Abstract
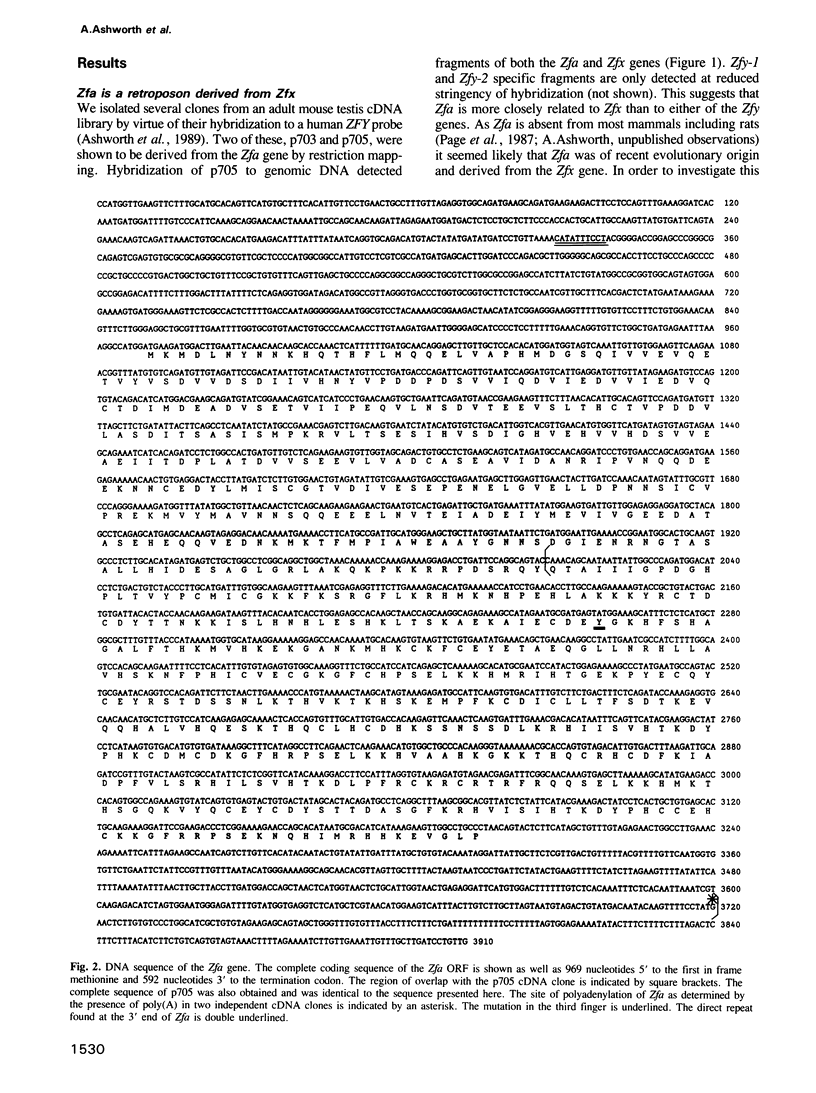
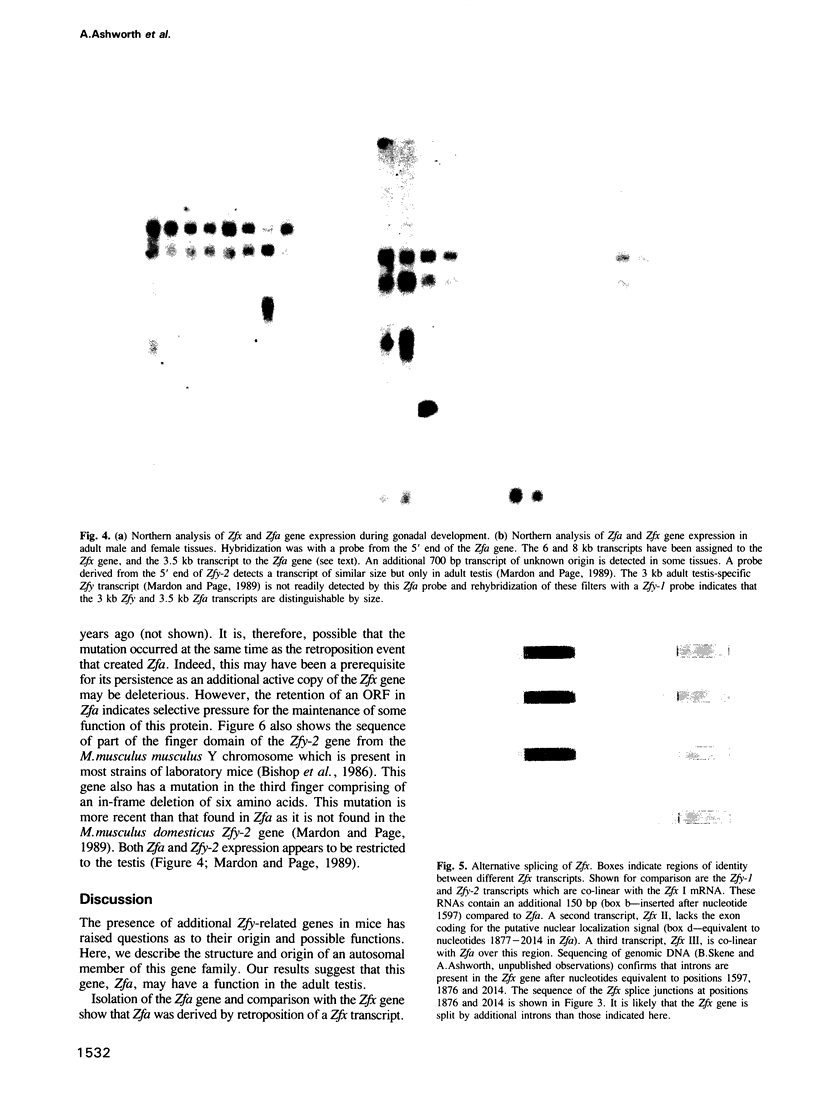
ZFY, a gene on the Y chromosome encoding a zinc finger protein, has been proposed as a candidate for the human testis determining gene. Sequences related to ZFY, called ZFX, are present on the X chromosome of a wide range of placental mammals. Unlike most mammals the mouse has four genes homologous to ZFY; two on the Y chromosome, Zfy-1 and Zfy-2, an X-linked gene, Zfx, and an autosomal gene, Zfa. We show here that Zfa has arisen recently by retroposition of one of at least three alternatively spliced mRNAs transcribed from the Zfx gene. Zfa is an unusual retroposon in that it has retained an open reading frame and is expressed, although its function may be limited or altered by the presence of a potentially inactivating mutation in the third of its zinc fingers. This mutation must have occurred at the same time or soon after the retroposition event as it is also present in the Zfa gene of Mus spretus. Interestingly the third finger of the M. musculus musculus Zfy-2 gene has also sustained a mutation suggesting that this gene family may be rapidly evolving in mice.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashworth A., Swift S., Affara N. Sequence of cDNA for murine Zfy-1, a candidate for Tdy. Nucleic Acids Res. 1989 Apr 11;17(7):2864–2864. doi: 10.1093/nar/17.7.2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Bird A. P. CpG-rich islands and the function of DNA methylation. Nature. 1986 May 15;321(6067):209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Bishop C. E., Boursot P., Baron B., Bonhomme F., Hatat D. Most classical Mus musculus domesticus laboratory mouse strains carry a Mus musculus musculus Y chromosome. Nature. 1985 May 2;315(6014):70–72. doi: 10.1038/315070a0. [DOI] [PubMed] [Google Scholar]
- Blumberg H., Eisen A., Sledziewski A., Bader D., Young E. T. Two zinc fingers of a yeast regulatory protein shown by genetic evidence to be essential for its function. 1987 Jul 30-Aug 5Nature. 328(6129):443–445. doi: 10.1038/328443a0. [DOI] [PubMed] [Google Scholar]
- Boulay J. L., Dennefeld C., Alberga A. The Drosophila developmental gene snail encodes a protein with nucleic acid binding fingers. 1987 Nov 26-Dec 2Nature. 330(6146):395–398. doi: 10.1038/330395a0. [DOI] [PubMed] [Google Scholar]
- Brown R. S., Sander C., Argos P. The primary structure of transcription factor TFIIIA has 12 consecutive repeats. FEBS Lett. 1985 Jul 8;186(2):271–274. doi: 10.1016/0014-5793(85)80723-7. [DOI] [PubMed] [Google Scholar]
- Burgoyne P. S., Buehr M., Koopman P., Rossant J., McLaren A. Cell-autonomous action of the testis-determining gene: Sertoli cells are exclusively XY in XX----XY chimaeric mouse testes. Development. 1988 Feb;102(2):443–450. doi: 10.1242/dev.102.2.443. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diakun G. P., Fairall L., Klug A. EXAFS study of the zinc-binding sites in the protein transcription factor IIIA. Nature. 1986 Dec 18;324(6098):698–699. doi: 10.1038/324698a0. [DOI] [PubMed] [Google Scholar]
- Dobner P. R., Kislauskis E., Wentworth B. M., Villa-Komaroff L. Alternative 5' exons either provide or deny an initiator methionine codon to the same alpha-tubulin coding region. Nucleic Acids Res. 1987 Jan 12;15(1):199–218. doi: 10.1093/nar/15.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairall L., Rhodes D., Klug A. Mapping of the sites of protection on a 5 S RNA gene by the Xenopus transcription factor IIIA. A model for the interaction. J Mol Biol. 1986 Dec 5;192(3):577–591. doi: 10.1016/0022-2836(86)90278-0. [DOI] [PubMed] [Google Scholar]
- Ferris S. D., Sage R. D., Prager E. M., Ritte U., Wilson A. C. Mitochondrial DNA evolution in mice. Genetics. 1983 Nov;105(3):681–721. doi: 10.1093/genetics/105.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorne T. A., Blumberg H., Young E. T. Sequence homology of the yeast regulatory protein ADR1 with Xenopus transcription factor TFIIIA. Nature. 1986 Mar 20;320(6059):283–287. doi: 10.1038/320283a0. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Kadonaga J. T., Carner K. R., Masiarz F. R., Tjian R. Isolation of cDNA encoding transcription factor Sp1 and functional analysis of the DNA binding domain. Cell. 1987 Dec 24;51(6):1079–1090. doi: 10.1016/0092-8674(87)90594-0. [DOI] [PubMed] [Google Scholar]
- Kawasaki E. S., Clark S. S., Coyne M. Y., Smith S. D., Champlin R., Witte O. N., McCormick F. P. Diagnosis of chronic myeloid and acute lymphocytic leukemias by detection of leukemia-specific mRNA sequences amplified in vitro. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5698–5702. doi: 10.1073/pnas.85.15.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman P., Gubbay J., Collignon J., Lovell-Badge R. Zfy gene expression patterns are not compatible with a primary role in mouse sex determination. Nature. 1989 Dec 21;342(6252):940–942. doi: 10.1038/342940a0. [DOI] [PubMed] [Google Scholar]
- Mardon G., Mosher R., Disteche C. M., Nishioka Y., McLaren A., Page D. C. Duplication, deletion, and polymorphism in the sex-determining region of the mouse Y chromosome. Science. 1989 Jan 6;243(4887):78–80. doi: 10.1126/science.2563173. [DOI] [PubMed] [Google Scholar]
- Mardon G., Page D. C. The sex-determining region of the mouse Y chromosome encodes a protein with a highly acidic domain and 13 zinc fingers. Cell. 1989 Mar 10;56(5):765–770. doi: 10.1016/0092-8674(89)90680-6. [DOI] [PubMed] [Google Scholar]
- McCarrey J. R. Nucleotide sequence of the promoter region of a tissue-specific human retroposon: comparison with its housekeeping progenitor. Gene. 1987;61(3):291–298. doi: 10.1016/0378-1119(87)90192-2. [DOI] [PubMed] [Google Scholar]
- McLaren A. Sex determination in mammals. Trends Genet. 1988 Jun;4(6):153–157. doi: 10.1016/0168-9525(88)90020-0. [DOI] [PubMed] [Google Scholar]
- Miller J., McLachlan A. D., Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985 Jun;4(6):1609–1614. doi: 10.1002/j.1460-2075.1985.tb03825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Monk M., McLaren A. X-chromosome activity in foetal germ cells of the mouse. J Embryol Exp Morphol. 1981 Jun;63:75–84. [PubMed] [Google Scholar]
- Moses K., Ellis M. C., Rubin G. M. The glass gene encodes a zinc-finger protein required by Drosophila photoreceptor cells. Nature. 1989 Aug 17;340(6234):531–536. doi: 10.1038/340531a0. [DOI] [PubMed] [Google Scholar]
- Nagai K., Nakaseko Y., Nasmyth K., Rhodes D. Zinc-finger motifs expressed in E. coli and folded in vitro direct specific binding to DNA. Nature. 1988 Mar 17;332(6161):284–286. doi: 10.1038/332284a0. [DOI] [PubMed] [Google Scholar]
- Nagamine C. M., Chan K. M., Kozak C. A., Lau Y. F. Chromosome mapping and expression of a putative testis-determining gene in mouse. Science. 1989 Jan 6;243(4887):80–83. doi: 10.1126/science.2563174. [DOI] [PubMed] [Google Scholar]
- Nagoshi R. N., McKeown M., Burtis K. C., Belote J. M., Baker B. S. The control of alternative splicing at genes regulating sexual differentiation in D. melanogaster. Cell. 1988 Apr 22;53(2):229–236. doi: 10.1016/0092-8674(88)90384-4. [DOI] [PubMed] [Google Scholar]
- Page D. C., Mosher R., Simpson E. M., Fisher E. M., Mardon G., Pollack J., McGillivray B., de la Chapelle A., Brown L. G. The sex-determining region of the human Y chromosome encodes a finger protein. Cell. 1987 Dec 24;51(6):1091–1104. doi: 10.1016/0092-8674(87)90595-2. [DOI] [PubMed] [Google Scholar]
- Palmer M. S., Sinclair A. H., Berta P., Ellis N. A., Goodfellow P. N., Abbas N. E., Fellous M. Genetic evidence that ZFY is not the testis-determining factor. Nature. 1989 Dec 21;342(6252):937–939. doi: 10.1038/342937a0. [DOI] [PubMed] [Google Scholar]
- Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988 Oct 20;335(6192):683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- Redemann N., Gaul U., Jäckle H. Disruption of a putative Cys-zinc interaction eliminates the biological activity of the Krüppel finger protein. Nature. 1988 Mar 3;332(6159):90–92. doi: 10.1038/332090a0. [DOI] [PubMed] [Google Scholar]
- Robinson M. O., McCarrey J. R., Simon M. I. Transcriptional regulatory regions of testis-specific PGK2 defined in transgenic mice. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8437–8441. doi: 10.1073/pnas.86.21.8437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J. H. The origin and evolution of retroposons. Int Rev Cytol. 1985;93:187–279. doi: 10.1016/s0074-7696(08)61375-3. [DOI] [PubMed] [Google Scholar]
- Rogers J., Early P., Carter C., Calame K., Bond M., Hood L., Wall R. Two mRNAs with different 3' ends encode membrane-bound and secreted forms of immunoglobulin mu chain. Cell. 1980 Jun;20(2):303–312. doi: 10.1016/0092-8674(80)90616-9. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Schneider-Gädicke A., Beer-Romero P., Brown L. G., Mardon G., Luoh S. W., Page D. C. Putative transcription activator with alternative isoforms encoded by human ZFX gene. Nature. 1989 Dec 7;342(6250):708–711. doi: 10.1038/342708a0. [DOI] [PubMed] [Google Scholar]
- Schneider-Gädicke A., Beer-Romero P., Brown L. G., Nussbaum R., Page D. C. ZFX has a gene structure similar to ZFY, the putative human sex determinant, and escapes X inactivation. Cell. 1989 Jun 30;57(7):1247–1258. doi: 10.1016/0092-8674(89)90061-5. [DOI] [PubMed] [Google Scholar]
- Sinclair A. H., Foster J. W., Spencer J. A., Page D. C., Palmer M., Goodfellow P. N., Graves J. A. Sequences homologous to ZFY, a candidate human sex-determining gene, are autosomal in marsupials. Nature. 1988 Dec 22;336(6201):780–783. doi: 10.1038/336780a0. [DOI] [PubMed] [Google Scholar]
- Stanojević D., Hoey T., Levine M. Sequence-specific DNA-binding activities of the gap proteins encoded by hunchback and Krüppel in Drosophila. Nature. 1989 Sep 28;341(6240):331–335. doi: 10.1038/341331a0. [DOI] [PubMed] [Google Scholar]
- Treisman J., Desplan C. The products of the Drosophila gap genes hunchback and Krüppel bind to the hunchback promoters. Nature. 1989 Sep 28;341(6240):335–337. doi: 10.1038/341335a0. [DOI] [PubMed] [Google Scholar]
- Vrana K. E., Churchill M. E., Tullius T. D., Brown D. D. Mapping functional regions of transcription factor TFIIIA. Mol Cell Biol. 1988 Apr;8(4):1684–1696. doi: 10.1128/mcb.8.4.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. I., Li W. H. Evidence for higher rates of nucleotide substitution in rodents than in man. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1741–1745. doi: 10.1073/pnas.82.6.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]