Abstract
Pyogenic vertebral osteomyelitis (PVO) may result in neurological deficits and sequelae, so early diagnosis and appropriate treatment are critical. Many previous studies on PVO exist, but our paper has aimed to comprehensively summarize the clinical aspects of PVO. Through review of the vast literature on the clinical research of PVO an overview of the clinical characteristics, diagnostic methods, treatment and prognosis is provided.
Keywords: Pyogenic vertebral osteomyelitis, Causative organism, Diagnosis, Antibiotic treatment, Prognosis
INTRODUCTION
Vertebral osteomyelitis (OM) is an uncommon infectious condition of the spine and various terms such as spondylodiscitis, septic discitis, and spinal OM have been used98). The incidence has been rising recently assumed to be due to aging society, increase in the number of immune compromised subjects1,41,63). The incidence has been reported to be 2.2 to 5.8 per 100,000 and highest in men aged 70–79 years33,41), and the overall incidence increases with aging. In the population older than 20 years, the male predominance in incidence increases until the age of 80 years33). Despite the great number of literature on the topic, there is still controversy regarding various aspects of diagnosis and treatment. Through this paper, the clinical features, methods of diagnosis, and steps of treatment will be reviewed based on up to date literatures. Especially, the review focused on pyogenic vertebral osteomyelitis (PVO) with the exception of spinal tuberculosis.
CLINICAL PRESENTATION
The most common symptom of PVO is axial back pain or neck pain. More than 80% of the patients present with rather severe pain not controlled by analgesics2,31,36). The onset of the pain is usually insidious and duration may be as long as several months. Epidural abscess should be considered in patients with severe, sharp, or lancinating type of back pain20,85,98). Local tenderness or spasm of the paraspinal muscles may be evident in physical examination13,63). Neurological deficits including motor weakness and sensory loss are not as common, ranging from 10% to 50%12,26,36,42,53,57). Fever may be present but is not a necessary condition. Various reports have shown 35% to 60% of the patients have fever on presentation69,79).
The fact that there may be a site of primary infection in PVO patients should be considered. Symptoms from the primary source may precede the typical back pain of vertebral myelitis itself. In approximately half of the patients, the primary infection site may be identified: skin, respiratory, oral, urinary tract, gastrointestinal tract, vascular access site, endocarditis or arthritis69). Endocarditis was found in as many as 1/3 of the PVO patients78). Several study reported about 19% to 47% of patients had undergone spinal surgery before PVO diagnosis16,62,96).
Many of the patients with PVO have underlying diseases such as diabetes mellitus, coronary artery diseases, immune-suppressed condition, and cancer62,69,96).
Differential diagnosis for back patients include degenerative spinal diseases, vertebral fractures or disc herniation, inflammatory spinal diseases, and metastatic tumor from systemic tumors13,98). In cases of back pain with fever, viral syndromes, pyelonephritis, and pancreatitis should also be considered98).
Because the symptoms and signs of PVO are usually nonspecific, and not infrequently fever is not seen, the correct diagnosis may not be made until almost 1 year since the onset of symptoms5,12,66,70). It is crucial that clinicians should always consider PVO as one of the disease entities of differential diagnosis.
LABORATORY FINDINGS
Leukocytosis or high proportion (>80%) of neutrophils is not sensitive for diagnosis of OM36,38). Nevertheless those laboratory results may be helpful as part of the routine work up for infection or fever, as well as markers to evaluate the treatment response2). In contrast, increase in erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) were noted to be very helpful, with sensitivity of 98% and 100%, respectively38,43).
Although ESR is not specific for infection, it rises in inflammatory condition, therefore may suggest the possibility of infection and may show the treatment response of the patient9). CRP has more specificity on infection than ESR because it rises within 6 hours of a bacterial infection9). It also normalizes more quickly than ESR after adequate treatment on the infection. These parameters are commonly increased after surgical procedures without any complications due to normal inflammatory reactions. However in these cases, ESR peaks at approximately 5 days after the operation and normalizes within 3 weeks, and CRP reaches maximal value at 2–3 days postoperatively and returns to normal limits during 6–14 postoperative days42,86). Therefore, Elevation of ESR and CRP is not pathognomonic feature of infection but those are useful as screening tests and also as monitoring parameters for treatment response.
RADIOLOGIC FINDINGS
Plain radiography is useful as the initial evaluation tool to screen a wide range of possible diseases because it is easily accessed, but is not sensitive for OM98). Blurring of end-plate and decrease in disc space may be detected in the early phase but because many patients have underlying degenerative changes in the spine, it is not easy to suspect OM based on these findings9).
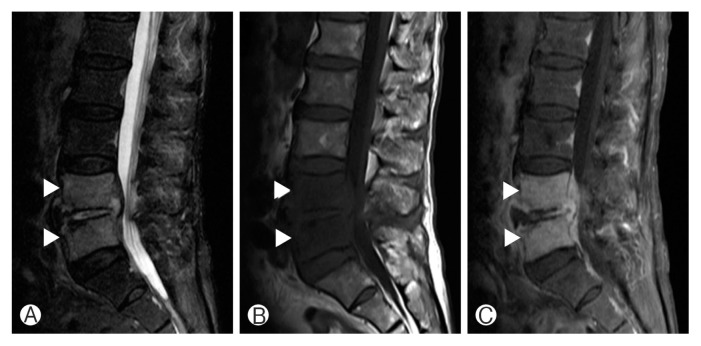
Magnetic resonance imaging (MRI) should be considered as the initial tool in patients with neurological deficits to rule out epidural abscess or herniation of the intervertebral disc98). MRI is the gold standard to diagnosis spinal infection with sensitivity, specificity, and accuracy of over 90%2). Destruction of endplate and marrow edema of the vertebral body results in decreased signal intensity (SI) of vertebral body, disc, and endplate on T1-weighted image (T1WI)22,26,91), increased SI of vertebral body and disc on T2-weighted image (T2WI), and contrast enhancement55,72). Typical cases show involvement the disc and 2 adjacent vertebral bodies (Fig. 1). In postoperative patients, the abovementioned features should be interpreted with care because slight signal changes may be seen in the remaining discs in cases of noncomplicated discectomy, making the differentiation of early discitis and normal postoperative change difficult. When the adjacent vertebral body shows low SI on T1WI along with contrast enhancement, infection may be more likely9). The MRI findings of a spinal tumor may be similar to PVO but may be differentiated by the fact that disc space is usually spared9). The MRI can be also helpful in distinguishing between PVO and tuberculous spondylitis. The findings on MRI are more frequently observed in PVO cases, which are less severe bony destruction, disc involvement, ill-defined postcontrast paraspinal abnormal signal margin, disc abscess with peridiscal rim enhancement and homogeneous enhancement of the vertebral body8). On the contrary, more severe bone destruction with relative disc preservation, focal heterogenous contrast enhancement of the vertebral body, well-defined abnormal SI in paraspinal areas, and vertebral intraosseous abscess with rim enhancement are distinctive MRI findings of tuberculous spondylitis8).
Fig. 1.
Magnetic resonance iamging of lumbar pyogenic vertebral ostemomyelitis case. T2-weighted 1 sagittal image (A), T1-weighted 1 (T1W1) sagittal image (B), and T1WI sagittal image (C) with enhancement. Arrowheads indicate signal change of adjacent vertebrae.
Although MRI is more sensitive than computed tomography (CT) especially for early diagnosis of OM, CT scan may be utilized for those patients in whom MRI is contraindicated or percutaneous biopsy is needed98). Also, CT may be helpful in deciding the extent of debridement of infected, necrotic tissues, because MRI may overestimate the extent of disease involvement9).
Three-phase technetium-99m bone scan show positive results, a few days after the symptom onset with high sensitivity of 90% but the specificity is rather low, 78%82). The scan may show increased activity for osteoporotic fractures, tumor and even after the spondylitis is cured with normalization of the laboratory findings9). Ga-67 scintigraphy with single-photon-emission CT seems to show similar accuracy with MRI, but is less sensitive for detecting epidural abscess64). Indium 111-labeled leukocyte scintigraphy and antigranulocyte scintigraphy are very sensitive for detecting PVO but have very low specificities (<20%)2,72). Not many studies on 18F-fluorodeoxyglucose positron emission tomography are available, but very high sensitivity (100%) and specificity (100%) for diagnosing disc space infection have been reported, and may be utilized to differentiate OM and degenerative changes88). It may be an especially better choice in patients with metallic implants98).
CAUSATIVE ORGANISMS
Most of PVO is caused by a single organism. However, polymicrobial infection is found in less than 10% of the patients69,87) usually with underlying decubitus ulcer, chronic debility and immune compromise12,42).
Staphylococcus aureus was the most common causative bacteria according to numerous reports14,20,30,93). Among the S. aureus, the prevalence of methicillin-resistant Staphylococcus aureus (MRSA) seems to be increasing recently compared to previous data, up to 40%–57.1%6,61,67,73). Male sex, multiple comorbidities and previous non-spine surgery were significant risk factors for PVO due to MRSA as compared to methicillin-sensitive Staphylococcus aureus (MSSA)37). Gram-negative rod was identified in 7%–33% of the patients, enterobacteriaceae being the most common species6,19,34,57). Gram-negative rod may be the causative organism more frequently in patients with genitourinary tract, gastrointestinal tract infection, old-age, compromised immune status, and diabetes13).
Coagulase-negative staphylococci are also found in 5%–16% of PVO, and staphyloccus epidermidis is most common. Frequently it is associated with postoperative infection or intracardiac device-related sepsis20), and is more likely to be found in patients with symptoms presenting more than 1 month after the operation32). Streptococci and enterococci may also be identified in 5%–20% of PVO cases34,57) and is frequently related with dental port of entry or endocarditis, whereas staphylococci are less commonly found in PVO patients with endocarditis13). The less virulent Staphylococcus epidermidis and viridans streptococci may cause indolent infections31). Although anaerobe is responsible for only 3% of PVO, it is more common in diabetes patients2).
IDENTIFICATION OF CAUSATIVE ORGANISMS
Identification of the causative organism is imperative because appropriate choice of the antimicrobial agent is crucial for treatment. Blood culture should be performed initially for all patients regardless of the presence of fever. Blood cultures are reported to have diagnostic value in 30%–78% of PVO cases69) meaning further invasive procedures may not be necessary in those patients with positive blood culture results. Other body fluids such as urine and sputum as well as swabs from any portals of entry should be evaluated and cultured to find the primary focus of the infection13). When causative organism is not identified with blood culture or other samples in patients who are suspected with PVO on radiological evaluation, biopsy to directly obtain the infected tissue is necessary. Percutaneous biopsy using CT or fluoroscope guidance can be performed, or biopsy with direct inspection of the involved tissue using endoscope or open surgery may be done. If polymicrobial OM is suspected, biopsy is mandatory regardless of the results of blood culture74,98). In patients with paravertebral, epidural, or psoas abscess, drainage of the abscess may be done instead of disc or bone biopsy to identify the organism98).
Biopsy specimens are known to have higher overall diagnostic yield (47%–100%), regardless of the method of biopsy69). Culture for aerobic, anaerobic bacteria, and fungi must be done for biopsy samples98). Evaluation for mycobacteria or brucella species should be done in patients living in endemic areas or those with subacute presentations12,77).
False negative blood culture or biopsy results are frequently found in patients who were treated with empirical antibiotics before microbiological diagnosis. Second biopsy should be performed when the initial culture results are negative23,27). With the exception of the acutely ill patients with definite symptoms or signs of sepsis or abscess, antimicrobial treatment should not be initiated until the causative organism is identified98). It is recommended that even those patients who were treated with antibiotics before identification of causative organism, if the patient is stable, biopsy should be postponed for at least 48 hours from the last injection or intake of antibiotic98). Some studies have shown that an even longer antibiotic-free period of 1–2 weeks will increase the yield rate of the biopsy, but it is not recommended in OM patients because of acutely-ill, critical condition of acute OM patients98). If the results of closed technique biopsy are repeatedly negative in patients in whom OM is highly likely, open biopsy should be considered31,98). Open biopsy showed positive culture results in over 75% of cases2,39,53,67,86). In a few prospective studies, the proportion of spine infections with negative culture results was reported to be 21%–34%14,66,87).
However, various reports have shown that the treatment duration, mortality, and recurrence rate do not seem to differ between the patients with identification of the causative organism and those without46,63,89,96). Similarly, the data from the authors’ institute also show that the yield of a second biopsy in patients with negative results from a first fluoroscopy-guided biopsy was only 7.6%(2 out of 26 patients)45). Although the importance of identification of the causative organism should not be underestimated, the above results show that the patient’s risk and economic burden of repetitive biopsy should be considered with caution. Histopathological examination may also provide useful information. The presence of white blood cells in the infected tissue may differentiate between infection and contamination, and granuloma may suggest atypical causes such as brucellosis or tuberculosis98).
Molecular diagnostics are not routinely utilized for OM. However, broad-range polymerase chain reaction (PCR) analysis of organisms may be used when available, if blood and biopsy culture results are negative21). PCR analysis may find microorganisms not detectable by classic culture methods21,54,92). A recent study reported that 16S rDNA PCR assay may be more sensitive than routine culture in etiological diagnosis OM11).
ANTIMICROBIAL TREATMENT
There are yet no randomized control trials regarding antibiotic treatment of PVO. The choice of antibiotics for PVO treatment should target the identified causative organism when possible, and factors such as bone and disc penetration capability, potential side effects, and administration feasibility should be considered13). For gram-positive bacteria, intravenous therapy is still the standard therapy. If causative organism is not identified, broad spectrum antibiotics with antistaphyloccocal coverage, as well as coverage for clinically suspected organism is recommended28,86). The outcome of early switch to oral antibiotics is controversial but oral bactericidal agents with high bioavailability and bone penetration such as clindamycin and fluoroquinolones may enable early administration of oral agents3,76,83). Beta-lactam antibiotics on the other hand, should not be used as an oral antibiotic for OM because of its low bioavailability.
No controlled trial data is available on the optimal treatment duration, but usually 4–6 weeks81,98), up to 3 months61,77) are recommended. In the presence of undrained abscess or spinal prosthesis, longer duration of antibiotic treatment is desired49,99,100). One study suggested the disappearance of inflammatory patterns and spinal pain, along with normalization of the body temperature, CRP and/or ESR, and improvement in plain radiography as indicators for termination of antibiotic administration57).
IMMOBILIZATION
Bed rest during the initial 2–4 weeks followed by ambulation with appropriate brace or corset is recommended in patients with severe, acute pain32,53). External immobilization helps stabilization of the spine, reduction of pain and prevents deformity9). The appropriate duration of bracing may vary from 3–6 weeks, up to 3 months, and should be decided according to individual patient’s degree of bone destruction and deformity9,13).
SURGERY
Mostly, the goal of surgery for OM is diagnosis (biopsy)69) but may itself have therapeutic roles in cases with compression of the cord or cauda equina showing progressive neurologic deficits. Urgent surgical decompression should be considered because preoperative neurologic status is the important predictor of the final neurologic outcome in spinal epidural abscess patients15,65).
Surgery should also be considered in cases where diagnosis is not confirmed, poor response to appropriate treatment is seen, or progressive deformity of the spine causing instability is noted. Surgical debridement is almost always required in infections associated with spinal prosthesis49). Removal of the prosthesis is further recommended for the late onset infection patients whose symptoms presented more than 30 days after the instrumentation surgery, because presence of prosthesis decreases the treatment success rate49).
Spinal infection may result in severe bone destruction and deformity, in which internal fixation of structural stabilization may be necessary. However, surgeons may be reluctant to instrumentation of an infected spine because prosthesis may hinder the antimicrobial treatment. Recent studies focusing on this issue have reported the usefulness and stability of internal fixation in active PVO52,56,60,80).
Autograft, allograft, titanium mesh cage may be used as materials for anterior column support and bony fusion, allograft and titanium mesh cages have not been popular due to the abovementioned reasons. Several studies have proved favorable outcomes in infection control as well as spinal stability of PVO patients who had allograft or titanium mesh cage implanted35,48,58,84). It should be emphasized that in such patients thorough, aggressive removal of the infected tissue combined with appropriate antibiotic treatment is mandatory to obtain good outcome.
The surgical approach for operation in PVO is worth considering. In many studies, the anterior and posterior approach (combined approach) was performed and proved to be safe and efficient24,48,52,58,75). One study had shown the combined approach had advantages in terms of hospitalization period and loss of correction compared to anterior or posterior only approach71). However another study proved superiority of ventral stabilization via single anterior approach versus ventro-dorsal fusion in long term outcome60). In addition there are several studies that good clinical outcome was achieved via single approach25,29,47,59,95). Thus the type of surgical approach needs to be tailored according to patient general medical condition, degree of bony destruction and location of compressive lesions.
The utilization of intrawound vancomycin powder during spinal surgery has become popular to prevent surgical site infection (SSI)18). Several meta-analyses had suggested that intrawound vancomycin powder could be effective to reduce SSI after spinal operation4,10,40,44). However those meta-analyses were limited in that they included studies with low level of evidence (grade III or IV) and had heterogeneity in the clinical settings, such as definition of SSI, method of vancomycin powder application, type of surgery, perioperative antibiotics regimen, etc. One prospective randomized controlled study proved intrawound usage of vancomycin powder did not significantly reduce the incidence of SSI in spinal surgery90). A recent study aimed at 9,823 patients revealed about 50% reduction of SSI in intrawound antibiotics using group on unadjusted analysis, but this difference was not statistically significant after adjustment17). So far, the evidence on the benefit of intrawound vancomycin in spinal surgery is uncertain. It will be interesting to see the results of ongoing prospective clinical trials related to application of intrawound vancomycin in spinal surgery (http://ClinicalTrials.gov; NCT01566422; NCT01977989)10,40).
FOLLOW-UP AND OUTCOMES
Response to treatment can be evaluated with improvement of clinical symptoms such as pain and fever or laboratory study, and radiologic imaging. When definite improvement in the clinical symptom and laboratory parameters is observed in response to treatment, follow-up evaluation with MRI or CT is usually not necessary. The correlation between improvement of MRI findings and clinical recovery is not strong7,97). In one study, there were 85% of the patients whose MRI taken 4–8 weeks after the initiation of treatment showed no change or improvement had improved clinically50), and no single MR finding was associated with the patients’ clinical status51). Therefore followup MRI should be selectively performed for those patients who do not show clinical improvement despite adequate treatment or when epidural abscess is suspected85). The disappearance of contrast enhancement and recovery of normal SI are reliable MRI features of complete healing. It should be kept in mind that even after complete resolution of the clinical infection, uptake of contrast on the MRI may reside for several months2,94). MRI should be repeated before terminating antibiotic treatment in case of a nonsurgically treated abscess98).
Various studies report successful treatment rate of 50%–91% with antibiotics for PVO42,53,81,94). Good prognosis is expected for those patients who show decrease in ESR and weekly decrement of CRP by 50% during the first month of treatment26,32). In contrast, no relief of symptoms or consistent CRP value of above 30mg/L may indicate treatment failure43,50).
PVO related mortality is reported to be 2%–11%12,39,53,68). The severity of comorbidity, age over 60 years, high CRP value at admission (≥100mg/L) are known related factors to higher mortality62,68). Another series reported that delay in diagnosis of more than 2 months, neurological deficit such as paralysis or paresis, and nosocomial infection were related to death or permanent deficits68). Some reports The PVO caused by MRSA showed more persistent bacteremia, relapse, increased hospital stay compared to those caused by MSSA73).
In one study of 253 patients, the relapse rate was approximately 14% and related factors were recurrent bacteremia, chronic draining sinuses, paravertebral abscesses68). In another study, the mortality between PVO patients with or without endocarditis was not different but the relapse rate was significantly higher for those with endocarditis (8% vs. 1.9%)78). Relapse of PVO may occur as late as 1 year after the completion of treatment13,39), therefore follow-up for sufficient period after treatment is mandatory.
CONCLUSION
The incidence of PVO has been increasing lately so although a rare condition, clinicians should consider it in patients with unremitting back pain and increase in inflammatory marker. When PVO is suspected, MRI should be performed promptly and culture study to identify the causative organism is crucial. Treatment should be specified according to culture results, so if the patient’s condition is tolerable, antimicrobial agents should not be administered before identification of the organism. Although data from randomized control trials regarding antibiotics regimen and administration period are lacking, 6-week period of treatment is routinely recommended and longer periods for patients with complicated infection or spinal implants.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Acosta FL, Jr, Chin CT, Quinones-Hinojosa A, Ames CP, Weinstein PR, Chou D. Diagnosis and management of adult pyogenic osteomyelitis of the cervical spine. Neurosurg Focus. 2004;17:E2. doi: 10.3171/foc.2004.17.6.2. [DOI] [PubMed] [Google Scholar]
- 2.An HS, Seldomridge JA. Spinal infections: diagnostic tests and imaging studies. Clin Orthop Relat Res. 2006;444:27–33. doi: 10.1097/01.blo.0000203452.36522.97. [DOI] [PubMed] [Google Scholar]
- 3.Babouee Flury B, Elzi L, Kolbe M, Frei R, Weisser M, Scharen S, et al. Is switching to an oral antibiotic regimen safe after 2 weeks of intravenous treatment for primary bacterial vertebral osteomyelitis? BMC Infect Dis. 2014;14:226. doi: 10.1186/1471-2334-14-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakhsheshian J, Dahdaleh NS, Lam SK, Savage JW, Smith ZA. The use of vancomycin powder in modern spine surgery: systematic review and meta-analysis of the clinical evidence. World Neurosurg. 2015;83:816–823. doi: 10.1016/j.wneu.2014.12.033. [DOI] [PubMed] [Google Scholar]
- 5.Bateman JL, Pevzner MM. Spinal osteomyelitis: a review of 10 years’ experience. Orthopedics. 1995;18:561–565. doi: 10.3928/0147-7447-19950601-10. [DOI] [PubMed] [Google Scholar]
- 6.Bhavan KP, Marschall J, Olsen MA, Fraser VJ, Wright NM, Warren DK. The epidemiology of hematogenous vertebral osteomyelitis: a cohort study in a tertiary care hospital. BMC Infect Dis. 2010;10:158. doi: 10.1186/1471-2334-10-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carragee EJ. The clinical use of magnetic resonance imaging in pyogenic vertebral osteomyelitis. Spine (Phila Pa 1976) 1997;22:780–785. doi: 10.1097/00007632-199704010-00015. [DOI] [PubMed] [Google Scholar]
- 8.Chang MC, Wu HT, Lee CH, Liu CL, Chen TH. Tuberculous spondylitis and pyogenic spondylitis: comparative magnetic resonance imaging features. Spine (Phila Pa 1976) 2006;31:782–788. doi: 10.1097/01.brs.0000206385.11684.d5. [DOI] [PubMed] [Google Scholar]
- 9.Cheung WY, Luk KD. Pyogenic spondylitis. Int Orthop. 2012;36:397–404. doi: 10.1007/s00264-011-1384-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiang HY, Herwaldt LA, Blevins AE, Cho E, Schweizer ML. Effectiveness of local vancomycin powder to decrease surgical site infections: a meta-analysis. Spine J. 2014;14:397–407. doi: 10.1016/j.spinee.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Choi SH, Sung H, Kim SH, Lee SO, Lee SH, Kim YS, et al. Usefulness of a direct 16S rRNA gene PCR assay of percutaneous biopsies or aspirates for etiological diagnosis of vertebral osteomyelitis. Diagn Microbiol Infect Dis. 2014;78:75–78. doi: 10.1016/j.diagmicrobio.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Colmenero JD, Jimenez-Mejias ME, Sanchez-Lora FJ, Reguera JM, Palomino-Nicas J, Martos F, et al. Pyogenic, tuberculous, and bru-cellar vertebral osteomyelitis: a descriptive and comparative study of 219 cases. Ann Rheum Dis. 1997;56:709–715. doi: 10.1136/ard.56.12.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cottle L, Riordan T. Infectious spondylodiscitis. J Infect. 2008;56:401–412. doi: 10.1016/j.jinf.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 14.D’Agostino C, Scorzolini L, Massetti AP, Carnevalini M, d’Ettorre G, Venditti M, et al. A seven-year prospective study on spondylodiscitis: epidemiological and microbiological features. Infection. 2010;38:102–107. doi: 10.1007/s15010-009-9340-8. [DOI] [PubMed] [Google Scholar]
- 15.Darouiche RO. Spinal epidural abscess. N Engl J Med. 2006;355:2012–2020. doi: 10.1056/NEJMra055111. [DOI] [PubMed] [Google Scholar]
- 16.Dufour V, Feydy A, Rillardon L, Redondo A, Le Page L, Bert F, et al. Comparative study of postoperative and spontaneous pyogenic spondylodiscitis. Semin Arthritis Rheum. 2005;34:766–771. doi: 10.1016/j.semarthrit.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Ehlers AP, Khor S, Shonnard N, Oskouian RJ, Jr, Sethi RK, Cizik AM, et al. Intra-wound antibiotics and infection in spine fusion surgery: a report from Washington State’s SCOAP-CERTAIN Collaborative. Surg Infect (Larchmt) 2016;17:179–186. doi: 10.1089/sur.2015.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emohare O, Ledonio CG, Hill BW, Davis RA, Polly DW, Jr, Kang MM. Cost savings analysis of intrawound vancomycin powder in posterior spinal surgery. Spine J. 2014;14:2710–2715. doi: 10.1016/j.spinee.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Euba G, Narvaez JA, Nolla JM, Murillo O, Narvaez J, Gomez-Vaquero C, et al. Long-term clinical and radiological magnetic resonance imaging outcome of abscess-associated spontaneous pyogenic vertebral osteomyelitis under conservative management. Semin Arthritis Rheum. 2008;38:28–40. doi: 10.1016/j.semarthrit.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Fantoni M, Trecarichi EM, Rossi B, Mazzotta V, Di Giacomo G, Nasto LA, et al. Epidemiological and clinical features of pyogenic spondylodiscitis. Eur Rev Med Pharmacol Sci. 2012;16(Suppl 2):2–7. [PubMed] [Google Scholar]
- 21.Fenollar F, Levy PY, Raoult D. Usefulness of broad-range PCR for the diagnosis of osteoarticular infections. Curr Opin Rheumatol. 2008;20:463–470. doi: 10.1097/BOR.0b013e3283032030. [DOI] [PubMed] [Google Scholar]
- 22.Forrester DM. Infectious spondylitis. Semin Ultrasound CT MR. 2004;25:461–473. doi: 10.1053/j.sult.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Friedman JA, Maher CO, Quast LM, McClelland RL, Ebersold MJ. Spontaneous disc space infections in adults. Surg Neurol. 2002;57:81–86. doi: 10.1016/s0090-3019(01)00681-4. [DOI] [PubMed] [Google Scholar]
- 24.Fukuta S, Miyamoto K, Masuda T, Hosoe H, Kodama H, Nishimoto H, et al. Two-stage (posterior and anterior) surgical treatment using posterior spinal instrumentation for pyogenic and tuberculotic spondylitis. Spine (Phila Pa 1976) 2003;28:E302–308. doi: 10.1097/01.BRS.0000083318.40123.5E. [DOI] [PubMed] [Google Scholar]
- 25.Fushimi K, Miyamoto K, Fukuta S, Hosoe H, Masuda T, Shimizu K. The surgical treatment of pyogenic spondylitis using posterior instrumentation without anterior debridement. J Bone Joint Surg Br. 2012;94:821–824. doi: 10.1302/0301-620X.94B6.28632. [DOI] [PubMed] [Google Scholar]
- 26.Gasbarrini AL, Bertoldi E, Mazzetti M, Fini L, Terzi S, Gonella F, et al. Clinical features, diagnostic and therapeutic approaches to haematogenous vertebral osteomyelitis. Eur Rev Med Pharmacol Sci. 2005;9:53–66. [PubMed] [Google Scholar]
- 27.Gaudias J. Considerations on antimicrobial therapy for pyogenic discitis. Joint Bone Spine. 2001;68:463–465. doi: 10.1016/s1297-319x(01)00322-0. [DOI] [PubMed] [Google Scholar]
- 28.Gillard J, Boutoille D, Varin S, Asseray N, Berthelot JM, Maugars Y. Suspected disk space infection with negative microbiological tests-report of eight cases and comparison with documented pyogenic discitis. Joint Bone Spine. 2005;72:156–162. doi: 10.1016/j.jbspin.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Gorensek M, Kosak R, Travnik L, Vengust R. Posterior instrumentation, anterior column reconstruction with single posterior approach for treatment of pyogenic osteomyelitis of thoracic and lumbar spine. Eur Spine J. 2013;22:633–641. doi: 10.1007/s00586-012-2487-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gouliouris T, Aliyu SH, Brown NM. Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother. 2010;65(Suppl 3):iii11–24. doi: 10.1093/jac/dkq303. [DOI] [PubMed] [Google Scholar]
- 31.Govender S. Spinal infections. J Bone Joint Surg Br. 2005;87:1454–1458. doi: 10.1302/0301-620X.87B11.16294. [DOI] [PubMed] [Google Scholar]
- 32.Grados F, Lescure FX, Senneville E, Flipo RM, Schmit JL, Fardellone P. Suggestions for managing pyogenic (non-tuberculous) discitis in adults. Joint Bone Spine. 2007;74:133–139. doi: 10.1016/j.jbspin.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Grammatico L, Baron S, Rusch E, Lepage B, Surer N, Desenclos JC, et al. Epidemiology of vertebral osteomyelitis (VO) in France: analysis of hospital-discharge data 2002–2003. Epidemiol Infect. 2008;136:653–660. doi: 10.1017/S0950268807008850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hadjipavlou AG, Mader JT, Necessary JT, Muffoletto AJ. Hematogenous pyogenic spinal infections and their surgical management. Spine (Phila Pa 1976) 2000;25:1668–1679. doi: 10.1097/00007632-200007010-00010. [DOI] [PubMed] [Google Scholar]
- 35.Hee HT, Majd ME, Holt RT, Pienkowski D. Better treatment of vertebral osteomyelitis using posterior stabilization and titanium mesh cages. J Spinal Disord Tech. 2002;15:149–156. doi: 10.1097/00024720-200204000-00010. discussion 156. [DOI] [PubMed] [Google Scholar]
- 36.Hopkinson N, Stevenson J, Benjamin S. A case ascertainment study of septic discitis: clinical, microbiological and radiological features. QJM. 2001;94:465–470. doi: 10.1093/qjmed/94.9.465. [DOI] [PubMed] [Google Scholar]
- 37.Inoue S, Moriyama T, Horinouchi Y, Tachibana T, Okada F, Maruo K, et al. Comparison of clinical features and outcomes of staphylococcus aureus vertebral osteomyelitis caused by methicillin-resistant and methicillin-sensitive strains. Springerplus. 2013;2:283. doi: 10.1186/2193-1801-2-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jensen AG, Espersen F, Skinhoj P, Frimodt-Moller N. Bacteremic Staphylococcus aureus spondylitis. Arch Intern Med. 1998;158:509–517. doi: 10.1001/archinte.158.5.509. [DOI] [PubMed] [Google Scholar]
- 39.Jimenez-Mejias ME, de Dios Colmenero J, Sanchez-Lora FJ, Palomino-Nicas J, Reguera JM, Garcia de la Heras J, et al. Postoperative spondylodiskitis: etiology, clinical findings, prognosis, and comparison with nonoperative pyogenic spondylodiskitis. Clin Infect Dis. 1999;29:339–345. doi: 10.1086/520212. [DOI] [PubMed] [Google Scholar]
- 40.Kang DG, Holekamp TF, Wagner SC, Lehman RA., Jr Intrasite vancomycin powder for the prevention of surgical site infection in spine surgery: a systematic literature review. Spine J. 2015;15:762–770. doi: 10.1016/j.spinee.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 41.Kehrer M, Pedersen C, Jensen TG, Lassen AT. Increasing incidence of pyogenic spondylodiscitis: a 14-year population-based study. J Infect. 2014;68:313–320. doi: 10.1016/j.jinf.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 42.Khan IA, Vaccaro AR, Zlotolow DA. Management of vertebral diskitis and osteomyelitis. Orthopedics. 1999;22:758–765. doi: 10.3928/0147-7447-19990801-07. [DOI] [PubMed] [Google Scholar]
- 43.Khan MH, Smith PN, Rao N, Donaldson WF. Serum C-reactive protein levels correlate with clinical response in patients treated with antibiotics for wound infections after spinal surgery. Spine J. 2006;6:311–315. doi: 10.1016/j.spinee.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 44.Khan NR, Thompson CJ, DeCuypere M, Angotti JM, Kalobwe E, Muhlbauer MS, et al. A meta-analysis of spinal surgical site infection and vancomycin powder. J Neurosurg Spine. 2014;21:974–983. doi: 10.3171/2014.8.SPINE1445. [DOI] [PubMed] [Google Scholar]
- 45.Kim BJ, Lee JW, Kim SJ, Lee GY, Kang HS. Diagnostic yield of fluoroscopy-guided biopsy for infectious spondylitis. AJNR Am J Neuroradiol. 2013;34:233–238. doi: 10.3174/ajnr.A3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim J, Kim YS, Peck KR, Kim ES, Cho SY, Ha YE, et al. Outcome of culture-negative pyogenic vertebral osteomyelitis: Comparison with microbiologically confirmed pyogenic vertebral osteomyelitis. Semin Arthritis Rheum. 2014;44:246–252. doi: 10.1016/j.semarthrit.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 47.Kim YM, Choi SM. Posterior only approach for lumbar pyogenic spondylitis with short instrumentation and prolonged suction drainage. Spine (Phila Pa 1976) 2016;41:E1022–1029. doi: 10.1097/BRS.0000000000001566. [DOI] [PubMed] [Google Scholar]
- 48.Korovessis P, Repantis T, Iliopoulos P, Hadjipavlou A. Beneficial influence of titanium mesh cage on infection healing and spinal reconstruction in hematogenous septic spondylitis: a retrospective analysis of surgical outcome of twenty-five consecutive cases and review of literature. Spine (Phila Pa 1976) 2008;33:E759–767. doi: 10.1097/BRS.0b013e318187875e. [DOI] [PubMed] [Google Scholar]
- 49.Kowalski TJ, Berbari EF, Huddleston PM, Steckelberg JM, Mandrekar JN, Osmon DR. The management and outcome of spinal implant infections: contemporary retrospective cohort study. Clin Infect Dis. 2007;44:913–920. doi: 10.1086/512194. [DOI] [PubMed] [Google Scholar]
- 50.Kowalski TJ, Berbari EF, Huddleston PM, Steckelberg JM, Osmon DR. Do follow-up imaging examinations provide useful prognostic information in patients with spine infection? Clin Infect Dis. 2006;43:172–179. doi: 10.1086/505118. [DOI] [PubMed] [Google Scholar]
- 51.Kowalski TJ, Layton KF, Berbari EF, Steckelberg JM, Huddleston PM, Wald JT, et al. Follow-up MR imaging in patients with pyogenic spine infections: lack of correlation with clinical features. AJNR Am J Neuroradiol. 2007;28:693–699. [PMC free article] [PubMed] [Google Scholar]
- 52.Kuklo TR, Potter BK, Bell RS, Moquin RR, Rosner MK. Singlestage treatment of pyogenic spinal infection with titanium mesh cages. J Spinal Disord Tech. 2006;19:376–382. doi: 10.1097/01.bsd.0000203945.03922.f6. [DOI] [PubMed] [Google Scholar]
- 53.Lam KS, Webb JK. Discitis. Hosp Med. 2004;65:280–286. doi: 10.12968/hosp.2004.65.5.13703. [DOI] [PubMed] [Google Scholar]
- 54.Lecouvet F, Irenge L, Vandercam B, Nzeusseu A, Hamels S, Gala JL. The etiologic diagnosis of infectious discitis is improved by amplification-based DNA analysis. Arthritis Rheum. 2004;50:2985–2994. doi: 10.1002/art.20462. [DOI] [PubMed] [Google Scholar]
- 55.Ledermann HP, Schweitzer ME, Morrison WB, Carrino JA. MR imaging findings in spinal infections: rules or myths? Radiology. 2003;228:506–514. doi: 10.1148/radiol.2282020752. [DOI] [PubMed] [Google Scholar]
- 56.Lee MC, Wang MY, Fessler RG, Liauw J, Kim DH. Instrumentation in patients with spinal infection. Neurosurg Focus. 2004;17:E7. doi: 10.3171/foc.2004.17.6.7. [DOI] [PubMed] [Google Scholar]
- 57.Legrand E, Flipo RM, Guggenbuhl P, Masson C, Maillefert JF, Soubrier M, et al. Management of nontuberculous infectious discitis. treatments used in 110 patients admitted to 12 teaching hospitals in France. Joint Bone Spine. 2001;68:504–509. doi: 10.1016/s1297-319x(01)00315-3. [DOI] [PubMed] [Google Scholar]
- 58.Liljenqvist U, Lerner T, Bullmann V, Hackenberg L, Halm H, Winkelmann W. Titanium cages in the surgical treatment of severe vertebral osteomyelitis. Eur Spine J. 2003;12:606–612. doi: 10.1007/s00586-003-0614-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin CP, Ma HL, Wang ST, Liu CL, Yu WK, Chang MC. Surgical results of long posterior fixation with short fusion in the treatment of pyogenic spondylodiscitis of the thoracic and lumbar spine: a retrospective study. Spine (Phila Pa 1976) 2012;37:E1572–1579. doi: 10.1097/BRS.0b013e31827399b8. [DOI] [PubMed] [Google Scholar]
- 60.Linhardt O, Matussek J, Refior HJ, Krodel A. Long-term results of ventro-dorsal versus ventral instrumentation fusion in the treatment of spondylitis. Int Orthop. 2007;31:113–119. doi: 10.1007/s00264-006-0140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Livorsi DJ, Daver NG, Atmar RL, Shelburne SA, White AC, Jr, Musher DM. Outcomes of treatment for hematogenous Staphylococcus aureus vertebral osteomyelitis in the MRSA ERA. J Infect. 2008;57:128–131. doi: 10.1016/j.jinf.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 62.Loibl M, Stoyanov L, Doenitz C, Brawanski A, Wiggermann P, Krutsch W, et al. Outcome-related co-factors in 105 cases of vertebral osteomyelitis in a tertiary care hospital. Infection. 2014;42:503–510. doi: 10.1007/s15010-013-0582-0. [DOI] [PubMed] [Google Scholar]
- 63.Lora-Tamayo J, Euba G, Narvaez JA, Murillo O, Verdaguer R, Sobrino B, et al. Changing trends in the epidemiology of pyogenic vertebral osteomyelitis: the impact of cases with no microbiologic diagnosis. Semin Arthritis Rheum. 2011;41:247–255. doi: 10.1016/j.semarthrit.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 64.Love C, Patel M, Lonner BS, Tomas MB, Palestro CJ. Diagnosing spinal osteomyelitis: a comparison of bone and Ga-67 scintigraphy and magnetic resonance imaging. Clin Nucl Med. 2000;25:963–977. doi: 10.1097/00003072-200012000-00002. [DOI] [PubMed] [Google Scholar]
- 65.Lu CH, Chang WN, Lui CC, Lee PY, Chang HW. Adult spinal epidural abscess: clinical features and prognostic factors. Clin Neurol Neurosurg. 2002;104:306–310. doi: 10.1016/s0303-8467(02)00020-3. [DOI] [PubMed] [Google Scholar]
- 66.Luzzati R, Giacomazzi D, Danzi MC, Tacconi L, Concia E, Vento S. Diagnosis, management and outcome of clinically- suspected spinal infection. J Infect. 2009;58:259–265. doi: 10.1016/j.jinf.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 67.Marschall J, Bhavan KP, Olsen MA, Fraser VJ, Wright NM, Warren DK. The impact of prebiopsy antibiotics on pathogen recovery in hematogenous vertebral osteomyelitis. Clin Infect Dis. 2011;52:867–872. doi: 10.1093/cid/cir062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McHenry MC, Easley KA, Locker GA. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis. 2002;34:1342–1350. doi: 10.1086/340102. [DOI] [PubMed] [Google Scholar]
- 69.Mylona E, Samarkos M, Kakalou E, Fanourgiakis P, Skoutelis A. Pyogenic vertebral osteomyelitis: a systematic review of clinical characteristics. Semin Arthritis Rheum. 2009;39:10–17. doi: 10.1016/j.semarthrit.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 70.Nolla JM, Ariza J, Gomez-Vaquero C, Fiter J, Bermejo J, Valverde J, et al. Spontaneous pyogenic vertebral osteomyelitis in nondrug users. Semin Arthritis Rheum. 2002;31:271–278. doi: 10.1053/sarh.2002.29492. [DOI] [PubMed] [Google Scholar]
- 71.Okada Y, Miyamoto H, Uno K, Sumi M. Clinical and radiological outcome of surgery for pyogenic and tuberculous spondylitis: comparisons of surgical techniques and disease types. J Neurosurg Spine. 2009;11:620–627. doi: 10.3171/2009.5.SPINE08331. [DOI] [PubMed] [Google Scholar]
- 72.Palestro CJ, Love C, Miller TT. Infection and musculoskeletal conditions: Imaging of musculoskeletal infections. Best Pract Res Clin Rheumatol. 2006;20:1197–1218. doi: 10.1016/j.berh.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 73.Park KH, Chong YP, Kim SH, Lee SO, Choi SH, Lee MS, et al. Clinical characteristics and therapeutic outcomes of hematogenous vertebral osteomyelitis caused by methicillin-resistant Staphylococcus aureus. J Infect. 2013;67:556–564. doi: 10.1016/j.jinf.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 74.Patzakis MJ, Rao S, Wilkins J, Moore TM, Harvey PJ. Analysis of 61 cases of vertebral osteomyelitis. Clin Orthop Relat Res. 1991;(264):178–183. [PubMed] [Google Scholar]
- 75.Pee YH, Park JD, Choi YG, Lee SH. Anterior debridement and fusion followed by posterior pedicle screw fixation in pyogenic spondylodiscitis: autologous iliac bone strut versus cage. J Neurosurg Spine. 2008;8:405–412. doi: 10.3171/SPI/2008/8/5/405. [DOI] [PubMed] [Google Scholar]
- 76.Perlroth J, Kuo M, Tan J, Bayer AS, Miller LG. Adjunctive use of rifampin for the treatment of Staphylococcus aureus infections: a systematic review of the literature. Arch Intern Med. 2008;168:805–819. doi: 10.1001/archinte.168.8.805. [DOI] [PubMed] [Google Scholar]
- 77.Perronne C, Saba J, Behloul Z, Salmon-Ceron D, Leport C, Vilde JL, et al. Pyogenic and tuberculous spondylodiskitis (vertebral osteomyelitis) in 80 adult patients. Clin Infect Dis. 1994;19:746–750. doi: 10.1093/clinids/19.4.746. [DOI] [PubMed] [Google Scholar]
- 78.Pigrau C, Almirante B, Flores X, Falco V, Rodriguez D, Gasser I, et al. Spontaneous pyogenic vertebral osteomyelitis and endocarditis: incidence, risk factors, and outcome. Am J Med. 2005;118:1287. doi: 10.1016/j.amjmed.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 79.Priest DH, Peacock JE., Jr Hematogenous vertebral osteomyelitis due to Staphylococcus aureus in the adult: clinical features and therapeutic outcomes. South Med J. 2005;98:854–862. doi: 10.1097/01.smj.0000168666.98129.33. [DOI] [PubMed] [Google Scholar]
- 80.Rayes M, Colen CB, Bahgat DA, Higashida T, Guthikonda M, Rengachary S, et al. Safety of instrumentation in patients with spinal infection. J Neurosurg Spine. 2010;12:647–659. doi: 10.3171/2009.12.SPINE09428. [DOI] [PubMed] [Google Scholar]
- 81.Roblot F, Besnier JM, Juhel L, Vidal C, Ragot S, Bastides F, et al. Optimal duration of antibiotic therapy in vertebral osteomyelitis. Semin Arthritis Rheum. 2007;36:269–277. doi: 10.1016/j.semarthrit.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 82.Rodiek SO. Diagnostic methods in spinal infections. Radiologe. 2001;41:976–986. doi: 10.1007/s001170170034. [DOI] [PubMed] [Google Scholar]
- 83.Schrenzel J, Harbarth S, Schockmel G, Genne D, Bregenzer T, Flueckiger U, et al. A randomized clinical trial to compare fleroxacin-rifampicin with flucloxacillin or vancomycin for the treatment of staphylococcal infection. Clin Infect Dis. 2004;39:1285–1292. doi: 10.1086/424506. [DOI] [PubMed] [Google Scholar]
- 84.Schuster JM, Avellino AM, Mann FA, Girouard AA, Grady MS, Newell DW, et al. Use of structural allografts in spinal osteomyelitis: a review of 47 cases. J Neurosurg. 2000;93(1 Suppl):8–14. doi: 10.3171/spi.2000.93.1.0008. [DOI] [PubMed] [Google Scholar]
- 85.Sendi P, Bregenzer T, Zimmerli W. Spinal epidural abscess in clinical practice. QJM. 2008;101:1–12. doi: 10.1093/qjmed/hcm100. [DOI] [PubMed] [Google Scholar]
- 86.Silber JS, Anderson DG, Vaccaro AR, Anderson PA, McCormick P NASS. Management of postprocedural discitis. Spine J. 2002;2:279–287. doi: 10.1016/s1529-9430(02)00203-6. [DOI] [PubMed] [Google Scholar]
- 87.Skaf GS, Domloj NT, Fehlings MG, Bouclaous CH, Sabbagh AS, Kanafani ZA, et al. Pyogenic spondylodiscitis: an overview. J Infect Public Health. 2010;3:5–16. doi: 10.1016/j.jiph.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 88.Stumpe KD, Zanetti M, Weishaupt D, Hodler J, Boos N, Von Schulthess GK. FDG positron emission tomography for differentiation of degenerative and infectious endplate abnormalities in the lumbar spine detected on MR imaging. AJR Am J Roentgenol. 2002;179:1151–1157. doi: 10.2214/ajr.179.5.1791151. [DOI] [PubMed] [Google Scholar]
- 89.Tachibana T, Moriyama T, Maruo K, Inoue S, Yoshiya S. Therapeutic impact of organism isolation in management of patients with pyogenic vertebral osteomyelitis. Springerplus. 2014;3:62. doi: 10.1186/2193-1801-3-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tubaki VR, Rajasekaran S, Shetty AP. Effects of using intravenous antibiotic only versus local intrawound vancomycin antibiotic powder application in addition to intravenous antibiotics on postoperative infection in spine surgery in 907 patients. Spine (Phila Pa 1976) 2013;38:2149–2155. doi: 10.1097/BRS.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 91.Varma R, Lander P, Assaf A. Imaging of pyogenic infectious spondylodiskitis. Radiol Clin North Am. 2001;39:203–213. doi: 10.1016/s0033-8389(05)70273-6. [DOI] [PubMed] [Google Scholar]
- 92.Weber U, Morf MH, Gubler JG, Altwegg M, Maibach RC. Spondylodiscitis as the first manifestation of Whipple’s disease -a removal worker with chronic low back pain. Clin Rheumatol. 2003;22:443–446. doi: 10.1007/s10067-003-0786-2. [DOI] [PubMed] [Google Scholar]
- 93.Weissman S, Parker RD, Siddiqui W, Dykema S, Horvath J. Vertebral osteomyelitis: retrospective review of 11 years of experience. Scand J Infect Dis. 2014;46:193–199. doi: 10.3109/00365548.2013.868600. [DOI] [PubMed] [Google Scholar]
- 94.Wirtz DC, Genius I, Wildberger JE, Adam G, Zilkens KW, Niethard FU. Diagnostic and therapeutic management of lumbar and thoracic spondylodiscitis--an evaluation of 59 cases. Arch Orthop Trauma Surg. 2000;120:245–251. doi: 10.1007/s004020050457. [DOI] [PubMed] [Google Scholar]
- 95.Yaldz C, Ozdemir N, Yaman O, Feran HG, Tansug T, Minoglu M. A retrospective study of 39 patients treated with anterior approach of thoracic and lumbar spondylodiscitis: clinical manifestations, anterior surgical treatment, and outcome. Medicine (Baltimore) 2015;94:e2110. doi: 10.1097/MD.0000000000002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yoon SH, Chung SK, Kim KJ, Kim HJ, Jin YJ, Kim HB. Pyogenic vertebral osteomyelitis: identification of microorganism and laboratory markers used to predict clinical outcome. Eur Spine J. 2010;19:575–582. doi: 10.1007/s00586-009-1216-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zarrouk V, Feydy A, Salles F, Dufour V, Guigui P, Redondo A, et al. Imaging does not predict the clinical outcome of bacterial vertebral osteomyelitis. Rheumatology (Oxford) 2007;46:292–295. doi: 10.1093/rheumatology/kel228. [DOI] [PubMed] [Google Scholar]
- 98.Zimmerli W. Clinical practice. Vertebral osteomyelitis N Engl J Med. 2010;362:1022–1029. doi: 10.1056/NEJMcp0910753. [DOI] [PubMed] [Google Scholar]
- 99.Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351:1645–1654. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]
- 100.Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA. 1998;279:1537–1541. doi: 10.1001/jama.279.19.1537. [DOI] [PubMed] [Google Scholar]