Abstract
Background and purpose
Prolonged electrocardiogram (ECG) monitoring after ischaemic stroke increases the diagnostic yield of paroxysmal atrial fibrillation (pAF). In order to facilitate the additional workload involved in ECG analysis due to prolonged monitoring times, we investigated the effectiveness of pAF detection with an automated software algorithm (SA) in comparison to the routine staff‐based analysis (RA) during standard stroke‐unit care. Therefore, patients with acute ischaemic stroke or transitory ischaemic attack presenting with sinus rhythmus on the admission ECG and no history of atrial fibrillation were prospectively included.
Methods
A 24‐h Holter ECG assessment was performed using either RA based on a computer‐aided evaluation and subsequent review by a cardiologist or a commercially available automated SA. In the case of discordant results concerning the occurrence of pAF between the two methods, the data underwent an independent external rating.
Results
Of 809 prospectively enrolled patients, 580 patients fulfilled the inclusion criteria. pAF was ultimately diagnosed in 3.3% of the cohort (19 patients). SA and RA correctly diagnosed pAF in 17 patients resulting in a comparable diagnostic effectiveness of the analysis methods (sensitivity: SA 89.5% vs. RA 89.5%; specificity: SA 99.3% vs. RA 99.1%; κ, 0.686; P < 0.001; 95% confidence interval, 0.525–0.847). RA revealed clinically relevant ECG abnormalities in an additional seven patients.
Conclusions
Although it should not completely replace RA, SA‐based evaluation of Holter ECG reaches a high diagnostic effectiveness for the detection of pAF and can be used for a rapid and resource‐saving analysis of ECG data to deal with prolonged monitoring times.
Keywords: cerebral infarction, Holter electrocardiogram, stroke, stroke unit, transitory ischaemic attack
Introduction
Atrial fibrillation (AF) remains one of the most common causes of ischaemic stroke and carries a 2.5‐fold higher risk of future ischaemic stroke 1. The detection of paroxysmal AF (pAF) can be challenging due to its short duration, frequently asymptomatic presentation and occurrence in a cluster 2. Although current guidelines recommend electrocardiogram (ECG) monitoring for at least 24 h after a stroke 3, recently published studies report higher detection rates (up to one quarter of patients with ischaemic stroke) with prolonged AF detection times and sequential cardiac monitoring methods 1. A prolongation of the Holter monitoring times might easily be achieved in routine practice and could lead to a higher detection rate of pAF and subsequent prescription of oral anticoagulation, resulting in a reduction of risk of recurrent stroke 4. Nevertheless, an extension of the monitoring time would require additional staff‐based capacities for ECG analysis that might be difficult to implement within the routine diagnostic workup, especially in primary and secondary hospitals, and could be challenging in terms of cost‐effective patient care. In this scenario, an automated computer ECG analysis strategy utilizing a software algorithm (SA) might facilitate and accelerate the analysis of increased ECG data due to prolonged detection times in a human‐resource‐preserving manner. In addition, SA might be used to extend Holter ECG times beyond the routinely used 24 h to increase the detection rate of pAF.
Here we assessed whether an automated SA is able to reach the diagnostic effectiveness of the commonly used routine staff‐based analysis (RA) with respect to pAF detection in 24‐h Holter ECG, obtained during routine diagnostic workup of patients with ischaemic stroke in a tertiary hospital.
Methods
Study design
This study was conducted at the board‐certified stroke unit of the Department of Neurology, University of Mainz. All patients with symptoms of acute ischaemic stroke or transitory ischaemic attack were prospectively included between August 2012 and July 2013. Patients presenting with sinus rhythmus on the admission ECG and no history of AF were eligible. The detailed patient selection procedure is depicted in Fig. 1. Ultimately, 580 patients fulfilled the inclusion criteria and completed 24‐h Holter ECG (Spacelabs, LifecardCF digital Holter®, Snoqualmie, WA, USA) during their hospital stay. The study was approved by the local Ethics Committee (Landesärztekammer Rheinland Pfalz).
Figure 1.
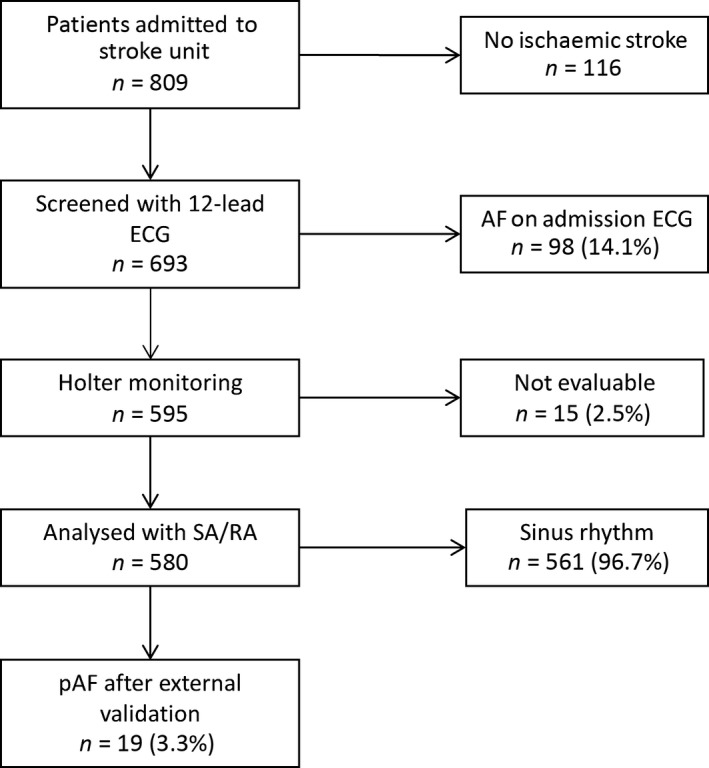
Patient recruitment and atrial fibrillation (AF) burden of the study population. ECG, electrocardiogram; pAF, paroxysmal AF; RA, routine analysis; SA, software algorithm.
Definition of atrial fibrillation
According to the 2010 European Society of Cardiology guideline on AF 5, we defined AF as showing absolutely irregular RR intervals without any repetitive ECG pattern, lacking a distinct p‐wave on surface ECG and showing an atrial cycle length of <200 ms (>300 beats/min). Only episodes lasting at least 30 s and not atrial flutter were included.
Data analysis
The RA used Pathfinder digital® software (version PA 8.602, Spacelabs, Snoqualmie, WA, USA) to identify episodes of suspected arrhythmia (e.g. pAF). A senior cardiologist performed a rating of the ECG data especially with regard to the occurrence of pAF and other relevant arrhythmias, which were documented within a structured report. Automated SA analyzed the same copied ECG dataset with the commercially available software SRAclinic® (Apoplex Medical Technologies GmbH, Pirmasens, Germany). Based on a time‐series analysis of multiple mathematical parameters, the software creates a report indicating whether pAF is present, including an extended Poincaré analysis 2, 6. In the case of pAF diagnosis by SA or a mismatch between the RA and SA report concerning the occurrence of pAF, the ECG data were analyzed in a dedicated core laboratory (M.W.‐K. and R.W.) with experience in cardiac electrophysiology and conduct of randomized trials 7, 8. The diagnostic workup of ECG data is depicted in Fig. 2. All investigators responsible for the rating of ECG data were blinded to the results of other methods, arrhythmias detected by telemetry on the stroke unit and the clinical data of the patients.
Figure 2.
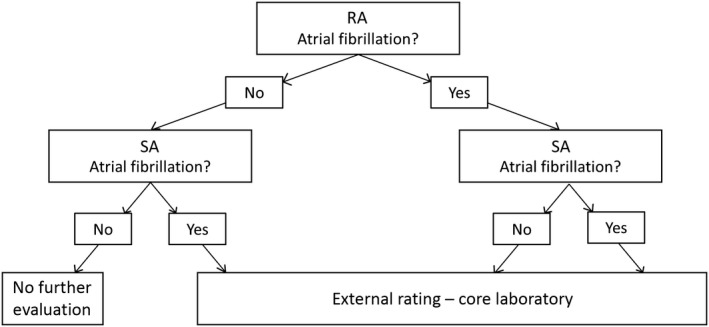
Diagnostic workup of 24‐h Holter ECG data. RA, routine analysis; SA, automated analysis.
Statistics
Data are presented as means (±SD) or numbers with percentages, if not otherwise indicated. For univariate analysis, the Mann–Whitney U‐test, Student's t‐test, chi‐square test or Fisher's exact test were used as appropriate. Inter‐rater reliability analysis used κ statistics to determine consistency between RA and SA. Agreement was quantified as fair (κ, 0.21–0.40), moderate (κ, 0.41–0.60), substantial (κ, 0.61–0.80) or almost perfect (κ, 0.81–0.99) 9. P < 0.05 was considered as significant. Statistical analysis was performed using SPSS (version 23, IBM, Armonk, NY, USA).
Results
During the study period, a total of 809 patients were admitted to the stroke unit, 693 of whom were identified as having had a stroke or transitory ischaemic attack. Because of AF documented by 12‐channel ECG (98 patients, 14.1%) and technical reasons (15 patients, 2.2%), the analysis of the Holter ECG (SA vs. RA) was performed in a total of 580 patients (mean age, 69.3 ± 12.6 years; male, 54.4%). Holter ECG was typically performed within the first 72 h after admission (mean, 28.5 ± 25.6 h) and in all cases during the hospital stay that was due to the qualifying event (stroke/transitory ischaemic attack).
After external validation, pAF could be detected during Holter ECG monitoring in 19 of 580 (3.3%) enrolled patients. The occurrence of pAF was associated with a history of diabetes mellitus [pAF, 9 (47.4%) vs. no AF, 137 (24.4%), P = 0.023] and greater age [pAF (mean), 81.6 ± 7.7 vs. no AF, 69.3 ± 12.6, P < 0.001]. For details see Table 1.
Table 1.
Baseline characteristics of the study population
| Variable | No AF | pAF | P‐value |
|---|---|---|---|
| n | 561 | 19 | |
| Male sex | 305 (54.4) | 11 (57.9) | 0.761 |
| Age (years) | 69.3 ± 12.6 | 81.6 ± 7.7 | <0.001 |
| History of stroke/TIA | 114 (20.3) | 1 (5.3) | 0.144 |
| Arterial hypertension | 444 (79.1) | 18 (94.7) | 0.144 |
| Diabetes mellitus | 137 (24.4) | 9 (47.4) | 0.023 |
| Coronary artery disease | 96 (17.1) | 6 (31.6) | 0.103 |
| Hyperlipidemia | 144 (25.7) | 6 (31.6) | 0.563 |
| Current tobacco use | 139 (24.8) | 2 (10.5) | 0.184 |
| NIH‐SS at admission | 2 (1–5) | 3 (2–11) | 0.101 |
| Qualifying event | 0.711 | ||
| Stroke | 422 (75.2) | 15 (79.0) | |
| TIA | 139 (24.8) | 4 (21.1) |
Data are presented as n (%) except for age [mean (±SD)] and National Institute of Health Stroke Scale (NIH‐SS) [mean (range)]. AF, atrial fibrillation; pAF, paroxysmal AF; TIA, transitory ischaemic attack.
The RA detected pAF in 22 patients (five false‐positive and two false‐negative results) leading to a sensitivity of 89.5% and a specificity of 99.1%. Similarly, SA was able to detect pAF in a total of 21 patients (four false‐positive and two false‐negative results), resulting in a sensitivity of 89.5% and a specificity of 99.3% (Table 2). The inter‐rater reliability for the two methods (RA vs. SA) was found to be substantial (κ, 0.686; P < 0.001; 95% confidence interval, 0.525–0.847) 9. For details see Table 3.
Table 2.
Detection rates of paroxysmal atrial fibrillation and test effectiveness of different analysis procedures
| RA | SA | |
|---|---|---|
| Correctly detected cases | 17 (89.4) | 17 (89.4) |
| False positive | 5 | 4 |
| False negative | 2 | 2 |
| Sensitivity | 89.5 | 89.5 |
| Specificity | 99.1 | 99.3 |
Data are presented as n (%). RA, routine analysis; SA, software algorithm.
Table 3.
Agreement between routine analysis (RA) and software algorithm (SA)
| SA | |||
|---|---|---|---|
| No AF | pAF | ||
| RA | No AF | 552 | 6 |
| pAF | 7 | 15 | |
AF, atrial fibrillation; pAF, paroxysmal AF. κ, 0.686; P < 0.001; 95% confidence interval, 0.525–0.847.
In two patients, the Holter ECG recording was interrupted because of insufficient ECG data due to low signal quality. However, the diagnosis of pAF was suspected based on the Poincaré plot and was corroborated after validation. These two patients were therefore included in the ‘pAF detected by SA’ group in the final data analysis.
In addition to pAF, RA was able to detect seven clinically relevant ECG abnormalities (e.g. sinoatrial blockades) leading to further cardiological diagnostics and pacemaker implantation in two cases. These clinically relevant arrhythmias were not identified during stroke‐unit telemetry or after reviewing the report of the SA.
Discussion
Within our patient cohort, SA‐based analysis of 24‐h Holter ECG data after acute ischaemic stroke was comparable to current routine ECG analysis in a tertiary hospital with regard to the detection of pAF. Concerning the diagnostic effectiveness of the two analysis methods (SA vs. RA), the accuracy of the diagnosis after an external blinded validation was high and yielded a sensitivity of 89.5% and specificity of at least 99.1% for each method (Table 2).
The detection of pAF in patients presenting with ischaemic stroke is of major clinical importance, as it changes the secondary preventive therapy from antiplatelet drugs to oral anticoagulation 10, leading to a 60–70% reduction of recurrent strokes in patients with AF compared to placebo 11. Because of this remarkable secondary preventive effect, recent studies have focused on an increased diagnostic yield of ECG monitoring. The authors of observational studies 1, 6, 7, 12, 13 and randomized trials 8, 14 achieved higher detection rates of pAF by simply prolonging monitoring times. Nevertheless, the analysis of prolonged monitoring data is costly in terms of time and resources, and therefore difficult to implement in clinical practice. In this regard, the SA might offer a cost‐effective and rapid option for the analysis of increased ECG data. It was recently shown that this software could be used for the evaluation of ECG telemetry recorded during stroke‐unit monitoring 6 and might be used for analyzing Holter ECG in the ambulatory setting beyond 24 h.
Our study examines, in a real‐world setting, the effectiveness of an SA and RA of 24‐h Holter ECG with regard to the detection of AF. A further strength of the study is the external validation of ECG data by the core laboratory in the case of AF detection by the SA or discordance between RA and SA. However, this study has some limitations, as the results of a monocentric study may not be directly transferrable to other healthcare systems and organizational structures. In addition, the central reading of ECG data was only performed in the case of pAF detection by the SA or discordant results between SA and RA. Therefore, in cases rated as false/false (no AF detection) by RA and SA there was no subsequent external rating and, although unlikely, there is the possibility that a diagnosis of pAF could have been missed. Moreover, no sample size calculation was performed and therefore this study is mainly exploratory and further studies addressing the role of automated analysis methods for the evaluation of increasing ECG data with prolonged monitoring times are needed.
Our study suggests that SA is an alternative to RA for the detection of pAF (κ, 0.686; P < 0.001; 95% confidence interval, 0.525–0.847) especially with regard to the cost‐effective and resource‐saving evaluation of increasing ECG data to improve the diagnostic yield of pAF after cerebral ischaemia. However, competent ECG readers must still verify positive SA diagnoses and a major limitation of solely SA analysis is the potential to overlook clinically relevant arrhythmias. This is reflected by the detection of seven such arrhythmias by RA resulting in further cardiological diagnostics and pacemaker implantation in two cases. Therefore, we would recommend using the SA approach as a complementary analysis tool to facilitate the prolongation of Holter ECG monitoring for patients with stroke in order to detect pAF.
Disclosure of conflicts of interest
T.U., A.G., S.G., A.M. and M.W.‐K. declare no financial or other conflicts of interest. R.W. has received personal fees from Bayer, Berlin Chemie, BMS, Boehringer Ingelheim, Boston Scientific, CVRx, Gilead, Johnson & Johnson, Medtronic, Novartis, Pfizer, Relypsa, Sanofi and Servier since 2003 outside the present work, and research grants from Boehringer Ingelheim, the European Union and Bundesministerium für Bildung und Forschung. K.G. received personal fees from Bayer, Boehringer Ingelheim, BMS, Daiichi Sankyo and Pfizer outside the present work.
Acknowledgements
The automated analysis technology (SRAclinic®) was kindly provided by Apoplex Medical Technologies GmbH (Pirmasens, Germany). We thank Cheryl Ernest for proofreading the manuscript.
References
- 1. Sposato LA, Cipriano LE, Saposnik G, Ruiz Vargas E, Riccio PM, Hachinski V. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta‐analysis. Lancet Neurol 2015; 14: 377–387. [DOI] [PubMed] [Google Scholar]
- 2. Schaefer JR, Leussler D, Rosin L, Pittrow D, Hepp T. Improved detection of paroxysmal atrial fibrillation utilizing a software‐assisted electrocardiogram approach. PLoS One 2014; 9: e89328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jauch EC, Saver JL, Adams HP Jr, et al Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44: 870–947. [DOI] [PubMed] [Google Scholar]
- 4. Palomaki A, Mustonen P, Hartikainen JE, et al Underuse of anticoagulation in stroke patients with atrial fibrillation – the FibStroke Study. Eur J Neurol 2016; 23: 133–139. [DOI] [PubMed] [Google Scholar]
- 5. European Heart Rhythm Association, European Association for Cardio‐Thoracic Surgery , Camm AJ, et al Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010; 31: 2369–2429. [DOI] [PubMed] [Google Scholar]
- 6. Rizos T, Guntner J, Jenetzky E, et al Continuous stroke unit electrocardiographic monitoring versus 24‐hour Holter electrocardiography for detection of paroxysmal atrial fibrillation after stroke. Stroke 2012; 43: 2689–2694. [DOI] [PubMed] [Google Scholar]
- 7. Stahrenberg R, Weber‐Krüger M, Seegers J, et al Enhanced detection of paroxysmal atrial fibrillation by early and prolonged continuous holter monitoring in patients with cerebral ischemia presenting in sinus rhythm. Stroke 2010; 41: 2884–2888. [DOI] [PubMed] [Google Scholar]
- 8. Wachter R, Gröschel K, Hamann GF, et al Holter‐electrocardiogram‐monitoring in patients with acute ischaemic stroke (Find‐AFRANDOMISED): an open‐label randomised controlled trial. Lancet Neurol 2017; 16: 282–290. [DOI] [PubMed] [Google Scholar]
- 9. Landis JR, Koch GG. Measurement of observer agreement for categorical data. Biometrics 1977; 33: 159–174. [PubMed] [Google Scholar]
- 10. January CT, Wann LS, Alpert JS, et al 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64: e1–e76. [DOI] [PubMed] [Google Scholar]
- 11. Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007; 146: 857–867. [DOI] [PubMed] [Google Scholar]
- 12. Wohlfahrt J, Stahrenberg R, Weber‐Krüger M, et al Clinical predictors to identify paroxysmal atrial fibrillation after ischaemic stroke. Eur J Neurol 2014; 21: 21–27. [DOI] [PubMed] [Google Scholar]
- 13. Poli S, Diedler J, Hartig F, et al Insertable cardiac monitors after cryptogenic stroke – a risk factor based approach to enhance the detection rate for paroxysmal atrial fibrillation. Eur J Neurol 2016; 23: 375–381. [DOI] [PubMed] [Google Scholar]
- 14. Higgins P, MacFarlane PW, Dawson J, McInnes GT, Langhorne P, Lees KR. Noninvasive cardiac event monitoring to detect atrial fibrillation after ischemic stroke: a randomized, controlled trial. Stroke 2013; 44: 2525–2531. [DOI] [PubMed] [Google Scholar]


