Abstract
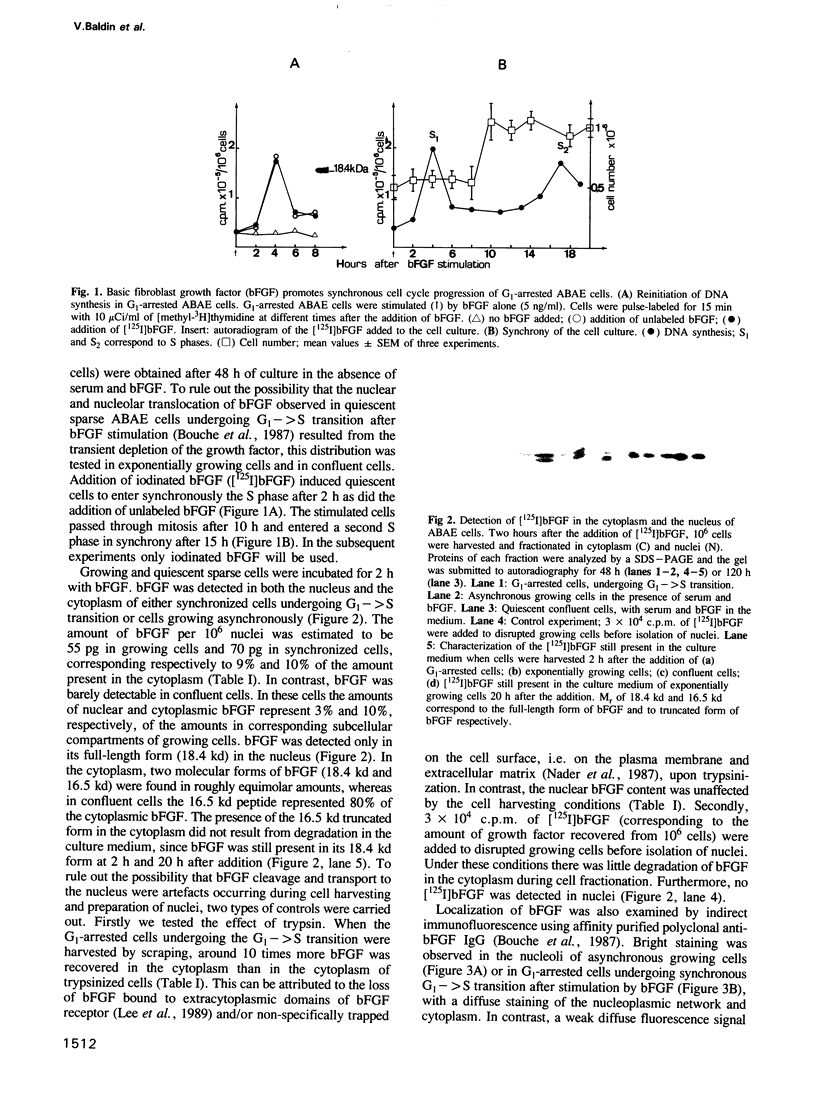
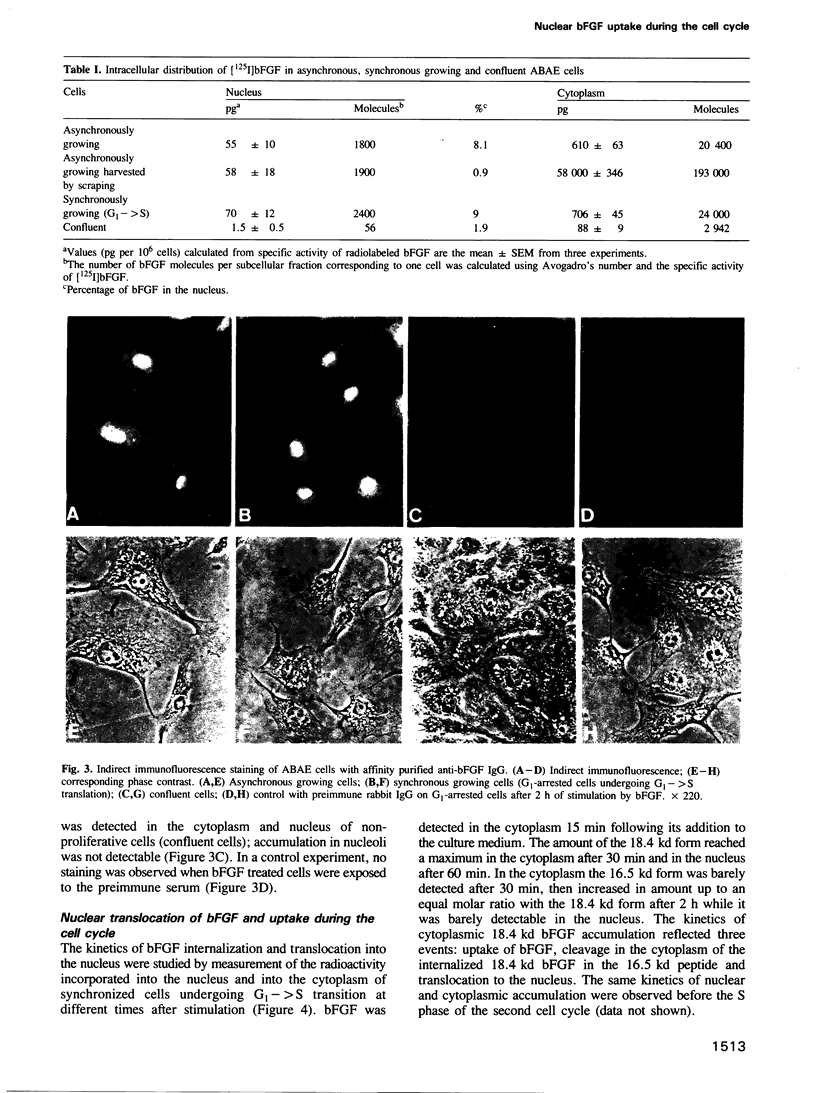
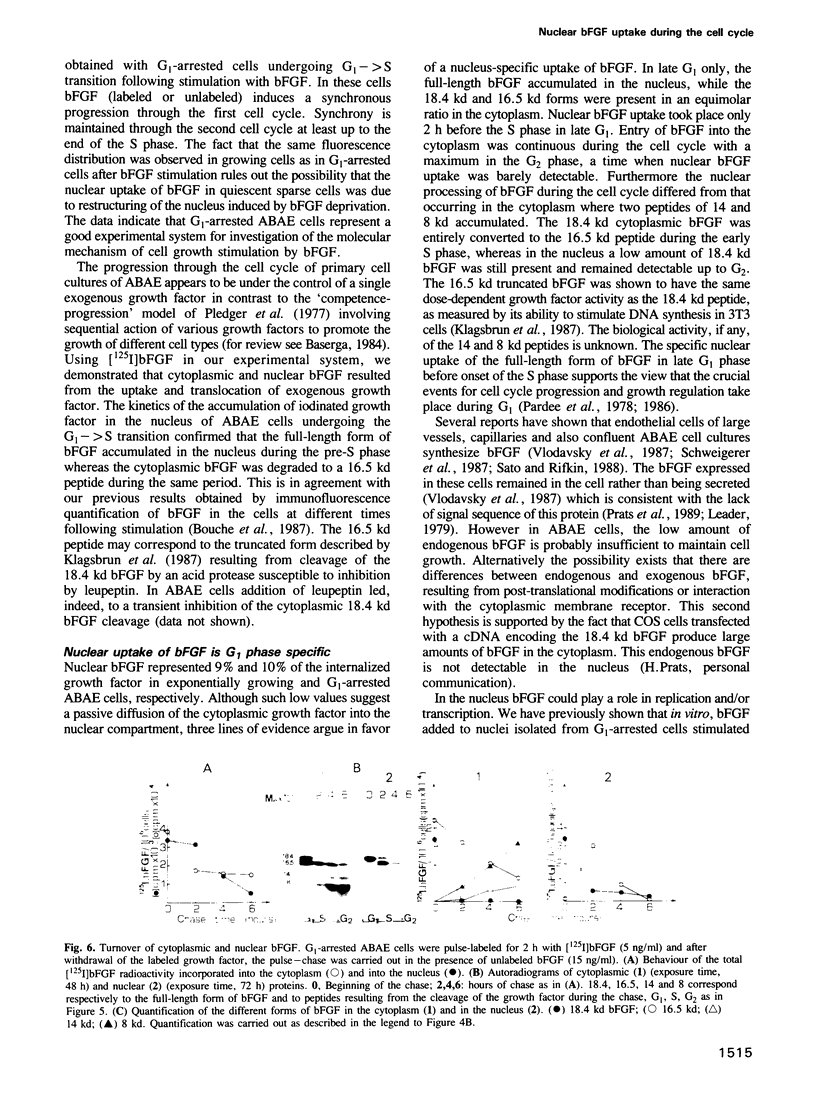
Primary cultures of adult bovine aortic endothelial (ABAE) cells require bFGF to grow. G1-arrested cells, obtained after 48 h without serum and bFGF, were found to enter S phase and grow synchronously for at least two generations on addition of bFGF. In growing cells bFGF was detected both in the cytoplasm (90%) and in the nucleus (10%) where it accumulates in the nucleolus. It was not detected in the nucleus of confluent cells. bFGF uptake was continuous in the cytoplasm throughout the cell cycle with a maximum in G2, while nuclear uptake occurred only in late G1. Cytoplasmic bFGF (18.4 kd) is cleaved into a 16.5 kd peptide in G1 (t1/2 = 30 min). In the nucleus the 18.4 kd form was the only one detected 2 h following bFGF addition and was then cleaved into the 16.5 kd in early S phase. These results are consistent with the possibility that in addition to the classical pathway of signal transduction, bFGF is directly translocated to the nucleus in late G1, and could play a role in replication and/or in transcription of rDNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amalric F., Nicoloso M., Zalta J. -P. A comparative study of "soluble" RNA polymerase activity of Zajdela hepatoma ascites cells and calf thymus. FEBS Lett. 1972 Apr 15;22(1):67–72. doi: 10.1016/0014-5793(72)80221-7. [DOI] [PubMed] [Google Scholar]
- Baserga R. Growth in size and cell DNA replication. Exp Cell Res. 1984 Mar;151(1):1–5. doi: 10.1007/978-3-642-67986-5_1. [DOI] [PubMed] [Google Scholar]
- Belenguer P., Baldin V., Mathieu C., Prats H., Bensaid M., Bouche G., Amalric F. Protein kinase NII and the regulation of rDNA transcription in mammalian cells. Nucleic Acids Res. 1989 Aug 25;17(16):6625–6636. doi: 10.1093/nar/17.16.6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R. M. Protein kinase C activation by diacylglycerol second messengers. Cell. 1986 Jun 6;45(5):631–632. doi: 10.1016/0092-8674(86)90774-9. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Cell signalling through phospholipid metabolism. J Cell Sci Suppl. 1986;4:137–153. doi: 10.1242/jcs.1986.supplement_4.9. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Blackshear P. J., Witters L. A., Girard P. R., Kuo J. F., Quamo S. N. Growth factor-stimulated protein phosphorylation in 3T3-L1 cells. Evidence for protein kinase C-dependent and -independent pathways. J Biol Chem. 1985 Oct 25;260(24):13304–13315. [PubMed] [Google Scholar]
- Bouche G., Gas N., Prats H., Baldin V., Tauber J. P., Teissié J., Amalric F. Basic fibroblast growth factor enters the nucleolus and stimulates the transcription of ribosomal genes in ABAE cells undergoing G0----G1 transition. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6770–6774. doi: 10.1073/pnas.84.19.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D. Localisation of a fibroblast growth factor and its effect alone and with hydrocortisone on 3T3 cell growth. Nature. 1974 May 10;249(453):123–127. doi: 10.1038/249123a0. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Moran J., Braun D., Birdwell C. Clonal growth of bovine vascular endothelial cells: fibroblast growth factor as a survival agent. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4120–4124. doi: 10.1073/pnas.73.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Neufeld G., Schweigerer L. Molecular and biological characterization of fibroblast growth factor, an angiogenic factor which also controls the proliferation and differentiation of mesoderm and neuroectoderm derived cells. Cell Differ. 1986 Jul;19(1):1–17. doi: 10.1016/0045-6039(86)90021-7. [DOI] [PubMed] [Google Scholar]
- Goustin A. S., Leof E. B., Shipley G. D., Moses H. L. Growth factors and cancer. Cancer Res. 1986 Mar;46(3):1015–1029. [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- James R., Bradshaw R. A. Polypeptide growth factors. Annu Rev Biochem. 1984;53:259–292. doi: 10.1146/annurev.bi.53.070184.001355. [DOI] [PubMed] [Google Scholar]
- Kimelman D., Kirschner M. Synergistic induction of mesoderm by FGF and TGF-beta and the identification of an mRNA coding for FGF in the early Xenopus embryo. Cell. 1987 Dec 4;51(5):869–877. doi: 10.1016/0092-8674(87)90110-3. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M., Smith S., Sullivan R., Shing Y., Davidson S., Smith J. A., Sasse J. Multiple forms of basic fibroblast growth factor: amino-terminal cleavages by tumor cell- and brain cell-derived acid proteinases. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1839–1843. doi: 10.1073/pnas.84.7.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. A., Maher D. W., Hannink M., Donoghue D. J. Identification of a signal for nuclear targeting in platelet-derived-growth-factor-related molecules. Mol Cell Biol. 1987 Oct;7(10):3527–3537. doi: 10.1128/mcb.7.10.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P. L., Johnson D. E., Cousens L. S., Fried V. A., Williams L. T. Purification and complementary DNA cloning of a receptor for basic fibroblast growth factor. Science. 1989 Jul 7;245(4913):57–60. doi: 10.1126/science.2544996. [DOI] [PubMed] [Google Scholar]
- Magnaldo I., L'Allemain G., Chambard J. C., Moenner M., Barritault D., Pouysségur J. The mitogenic signaling pathway of fibroblast growth factor is not mediated through polyphosphoinositide hydrolysis and protein kinase C activation in hamster fibroblasts. J Biol Chem. 1986 Dec 25;261(36):16916–16922. [PubMed] [Google Scholar]
- Maher D. W., Lee B. A., Donoghue D. J. The alternatively spliced exon of the platelet-derived growth factor A chain encodes a nuclear targeting signal. Mol Cell Biol. 1989 May;9(5):2251–2253. doi: 10.1128/mcb.9.5.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. S. Stimulation of RNA and protein synthesis by intracellular insulin. Science. 1988 Apr 22;240(4851):506–509. doi: 10.1126/science.2451860. [DOI] [PubMed] [Google Scholar]
- Nader H. B., Dietrich C. P., Buonassisi V., Colburn P. Heparin sequences in the heparan sulfate chains of an endothelial cell proteoglycan. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3565–3569. doi: 10.1073/pnas.84.11.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B., Coppock D. L., Yang H. C. Regulation of cell proliferation at the onset of DNA synthesis. J Cell Sci Suppl. 1986;4:171–180. doi: 10.1242/jcs.1986.supplement_4.11. [DOI] [PubMed] [Google Scholar]
- Pardee A. B., Dubrow R., Hamlin J. L., Kletzien R. F. Animal cell cycle. Annu Rev Biochem. 1978;47:715–750. doi: 10.1146/annurev.bi.47.070178.003435. [DOI] [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. Induction of DNA synthesis in BALB/c 3T3 cells by serum components: reevaluation of the commitment process. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4481–4485. doi: 10.1073/pnas.74.10.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prats H., Kaghad M., Prats A. C., Klagsbrun M., Lélias J. M., Liauzun P., Chalon P., Tauber J. P., Amalric F., Smith J. A. High molecular mass forms of basic fibroblast growth factor are initiated by alternative CUG codons. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1836–1840. doi: 10.1073/pnas.86.6.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakowicz-Szulczynska E. M., Rodeck U., Herlyn M., Koprowski H. Chromatin binding of epidermal growth factor, nerve growth factor, and platelet-derived growth factor in cells bearing the appropriate surface receptors. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3728–3732. doi: 10.1073/pnas.83.11.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper S. E., Burwen S. J., Barker M. E., Jones A. L. Translocation of epidermal growth factor to the hepatocyte nucleus during rat liver regeneration. Gastroenterology. 1987 May;92(5 Pt 1):1243–1250. doi: 10.1016/s0016-5085(87)91084-5. [DOI] [PubMed] [Google Scholar]
- Sato Y., Rifkin D. B. Autocrine activities of basic fibroblast growth factor: regulation of endothelial cell movement, plasminogen activator synthesis, and DNA synthesis. J Cell Biol. 1988 Sep;107(3):1199–1205. doi: 10.1083/jcb.107.3.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweigerer L., Neufeld G., Friedman J., Abraham J. A., Fiddes J. C., Gospodarowicz D. Capillary endothelial cells express basic fibroblast growth factor, a mitogen that promotes their own growth. Nature. 1987 Jan 15;325(6101):257–259. doi: 10.1038/325257a0. [DOI] [PubMed] [Google Scholar]
- Shing Y., Folkman J., Haudenschild C., Lund D., Crum R., Klagsbrun M. Angiogenesis is stimulated by a tumor-derived endothelial cell growth factor. J Cell Biochem. 1985;29(4):275–287. doi: 10.1002/jcb.240290402. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I., Fridman R., Sullivan R., Sasse J., Klagsbrun M. Aortic endothelial cells synthesize basic fibroblast growth factor which remains cell associated and platelet-derived growth factor-like protein which is secreted. J Cell Physiol. 1987 Jun;131(3):402–408. doi: 10.1002/jcp.1041310312. [DOI] [PubMed] [Google Scholar]
- Zeytin F. N., Rusk S. F. Fibroblast growth factor is a permissive factor mediating the cellular action of rat hypothalamic growth hormone-releasing factor. Endocrinology. 1988 Mar;122(3):1129–1132. doi: 10.1210/endo-122-3-1129. [DOI] [PubMed] [Google Scholar]
- Zeytin F. N., Rusk S. F., Raymond V., Mandell A. J. Fibroblast growth factor stabilizes ribonucleic acid and regulates differentiated functions in a multipeptide-secreting neuroendocrine cell line. Endocrinology. 1988 Mar;122(3):1121–1128. doi: 10.1210/endo-122-3-1121. [DOI] [PubMed] [Google Scholar]