075.1988.tb02876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982 Oct 22;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Vectors for P element-mediated gene transfer in Drosophila. Nucleic Acids Res. 1983 Sep 24;11(18):6341–6351. doi: 10.1093/nar/11.18.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüpbach T. Germ line and soma cooperate during oogenesis to establish the dorsoventral pattern of egg shell and embryo in Drosophila melanogaster. Cell. 1987 Jun 5;49(5):699–707. doi: 10.1016/0092-8674(87)90546-0. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. The effect of chromosomal position on the expression of the Drosophila xanthine dehydrogenase gene. Cell. 1983 Aug;34(1):47–57. doi: 10.1016/0092-8674(83)90135-6. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982 Oct 22;218(4570):341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- Wakimoto B. T., Kalfayan L. J., Spradling A. C. Developmentally regulated expression of Drosophila chorion genes introduced at diverse chromosomal positions. J Mol Biol. 1986 Jan 5;187(1):33–45. doi: 10.1016/0022-2836(86)90404-3. [DOI] [PubMed] [Google Scholar]
- Waring G. L., Mahowald A. P. Identification and time of synthesis of chorion proteins in Drosophila melanogaster. Cell. 1979 Mar;16(3):599–607. doi: 10.1016/0092-8674(79)90033-3. [DOI] [PubMed] [Google Scholar]