Abstract
Background
Neurofibromatosis type 1 (NF1) is a monogenic disorder affecting cognitive function. About one third of children with NF1 have attentional disorders, and the cognitive phenotype is characterized by impairment in prefrontally-mediated functions. Mouse models of NF1 show irregularities in GABA release and striatal dopamine metabolism. We hypothesized that youth with NF1 would show abnormal behavior and neural activity on a task of risk-taking reliant on prefrontal-striatal circuits.
Methods
Youth with NF1 (N=29) and demographically comparable healthy controls (N=22), ages 8-19, were administered a developmentally sensitive gambling task, in which they chose between low-risk gambles with a high probability of obtaining a small reward, and high-risk gambles with a low probability of obtaining a large reward. We used functional magnetic resonance imaging (fMRI) to investigate neural activity associated with risky decision making, as well as age-associated changes in these behavioral and neural processes.
Results
Behaviorally, youth with NF1 tended to make fewer risky decisions than controls. Neuroimaging analyses revealed significantly reduced neural activity across multiple brain regions involved in higher-order semantic processing and motivation (i.e., anterior cingulate, paracingulate, supramarginal, and angular gyri) in patients with NF1 relative to controls during the task. We also observed atypical age-associated changes in neural activity in patients with NF1, such that during risk taking, neural activity tended to decrease with age in controls, whereas it tended to increase with age in patients with NF1.
Conclusions
Findings suggest that developmental trajectories of neural activity during risky decision-making may be disrupted in youth with NF1.
Keywords: Neurofibromatosis Type I, Development, Decision-Making, Functional MRI, Phenotype-Genotype, Psychiatric Disorders
Introduction
Neurofibromatosis type 1 (NF1; MIM 162200), a monogenic disorder caused by mutations in the neurofibromin gene on chromosome 17, is one of the most common single gene disorders affecting cognitive function (prevalence 1:3500) (1). Physical features include the formation of peripheral nerve sheath tumors, café-au-lait spots, and Lisch nodules (2). About one-third of children with NF1 meet diagnostic criteria for attention deficit hyperactivity disorder (ADHD), and the cognitive phenotype includes impairment in prefrontally-mediated functions that encompass attention, working memory, and inhibitory control (3).
The Nf1 gene codes for the neurofibromin protein, which acts as a tumor suppressor that modulates the Ras signaling transduction pathway (4). Neurofibromin, a large cytoplasmic protein, is a negative regulator of Ras, and acts to keep it in its inactive, GDP-bound state. Mutations in the Nf1 gene lead to compromised neurofibromin activity, and thus overactivity of Ras, resulting in dysregulation of cell growth and proliferation.
Mouse models of NF1 have uncovered Ras-signaling dependent increases in GABA release (5-7) and deficits in plasticity that contribute to their learning and memory deficits. These mouse models have also shed light on irregularities in dopaminergic metabolism in this disorder (8). In NF1, tyrosine hydroxylase (TH), a precursor to dopamine, is reduced, leading to lower dopaminergic signaling (3). In addition to increases in GABA release in the striatum (7), Brown et al. (8) found reduced levels of dopamine in this structure in Nf1 mutant mice, and reduced rearing in response to novel objects, suggesting a dampened response to novel stimuli. In addition to treatments that target Ras signaling (9; 10), drugs that increased dopaminergic levels (i.e. methylphenidate or L-DOPA) rescued these behavioral deficits (8). Another study in a mouse model of NF1 found that deficiencies in dopaminergic signaling also appear to contribute to deficits in learning and memory (11). In humans, the stimulant methylphenidate (MPH) has been used to treat attentional deficits in patients with NF1, which acts by blocking dopamine reuptake, thus increasing extracellular dopamine (12; 13). Although it is still unknown how neurofibromin regulates dopamine homeostasis in the brain, a recent review of cognitive dysfunction in NF1 points towards dopamine as a possible molecular target for remediating cognitive and psychiatric symptoms in patients with NF1 (3). Recent evidence from animal models suggests that dopaminergic neurotransmission in the striatum plays a direct role in risky decision-making, in that it signals preference for making a risky or safe decision (14; 15). Further, individual differences in dopaminergic neurotransmission have been thought to underlie variability in risky decision-making in humans (16). Lastly, while little work has been done on inhibitory control in patients with NF1, a recent study found impaired impulse control in children and adolescents with NF1 on a visual go/no-go task, which was associated with reduced electroencephalography (EEG) correlates of inhibitory control (17). These authors also found reduced relative GABA levels in medial frontal regions of the brain in patients with NF1, as measured by magnetic resonance spectroscopy (MRS), which were directly related to severity of impulse control problems. This work warrants further investigation of decision-making processes in NF1, and their underlying neural substrates.
Based on these findings, we hypothesized that patients with NF1 would show abnormal behavior on a task of risk-taking shown to be reliant on the striatum and orbitofrontal cortex (OFC). The Cake Gambling Task (18) was developed as a child-friendly task to measure risky decision-making with varying amounts of potential reward. Participants are asked to choose between low-risk gambles with a high probability of receiving a small reward, and high-risk gambles with a low probability of obtaining a large reward. To date, this task has been used to investigate risky decision making in typical development; findings indicate that healthy youth tend to make riskier decisions as the potential reward is increased, and risk-taking in the low reward conditions tends to decrease with age (18). Functional MRI (fMRI) results revealed that risky decisions were associated with increased activation in the ventromedial prefrontal cortex (vmPFC) and ventral striatum (VS), while cautious choices were associated with activation in the dorsolateral prefrontal cortex (dlPFC). Interestingly, this study also observed an adolescent-specific peak in vmPFC activation while making risky decisions, and an adolescent-specific peak in the VS when receiving reward feedback. The authors also found that older participants showed decreased activation in the dorsal anterior cingulate (ACC) during risky decision-making. Further, the tendency to make risky decisions was associated with decreased activation in the ACC and lateral OFC (19; 20).
To our knowledge, no studies of reward-based decision-making have been conducted in youth with NF1. Given the neurophysiological results in mouse models and the behavioral profile of NF1, we predicted that youth with NF1 would be more risk-averse than healthy individuals, particularly when the potential reward is high. Because of the increases in GABA-mediated inhibition previously documented in the prefrontal cortex of NF1 mouse models and associated hypoactivation of specific prefrontal structures in NF1 subjects (7), we hypothesized that patients with NF1 would not show the expected increases in neural activity in vmPFC during risky decision-making, nor increases in DLPFC activity during safe decision-making. Consequently, we also anticipated altered age-related trajectories during both risky and safe decision-making in patients with NF1. Additionally, we predicted that the relationship between individual risky decision-making and neural activity may differ in patients with NF1 versus controls. Lastly, based on findings in the mouse model indicating reduced dopamine levels and abnormal reward sensitivity (8), we predicted that patients with NF1 wouldn’t show an increase in VS activity when receiving positive feedback.
Methods and Materials
Participants
51 participants (29 patients with NF1 and 22 healthy controls, aged 8-19, were included in the study. Participants in the study were recruited from three primary sources: 1) The Children’s Hospital Los Angeles Neurofibromatosis Clinic, a major NF1 referral center for the greater Los Angeles region; 2) local Children’s Tumor Foundation and NF Network family educational symposia; 3) NF-related websites as well as www.clinicaltrials.gov. All aspects of the research study were granted IRB approval by the University of California, Los Angeles prior to the collection of any data. All participants underwent verbal and written consent after study procedures were fully explained, and their parents or guardians also completed written consent.
Patients with NF1 were screened and enrolled by a pediatric neurologist (T.R.), and had a confirmed diagnosis of Neurofibromatosis Type I, according to NIH criteria (21). Exclusion criteria are listed in Supplementary Information. Demographic information for the sample is presented in Table 1.
Table 1.
Demographic characteristics of study participants.
| NF1 (N=29) | Controls (N=22) | p value | |
|---|---|---|---|
| Age (mean, SD, range) | 11.93 (2.64), 8-16 | 12.73 (3.49), 8-19 | 0.381 |
| Gender | 14M, 15F | 13M, 9F | 0.443 |
| Ethnicity (% Latino) | 38% | 32% | 0.651 |
| Full Scale IQ (mean, SD) | 93.79 (2.95) | 112.50 (3.33) | <0.001** |
| ADHD Diagnosis (%) | 41% | --- | --- |
| Participant Education (years) | 6.55 | 6.95 | 0.637 |
| Highest Parental Education (years) | 15.65 | 16.59 | 0.278 |
*Usable fMRI data were available on 36 participants (NF1: N=18, Control: N=18)
*Seven of the NF1 patients with ADHD were taking psychostimulant medication at the time of testing
Cognitive and Psychiatric Testing
Supervised clinical psychology doctoral students administered neurocognitive and psychiatric evaluations to study participants. IQ data were acquired with the WASI (22). Psychiatric diagnostic information was determined via parental interview using the Computerized Diagnostic Interview Schedule for Children (C-DISC) (23) and/or participant interview via the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (24).
Procedure
All participants first received extensive instructions in a quiet testing room, and performed 11 practice trials before their scan. They were told that they should attempt to win as many trials as possible, and that at the end of the scan they would receive a prize corresponding to their performance on two randomly selected outcomes. Participants all received the same amount of money at the end of the scan; younger participants were given the option to choose a reward from a prize box.
Cake Task Description
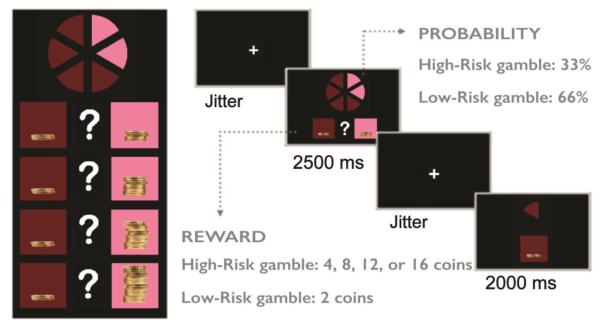
The fMRI task was adapted from the Cake Gambling Task (18), a child-friendly gambling task in which subjects are asked to choose between a low- and a high-risk gamble, associated with varying probability of reward (Figure 1). These probabilities are represented visually, as a circle with six distinct "wedges", which are brown and pink (4:2 ratio). Two squares are located beneath the circle, in which the rewards associated with the colors are presented as stacks of coins. On each trial, subjects choose a color, and receive a reward based on the probability described above (66% for low-risk gambles and 33% for high-risk gambles). The reward value associated with the high-risk choice varies (4, 8, 12, or 16 coins) and remains constant for the low-risk choice (2 coins). There were 84 total trials, with 21 trials per condition, which were separated into two blocks.
Figure 1.
The Cake Gambling Task. Participants are asked to choose between low-risk gambles with a high probability of obtaining a small reward (2 coins), versus high-risk gambles with a smaller probability of obtaining a higher reward (4-16 coins). In total, there were 84 trials, with 21 trials per condition. The trials were presented in an event-related fashion, and the order was consistent among subjects.
fMRI Acquisition
Neuroimaging data were collected on a 3T Siemens Trio MRI scanner at the Center for Cognitive Neuroscience at the University of California, Los Angeles. Functional data were acquired using T2*-weighted echoplanar images. While participants completed the task, 400 functional T2* echoplanar images were collected. As in Van Leijenhorst et al (2010) (25), two separate scans were acquired (seven minutes each), in order to check in with participants mid-scan. The two scans were subsequently combined for analysis (described below).
Each trial had the following structure: A jittered fixation cross was presented, varying between 300 and 5250 ms [using fsloptseq2; see http://surfer.nmr.mgh.harvard.edu/optseq/ (26)], followed by the stimulus for 2500 ms. Participants were instructed to respond during the stimulus presentation using either their index finger (brown choice) or middle finger (pink choice). Another jitter crosshair appeared, and then response feedback was given for 2000 ms. For correct outcomes (win), participants were shown the color that they chose, and the amount of money earned. For incorrect outcomes (loss), they were shown a stack of coins with a grey cross through it.
Additionally, a T2-weighted matched-bandwidth high resolution anatomical scan, and MPRAGE were collected (see Supplementary Information). Foam inserts were placed around the participants’ head to minimize head motion.
fMRI Preprocessing
fMRI analyses were performed using the FMRI Software Library (FSL) (www.fmrib.ox.ac.uk/fsl), version 5.0 (27). We excluded participants with translational motion that exceeded 4mm (NF1=11, Controls=5).
For participants retained in the analysis, images were first realigned to compensate for small head movements (28) Data were spatially smoothed using a 5-mm full-width-half-maximum Gaussian kernel. The data were filtered in the temporal domain using a nonlinear high-pass filter with a 100 second cutoff. The registration process first included registering the EPI images to the matched-bandwidth high-resolution scan, then to the structural MPRAGE image, and lastly into standard (Montreal Neurological Institute (MNI)) space, using nonlinear transformations. We ran each of the scans through a separate first-level analysis. These were then combined into a second-level analysis, before running group analyses. The two scans did not systemically differ from one another with regard to behavioral performance, nor patterns of neural activity (see Supplementary Information).
Behavioral Analyses
Cognitive and clinical data were processed using SPSS software v. 23 (IBM). We compared demographic characteristics between groups using independent-sample t-tests for continuous variables, and chi-square tests for categorical variables. Primary analyses of behavioral response data assessed how risk-taking changes as potential reward increases, and the relationship of risky decision-making to age. We used repeated-measures ANOVA to compare overall risk-taking across conditions between groups, and a univariate ANOVA to compare risk-taking between groups within each separate condition (4, 8, 12, or 16 coins). Additionally, paired t-tests were used to compare risk taking for the lowest (4 coins) vs. highest reward condition (16 coins) within each group. Age and gender were included as covariates in all behavioral analyses. To explore the relationship between risk-taking and age we used Pearson partial correlations controlling for gender. We also investigated post-feedback behavior, looking specifically at the tendency to make risky vs. safe decisions after receiving either a win or a loss. Separate univariate ANOVAs for post-win and post-loss were used to compare the percentage of trials in which the subject made a risky vs. safe choice, between groups. Secondary analyses investigated whether individual differences in IQ or diagnosis of ADHD may contribute to decision-making behavior.
fMRI Analyses
Standard model fitting was conducted for all subjects. After convolution with a canonical gamma hemodynamic response function, the following events were modeled: all risky decisions > baseline, all safe > baseline, all risky > all safe, all safe > all risky. We modeled both the decision-phase of the task (length determined as the reaction time between stimulus onset and participant response; <2500 ms), and the outcome-phase of the task (2000 ms). The six motion parameters as well as a motion outlier confound matrix produced by FSL motion outliers, designed to detect individual timepoints in the dataset that have been corrupted by excessive motion (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLMotionOutliers), were included as covariates of no interest. Among participants included in the fMRI analyses, there were no differences in translational motion between patients with NF1 and controls (p=0.15).
For group-level statistics, we investigated neural activity for the following contrasts: mean NF1 > baseline, mean control > baseline, NF1 > control, control > NF1, and age by group interactions in both directions. We investigated these for both the decision phase of the task (defined by the time between when the stimulus was first displayed, and when the participant gave a response) and the outcome phase of the task (during feedback; 2000 ms). We also ran a separate analysis, including the overall percentage of risky decision-making (defined as the percentage of time participants chose the low probability option) as a covariate, in order to determine the relationship between neural activity and behavioral propensity to make risky decisions, both between and across groups.
Group-level statistics images were thresholded with a cluster-forming threshold of z > 2.3 and a cluster probability of p<0.05, corrected for whole-brain multiple comparisons using Gaussian random field theory. Brain regions were identified using the Harvard-Oxford cortical and subcortical probabilistic atlases. For reporting of clusters, we used the cluster command in FSL, and calculated percent signal change in these regions, according to methods described by Mumford and colleagues (29). A list of clusters for each contrast is described in Supplementary Tables S1-S5. Figures were visualized using BrainNet Viewer, a MATLAB graphical user interface (30).
Results
Behavioral
Demographic characteristics
The total sample consisted of 51 participants (N=29 NF1, 22 controls). After excluding 16 subjects for excessive motion, as described above, fMRI data were available for analysis for 36 participants (N=18 NF1, 18 controls). As shown in Table 1, patients with NF1 were demographically matched with controls on age, gender, education, ethnicity, and parental education. Consistent with previous literature (3), patients with NF1 showed significantly decreased IQ as compared to controls (p<0.001**).
Cake Task
Behavioral Results
Overall, patients with NF1 showed a non-significant tendency to make fewer risky decisions across all reward categories, as compared to controls (p=0.105; Figure 2a), a finding which appeared to be driven by high-reward categories (12 coins: p=0.030, 16 coins: p=0.081).
Figure 2.
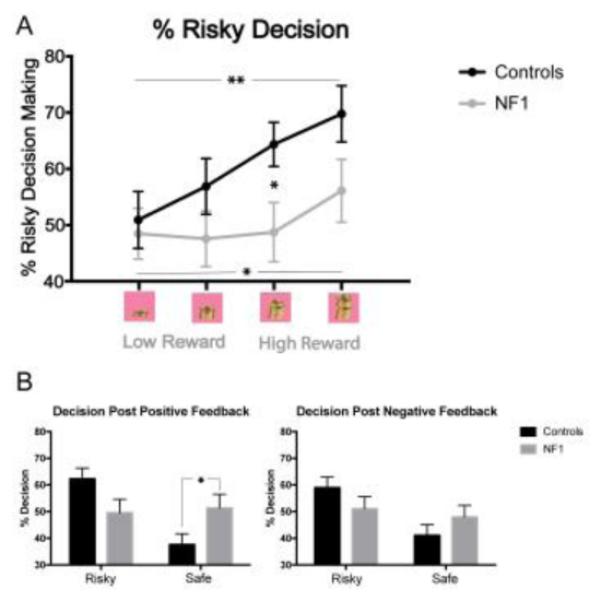
Behavioral results on the Cake Gambling Task.
A. Overall Performance. Patients with NF1 showed reduced risky decision-making relative to controls in the 12 coin condition (p=0.030), and a trend towards reduced risky decision-making in the 16 coin condition (p=0.081). Both groups showed an increased tendency to make risky decisions with higher reward (p=0.004 in controls; p=0.014 in NF1 patients).
B. Post Feedback Behavior. After receiving positive feedback (win), patients with NF1 were more likely than controls to make a safe decision (p=0.050). The groups did not differ on their responses after a loss.
Relationship with Age
We did not find a behavioral relationship between risky decision-making and age in patients with NF1 or controls (see Supplementary Figure S4).
Post-Feedback Behavior
Next we investigated how wins and losses affect subsequent trial behavior in patients with NF1 and controls. After receiving positive feedback (win), patients with NF1 were more likely than controls to make a safe decision (p=0.05; Figure 2b). Patients and controls did not differ in behavioral responses after receiving negative feedback (loss).
fMRI Results
Decision Phase
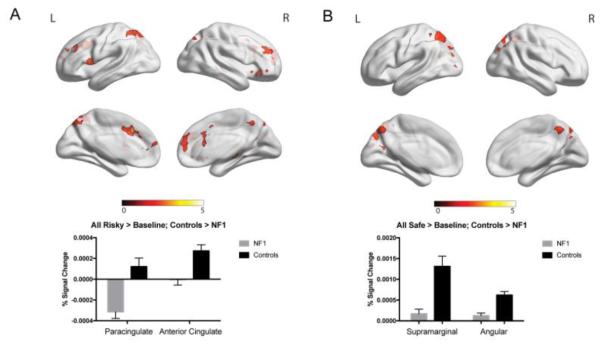
When making risky decisions, patients with NF1 showed decreased neural activity as compared to controls in multiple regions, including the paracingulate cortex and anterior cingulate cortex (Figure 3a). Similarly, when making safe decisions, NF1 patients showed decreased activity relative to controls in the supramarginal gyrus and angular gyrus (Figure 3b). No regions were found in either of these contrasts in which patients with NF1 showed increased neural activity compared to controls. When investigating the effect of age, we found an age by group interaction for risky decisions versus safe decisions in several regions, including the middle frontal gyrus and frontal pole. Specifically, older controls showed decreased activity in these regions during risky decision-making, whereas patients with NF1 showed the opposite pattern (Figure 4). When investigating the relationship between neural activity and risky decision-making (overall percentage of risky decisions made throughout the entire task), we found that controls with a higher tendency to make risky decisions showed decreased activity in the posterior cingulate cortex and frontal pole, whereas patients with NF1 showed increased activity in these regions in relation to risky decision making (Figure 5).
Figure 3.
fMRI results for neural activity during risky and safe decision making. Clusters represent regions where controls show significantly increased activity as compared to patients with NF1. Graphs represent percent signal change.
A. Risky choice. We found significantly increased activity in the paracingulate cortex (p=6.56e-07) and anterior cingulate cortex (p=9.67e-05) in controls relative to NF1 patients.
B. Safe choice. Controls showed significantly increased activity in the supramarginal gyrus (p-5.32E-11) and angular gyrus (p=4.83E-06) relative to NF1 patients.
Figure 4.
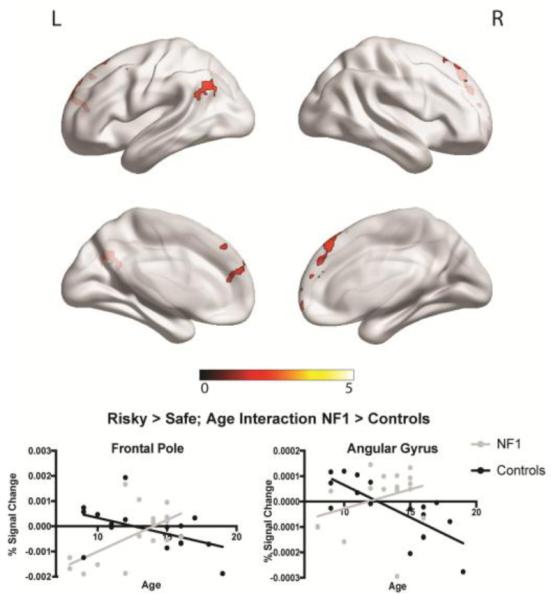
fMRI results for neural activity during risky vs. safe decision making, and relationship with age. Clusters represent regions that showed a significant group by age interaction effect for risky vs. safe decisions. We found a significant interaction in the middle frontal gyrus and frontal pole, such that controls showed a negative relationship between neural activity and age, whereas patients with NF1 showed increasing neural activity with increasing age. Graphs represent percent signal change. Pearson correlation values: frontal pole: NF1: r=0.60, p=0.01; Controls: r=−0.49, p=0.04; angular gyrus: NF1: r=0.34, p=0.18; Controls: r=−0.76, p<0.01.
Figure 5.
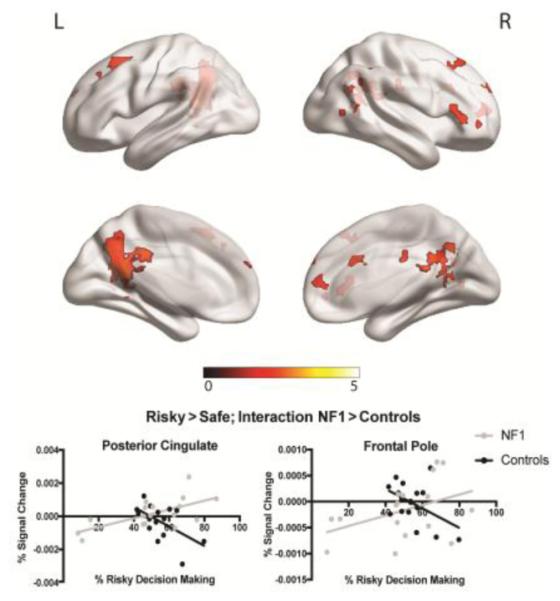
fMRI results for neural activity during risky vs. safe decision making, and relationship with individual propensity to make risky decisions. Clusters represent regions that showed a significant group interaction .Specifically, we found a significant interaction in the posterior cingulate cortex and frontal pole, such that controls showed a negative relationship between neural activity and risky decision-making, whereas patients with NF1 showed a positive relationship between neural activity and risky decision-making. Graphs represent percent signal change. Pearson correlation values: posterior cingulate: NF1: r=0.64, p<0.01; Controls: r=−0.57, p=0.02; frontal pole: NF1: r=0.41, p=0.10; Controls: r=−0.47, p=0.06.
Outcome Phase
When receiving positive feedback (win), as compared to negative feedback (loss), controls showed increased activity in the nucleus accumbens, caudate, and putamen; in contrast, patients with NF1 did not show any differences in neural activity for win vs. loss (Supplementary Figure S6).
Secondary Analyses
We conducted a secondary analysis in order to determine whether individual differences in IQ or ADHD diagnosis contributed to differences in risky decision-making. We did not find a significant relationship between IQ and risky decision-making in either patients with NF1 (r=−0.05, p=0.803) or controls (r=0.35, p=0.13). Finally, there was no significant effect of ADHD status on risky decision-making in patients with NF1 (p=0.91).
Discussion
This is the first known study to investigate risky decision-making in patients with NF1, a highly relevant area of investigation given the high rates of ADHD, hypothesized dopaminergic and GABAergic dysregulation in corticostriatal networks in this clinical population. Behaviorally, patients with NF1 tended to be less likely than controls to make risky decisions when the potential reward was high. They showed reduced neural activity in regions associated with reward, both during the decision-making and the outcome phases. Further, they did not show the typical age-related neural trajectories seen in controls, particularly in prefrontal regions.
Tendency towards decreased risky decision-making in patients with NF1
Our behavioral findings are suggestive of lower reward sensitivity in patients with NF1 as compared to controls, consistent with the notion that lower striatal dopamine levels are associated with decreased sensitivity to reward (31). In a mouse model of NF1, lower dopaminergic levels were found in the striatum (8), which may contribute to abnormalities in attentional and reward-based cognitive functions. The mice were given drugs that increased dopaminergic levels, which rescued the behavioral deficits, suggesting that abnormal dopaminergic homeostasis has a role in this behavior in NF1 patients (8). Relatedly, patients with Parkinson’s disease, who have decreased dopamine levels in the striatum, show deficits on a variety of reward-based tasks, which are remediated with dopamine agonist therapy (32).
Neural activity during risky and cautious decision-making
When making risky (relative to safe) decisions, patients with NF1 showed decreased neural activity in the paracingulate and anterior cingulate cortex, whereas controls showed increased activity. Both of these regions have been implicated in reward processing (33; 34). When making a safe decision, controls showed increased supramarginal and angular gyrus activity, relative to patients with NF1. In a previous study of healthy individuals, risky decision-making was associated with increased regional activity in the medial PFC, whereas increased activity in dorsolateral PFC was observed during safe decision-making (25). The angular gyrus is considered a functional hub within the default mode network, involved in self-referential mentation (35). It is also implicated in spatial representations of numbers (36). The decrease we observed in task-based neural activity within this region has also been observed in the resting state in patients with NF1 (37). Consistent with the directionality of our findings, prior fMRI studies have also found decreased neural activity in patients with NF1 relative to controls within the context of visual processing (37-39), and spatial working memory tasks (7).
The relationship between age and neural activity during risk-taking
When making risky decisions, patients with NF1 showed atypical age-related neural trajectories as compared to controls. In controls, age-associated decreases during risky decision-making were seen in the middle frontal gyrus and frontal pole, whereas patients with NF1 showed the opposite pattern. The prior study employing this task in healthy individuals found age-related decreases in neural activity in the anterior cingulate (25), which they attribute to a decreased need for cognitive control with increasing age. Frontal pole engagement has been linked the integration of higher-level cognitive processes (40), and specifically to processing effort and risk costs (41). The decreased activity we see in healthy individuals is consistent with the hypothesis of a reduced need for neural effort with increasing age. Patients with NF1 do not show this pattern, suggesting an altered maturational trajectory (42).
Individual differences in risk-taking and neural activity
In controls, a tendency to make risky decisions was associated with decreased activity in the frontal pole and posterior cingulate, whereas the opposite pattern was found in patients with NF1. The directionality of our findings in healthy individuals is consistent with that observed by Van Leijenhorst et al. (25). Decreased activity in the frontal pole (involved in cognitive control and integration of higher-order functions) and the posterior cingulate (a functional hub of the default mode network) in individuals with high levels of risk-taking may reflect a lack of control and inhibition, resulting in riskier behavior. The positive relationship between risk-taking and neural activity in patients with NF1 suggests that deciding to make a risky decision may be a more cognitively demanding process for them.
Neural activity during positive outcome
When receiving positive feedback after making a decision, controls showed increased neural activity in the nucleus accumbens, caudate, and putamen, whereas patients with NF1 did not show this pattern. Similarly, Van Leijenhorst et al (25) found an adolescent-specific peak in the VS in response to a reward, which aligns with the average age of participants in our study. Consistent with this, reward anticipation is associated with activation of a cortico-basal ganglia circuit, known to be modulated by dopamine in the VS (43). The lack of increased striatal activity in patients with NF1 when winning a reward may reflect decreased reward sensitivity in this group, consistent with findings of enhanced GABAergic (7) and altered dopaminergic function in striatal regions in an NF1 mouse model (8).
Neuroimaging findings in idiopathic ADHD
Given the high rates of ADHD in patients with NF1 (41% of our patient sample; 58% of those with the “inattentive type”, 42% with the “combined type”, and none with the “hyperactive type”), it is worth noting general patterns from task-based functional neuroimaging studies of idiopathic ADHD. Several fMRI studies of executive function tasks have found globally decreased activation in patients with idiopathic ADHD compared to controls (44) . Smith et. al found decreased insula and inferior frontal gyrus activity in patients with ADHD during a response inhibition task (45), and Scheres et al found that patients with ADHD showed decreased striatal activity during reward anticipation on a monetary incentive task (46). Our findings of hypoactivation during decision-making are consistent with the directionality of this prior work in idiopathic ADHD.
Limitations
Several limitations of the current study must be noted. First, the size of our sample with usable imaging data is relatively small, which reduces our statistical power. Given the prevalence of NF1 (1:3500), accruing large samples is challenging; our sample size is similar to prior neuroimaging studies in this patient population. Secondly, given the age range of our sample, and the high rate of attentional problems in those with NF1, many participants had to be excluded due to excess motion. Notably, however, there were no significant differences in motion in patients with NF1 vs. controls included in the analysis, indicating that our results are not an artifact of differential motion. Lastly, the link between GABA and hemodynamic response, as measured by the BOLD signal, is complex. GABA concentration has been shown to be reduced in patients with NF1 (47), and more research is warranted to determine how this may play a role in the BOLD response in patients with NF1 (48).
Conclusions
In the first study to investigate the functional neuroanatomy of risky decision-making in youth with NF1, we found a tendency toward more conservative decision making, concomitant with reduced activity of brain regions critical for higher-order semantic processing and motivation, in patients with NF1 relative to typically developing controls. Future PET studies are warranted to investigate dopamine receptor occupancy in the brains of patients with NF1, and the relationship to reward-related behavior.
Further work should also investigate how differences in risk-taking develop across the lifespan, and how they may interact with clinical symptomatology of NF1. Additionally, given pre-clinical evidence for reduced striatal dopamine and enhanced GABAergic function in NF1, studies that directly investigate the role of striatal dopaminergic and GABAergic function in reward processing in human patients with NF1 are warranted.
Supplementary Material
Acknowledgements
We wish to thank our funding sources, NRSA F31M102999, and DOD W81XWH-12-1-0081. We also wish to thank Dr. Adriana Galvan for her help with task design and interpretation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
Financial Disclosures
All authors report no biomedical financial interests or other potential conflicts of interest.
References
- 1.Rad E, Tee AR. Neurofibromatosis type 1: Fundamental insights into cell signalling and cancer. Seminars in Cell and Developmental Biology. 2016;52:39–46. doi: 10.1016/j.semcdb.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Huson SM. Recent developments in the diagnosis and management of neurofibromatosis. Archives of Disease in Childhood. 1989;64:745–749. doi: 10.1136/adc.64.5.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diggs-Andrews KA, Gutmann DH. Modeling cognitive dysfunction in neurofibromatosis-1. Trends in Neurosciences. 2013;36:237–247. doi: 10.1016/j.tins.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cichowski K, Jacks T. NF1 tumor suppressor gene function: narrowing the GAP. Cell. 2001;104:593–604. doi: 10.1016/s0092-8674(01)00245-8. [DOI] [PubMed] [Google Scholar]
- 5.Costa RM, Federov NB, Kogan JH, Murphy GG, Stern J, Ohno M, et al. Mechanism for the learning deficits in a mouse model of neurofibromatosis type 1. Nature. 2002;415:526–530. doi: 10.1038/nature711. [DOI] [PubMed] [Google Scholar]
- 6.Cui Y, Costa RM, Murphy GG, Elgersma Y, Zhu Y. Neurofibromin regulation of ERK signaling modulates GABA release and learning. Cell. 2008 doi: 10.1016/j.cell.2008.09.060. doi: 10.1016/j.cell.2008.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shilyansky C, Karlsgodt KH, Cummings DM, Sidiropoulou K, Hardt M, James AS, et al. Neurofibromin regulates corticostriatal inhibitory networks during working memory performance. Proc Natl Acad Sci USA. 2010;107:13141–13146. doi: 10.1073/pnas.1004829107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown JA, Emnett RJ, White CR, Yuede CM, Conyers SB, O'Malley KL, et al. Reduced striatal dopamine underlies the attention system dysfunction in neurofibromatosis-1 mutant mice. Human Molecular Genetics. 2010;19:4515–4528. doi: 10.1093/hmg/ddq382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li W, Cui Y, Kushner SA, Brown RAM, Jentsch JD, Frankland PW, et al. The HMG-CoA Reductase Inhibitor Lovastatin Reverses the Learning and Attention Deficits in a Mouse Model of Neurofibromatosis Type 1. Current Biology. 2005;15:1961–1967. doi: 10.1016/j.cub.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 10.Bearden CE, Hellemann GS, Rosser T, Montojo C, Jonas R, Enrique N, et al. A randomized placebo-controlled lovastatin trial for neurobehavioral function in neurofibromatosis I. Ann Clin Transl Neurol. 2016;3:266–279. doi: 10.1002/acn3.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diggs-Andrews KA, Tokuda K, Izumi Y, Zorumski CF, Wozniak DF, Gutmann DH. Dopamine deficiency underlies learning deficits in neurofibromatosis-1 mice. Ann Neurol. 2013;73:309–315. doi: 10.1002/ana.23793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mautner V-F, Kluwe L, Thakker SD, Leark RA. Treatment of ADHD in neurofibromatosis type 1. Dev Med Child Neurol. 2002;44:164–170. doi: 10.1017/s0012162201001876. [DOI] [PubMed] [Google Scholar]
- 13.Volkow ND, Wang G, Fowler JS, Logan J, Gerasimov M, Maynard L, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci. 2001;21:RC121. doi: 10.1523/JNEUROSCI.21-02-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohno M, Ghahremani DG, Morales AM, Robertson CL, Ishibashi K, Morgan AT, et al. Risk-taking behavior: dopamine D2/D3 receptors, feedback, and frontolimbic activity. Cereb Cortex. 2015;25:236–245. doi: 10.1093/cercor/bht218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugam JA, Day JJ, Wightman RM, Carelli RM. Phasic Nucleus Accumbens Dopamine Encodes Risk-Based Decision-Making Behavior. BPS. 2012;71:199–205. doi: 10.1016/j.biopsych.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rahman S, Sahakian BJ, Cardinal RN. Decision making and neuropsychiatry. Trends in cognitive …. 2001;5:271–277. doi: 10.1016/s1364-6613(00)01650-8. [DOI] [PubMed] [Google Scholar]
- 17.Ribeiro MJ, Violante IR, Bernardino I, Edden RAE, Castelo-Branco M. Abnormal relationship between GABA, neurophysiology and impulsive behavior in neurofibromatosis type 1. Cortex. 2015;64:194–208. doi: 10.1016/j.cortex.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Leijenhorst L, Westenberg PM, Crone EA. A Developmental Study of Risky Decisions on the Cake Gambling Task: Age and Gender Analyses of Probability Estimation and Reward Evaluation. Developmental Neuropsychology. 2008;33:179–196. doi: 10.1080/87565640701884287. [DOI] [PubMed] [Google Scholar]
- 19.Van Leijenhorst L, Crone EA, Bunge SA. Neural correlates of developmental differences in risk estimation and feedback processing. Neuropsychologia. 2006;44:2158–2170. doi: 10.1016/j.neuropsychologia.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Van Leijenhorst L, Moor BG, de Macks ZAO, Rombouts SARB, Westenberg PM, Crone EA. Adolescent risky decision-making: Neurocognitive development of reward and control regions. NeuroImage. 2010;51:345–355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- 21.Stumpf DA, Alksne JF, Annegers JF. Neurofibromatosis. Conference statement. Archives of Neurology. 1988 [Google Scholar]
- 22.Wechsler D. Manual for the Wechsler abbreviated intelligence scale (WASI) San Antonio; 1999. [Google Scholar]
- 23.Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwan-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, Differences From Previous Versions, and Reliability of Some Common Diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 24.First MB, Spitzer RL, Gibbon M, Williams J. First: Structured clinical interview for Axis I DSM-IV… - Google Scholar. Biometrics Research; New York: 1994. [Google Scholar]
- 25.Van Leijenhorst L, Moor BG, de Macks ZAO, Rombouts SARB, Westenberg PM, Crone EA. Adolescent risky decision-making: Neurocognitive development of reward and control regions. NeuroImage. 2010;51:345–355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- 26.Dale AM. Optimal experimental design for event-related fMRI. Hum Brain Mapp. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 28.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 29.Mumford JA, Poldrack RA. Modeling group fMRI data. Soc Cogn Affect Neurosci. 2007;2:251–257. doi: 10.1093/scan/nsm019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xia M, Wang J, He Y. Csermely P, editor. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS ONE. 2013;8:e68910. doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cools R, Frank MJ, Gibbs SE, Miyakawa A, Jagust W, D'Esposito M. Striatal dopamine predicts outcome-specific reversal learning and its sensitivity to dopaminergic drug administration. J Neurosci. 2009;29:1538–1543. doi: 10.1523/JNEUROSCI.4467-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bódi N, Kéri S, Nagy H, Moustafa A, Myers CE, Daw N, et al. Reward-learning and the novelty-seeking personality: a between- and within-subjects study of the effects of dopamine agonists on young Parkinson's patients. Brain. 2009;132:2385–2395. doi: 10.1093/brain/awp094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walter H, Abler B, Ciaramidaro A, Erk S. Motivating forces of human actions. Brain Res Bull. 2005;67:368–381. doi: 10.1016/j.brainresbull.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 34.Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proceedings of the National Academy of Sciences. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seghier ML. The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist. 2013;19:43–61. doi: 10.1177/1073858412440596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Göbel S, Walsh V, Rushworth MFS. The Mental Number Line and the Human Angular Gyrus. NeuroImage. 2001;14:1278–1289. doi: 10.1006/nimg.2001.0927. [DOI] [PubMed] [Google Scholar]
- 37.Tomson SN, Schreiner MJ, Narayan M, Rosser T, Enrique N, Silva AJ, et al. Resting state functional MRI reveals abnormal network connectivity in neurofibromatosis 1. Hum Brain Mapp. 2015;36:4566–4581. doi: 10.1002/hbm.22937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Billingsley RL, Jackson EF, Slopis JM, Swank PR, Mahankali S, Moore BD. Functional magnetic resonance imaging of phonologic processing in neurofibromatosis 1. J Child Neurol. 2003;18:731–740. doi: 10.1177/08830738030180110701. [DOI] [PubMed] [Google Scholar]
- 39.Violante IR, Ribeiro MJ, Cunha G, Bernardino I, Duarte JV, Ramos F, et al. Zang Y-F, editor. Abnormal Brain Activation in Neurofibromatosis Type 1: A Link between Visual Processing and the Default Mode Network. PLoS ONE. 2012;7:e38785–11. doi: 10.1371/journal.pone.0038785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prabhakaran V, Narayanan K, Zhao Z. Integration of diverse information in working memory within the frontal lobe. Nature. 2000;3:85–90. doi: 10.1038/71156. [DOI] [PubMed] [Google Scholar]
- 41.Burke CJ, Brunger C, Kahnt T, Park SQ, Tobler PN. Neural Integration of Risk and Effort Costs by the Frontal Pole: Only upon Request. Journal of Neuroscience. 2013;33:1706–1713. doi: 10.1523/JNEUROSCI.3662-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lorenzo J, Barton B, Arnold SS, North KN. Developmental trajectories of young children with neurofibromatosis type 1: a longitudinal study from 21 to 40 months of age. J Pediatr. 2015;166:1006–12.e1. doi: 10.1016/j.jpeds.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 43.Haber SN, Knutson B. The Reward Circuit: Linking Primate Anatomy and Human Imaging. Neuropsychopharmacology. 2009;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cortese S, Kelly C, Chabernaud C, Proal E, Di Martino A, Milham MP, Castellanos FX. Toward Systems Neuroscience of ADHD: A Meta-Analysis of 55 fMRI Studies. Am J Psychiatry. 2012;169:1038–1055. doi: 10.1176/appi.ajp.2012.11101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith AB, Taylor E, Brammer M, Toone B, Rubia K. Task-specific hypoactivation in prefrontal and temporoparietal brain regions during motor inhibition and task switching in medication-naive children and adolescents with attention deficit hyperactivity disorder. Am J Psychiatry. 2006;163:1044–1051. doi: 10.1176/ajp.2006.163.6.1044. [DOI] [PubMed] [Google Scholar]
- 46.Scheres A, Milham MP, Knutson B, Castellanos FX. Ventral Striatal Hyporesponsiveness During Reward Anticipation in Attention-Deficit/Hyperactivity Disorder. Biol Psychiatry. 2007;61:720–724. doi: 10.1016/j.biopsych.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 47.Violante IR, Patricio M, Bernardino I, Rebola J, Abrunhosa AJ, Ferreira N, Castelo-Branco M. GABA deficiency in NF1: A multimodal [11C]-flumazenil and spectroscopy study. Neurology. 2016;87:897–904. doi: 10.1212/WNL.0000000000003044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harris AD, Puts NAJ, Anderson BA, Yantis S, Pekar JJ, Barker PB, Edden RAE. Luo X, editor. Multi-Regional Investigation of the Relationship between Functional MRI Blood Oxygenation Level Dependent (BOLD) Activation and GABA Concentration. PLoS ONE. 2015;10:e0117531–17. doi: 10.1371/journal.pone.0117531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.