Abstract
Peroxisome Proliferator Activated Receptor (PPAR)-α is a key regulator of lipid metabolism and recent studies reveal it also regulates inflammation in several different disease models. Gemfibrozil, an agonist of PPAR-α, is a FDA approved drug for hyperlipidemia and has been shown to inhibit clinical signs in a rodent model of multiple sclerosis. Since many studies have shown improved outcome from spinal cord injury (SCI) by anti-inflammatory and neuroprotective agents, we tested the efficacy of oral gemfibrozil given before or after SCI for promoting tissue preservation and behavioral recovery after spinal contusion injury in mice. Unfortunately, the results were contrary to our hypothesis; in our first attempt, gemfibrozil treatment exacerbated locomotor deficits and increased tissue pathology after SCI. In subsequent experiments, the behavioral effects were not replicated but histological outcomes again were worse. We also tested the efficacy of a different PPAR-α agonist, fenofibrate, which also modulates immune responses and is beneficial in several neurodegenerative disease models. Fenofibrate treatment did not improve recovery, although there was a slight trend for a modest increase in histological tissue sparing. Based on our results, we conclude that PPAR-α agonists yield either no effect or worsen recovery from spinal cord injury, at least at the doses and the time points of drug delivery tested here. Further, patients sustaining spinal cord injury while taking gemfibrozil might be prone to exacerbated tissue damage.
Keywords: PPAR alpha, Spinal cord injury, Inflammation, Neuroprotection, Myelin, T cells, Macrophage, White matter sparing, Locomotor recovery
Introduction
There is a vital need for therapeutic treatments for spinal cord injured patients. To date, methylprednisolone is the only clinically approved treatment for spinal cord injury (SCI), and its use remains controversial (Hulbert and Hamilton, 2008; Ito et al., 2009). SCI involves tissue destruction and cell death due to physical injury to the spinal cord followed by secondary injury cascades initiated after the trauma (Profyris et al., 2004). These include breach of the blood–brain barrier and a robust influx of immune cells, including blood-derived monocytes, neutrophils and T cells, which are thought to exacerbate tissue damage (Donnelly and Popovich, 2008; Profyris et al., 2004). The recruitment of reactive inflammatory cells and subsequent proinflammatory cascades offer a prime target for neuroprotective agents, which may regulate the inflammatory component of SCI and in turn salvage the remaining healthy tissue.
Agonists of the transcription factor peroxisome proliferator activated receptor (PPAR) have been recognized for their neuroprotective functions, in part through their anti-inflammatory actions (Bordet et al., 2006). There are three isoforms of the PPAR receptors, α, (β/δ and -γ that are differentially distributed throughout the body and exert pleiotropic functions. PPAR-α, well known for its role in lipid metabolism, also displays beneficial effects in a number of neurodegenerative models through anti-inflammatory and anti-oxidant mechanisms (Pyper et al., 2010). Agonists of PPAR-α such as fenofibrate and Wy-14643 confer protection in a cerebral ischemia model and reduce the incidence of stroke in mice (Inoue et al., 2003; Deplanque et al., 2003). PPAR-α agonists tested in an Alzheimer’s model inhibited release of proinflammatory cytokines such as TNF-α and IL-6 in response to amyloid β peptide (Combs et al., 2000). Using a SCI model, Genovese et al (2009) demonstrated that PPAR-α knockout mice have impaired behavioral recovery compared to wild type mice. The same group examined the effect of palmitoylethanolamide, a PPAR-α agonist, in spinal cord injured mice, and observed improved functional recovery and anatomical changes such as decreased pro-inflammatory cytokines and fewer infiltrating cells (Genovese et al., 2008). Thus, activation of PPAR-α in multiple models suggests this molecule may be a potent neuroprotective agent.
The current study builds on this previous work by examining the efficacy of gemfibrozil, a commercially available agonist of PPAR-α that is FDA approved for treating hyperlipidemia. Work by several groups, including the Lovett-Racke lab, has demonstrated beneficial effects of gemfibrozil in a mouse experimental autoimmune encephalomyelitis (EAE) model, which is a model for multiple sclerosis (MS). EAE animals treated with gemfibrozil displayed significantly better clinical scores and had fewer demyelinating lesions (Lovett-Racke et al., 2004; Gocke et al., 2009; Yang et al., 2008; Dasgupta et al., 2007). Gemfibrozil reduced tissue pathology by up-regulating the transcription factor GATA-3, a key regulator of IL-4. This resulted in a decreased Th1 proinflammatory response and caused a shift towards the Th2 anti-inflammatory phenotype, thereby ameliorating EAE. Additionally, in vitro treatment of microglia and astrocytes with gemfibrozil significantly reduced proinflammatory cytokine production (Gocke et al., 2009). Thus, the beneficial actions of gemfibrozil in various models of CNS disease and injury indicate it may be a good therapeutic agent for SCI, as modulation of the immune response led to improved functional outcomes after SCI in several studies (Popovich et al., 1999; Jones et al., 2005; Fleming et al., 2006; Ankeny et al., 2009).
To this end, our first experiment mimicked the paradigm used successfully in the EAE model. That is, to maximize the potential of obtaining a beneficial effect, we began by studying oral pre-treatment of gemfibrozil in mice receiving a spinal contusion injury. This is also relevant as a large population of patients currently take gemfibrozil (known as Lopid) or similar fibrates to control hyperlipidemia. Our hypothesis was that animals receiving pre-injury gemfibrozil would have improved locomotor recovery and reduced histopathology. Surprisingly, animals treated with gemfibrozil for 3 days prior to SCI and continuing after injury had markedly worse locomotor recovery compared to vehicle controls. This was matched by a trend for reduced tissue sparing and a significant increase in T cell infiltration in drug-treated animals compared to controls. To confirm this result, the study was replicated; in the repeat study, however, there was no difference in locomotor recovery or tissue sparing between gemfibrozil and vehicle-treated mice. Two subsequent studies also tested gemfibrozil treatment with a lower injury severity and a higher drug dose, respectively. Neither of these studies showed behavioral changes but did show evidence of increased tissue pathology with gemfibrozil treatment. To determine if the histological deterioration was specific to gemfibrozil, we also tested a second PPAR-α agonist (fenofibrate) after SCI; mice treated with this drug showed no differences compared to control. Overall, PPAR-α agonists were found to be an ineffective and/or deleterious drug therapy after SCI at the doses tested in these studies.
Materials and methods
Spinal cord injury
Adult female C57/BL 6 mice (17–23 g, Jackson Labs) were anesthetized with ketamine (80 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) and a dorsal laminectomy was performed at the T9 vertebral level. Mice then received a moderate spinal contusion injury using the Infinite Horizons device (Precision Systems and Instrumentation, Lexington, KY) with a pre-set force of 75 kdyn (Studies I, II, IV; actual forces ranged between 75 and 81 kdyn) or 65 kdyn (Study III; actual forces ranged between 65 and 72 kdyn). The muscles overlying the spinal cord were then sutured and the skin was closed using surgical clips. Animals were given 2 cc of saline and placed into warm recovery cages. Post-surgical care included 5 days treatment with antibiotics (Gentomicin, 5 mg/kg) and saline to maintain hydration, and twice a day manual bladder expression until spontaneous voiding returned. All procedures conformed to NIH and The Ohio State University approved animal use protocols.
Drug administration
Study I
Mice were given vehicle or gemfibrozil (Sigma-Aldrich) beginning 3 days before SCI to ensure drug availability at the time of the injury (n=5–6/group). The drug was delivered orally by dissolving it in ethanol (0.25% w/w of gemfibrozil) and coating food pellets such that each animal received ~62 mg/kg/day; chow for control groups was treated with ethanol. Ethanol for each group was allowed to completely evaporate before giving the food to the animals. In addition, animals received an intraperitoneal injection of vehicle or gemfibrozil (500 µg dissolved in 200 µl PBS) 1 h after the injury, and then continued to receive the drug in their food until the end of the study (28 days post-injury). It does not appear that the drug or vehicle caused an altered or novel taste to the chow, as animals consumed an expected amount of chow each day compared to previous studies we have performed using the mouse SCI model. In addition, they gained weight along an expected post-injury time course.
Study II
Mice were treated as in Study I with the exception of receiving an intraperitoneal injection 1 h post-injury (n=6–7/group). To determine if starting the drug after injury would be beneficial, a separate group of animals did not receive vehicle or drug in their chow until the day of injury when chow was placed in their recovery cages (n=6–7/group). Mice typically begin to consume chow the first night after injury so animals on average began receiving the drug 6– 8 h post-injury. Animals were sacrificed at 3, 7 and 28 days post-injury.
Study III
Despite using the same injury severity (75 kdyn) in Studies I and II, animals in Study I appeared to have milder injuries based on final locomotor scores. This suggested that there might have been a threshold effect of the drug, where the deleterious actions were only detectable after a mild injury. To test this, a milder injury severity was intentionally produced, using 65 kdyn rather than 75 kdyn. To provide a more stringent drug treatment rather than relying on animals within a cage to consume similar amounts of food, animals were randomly divided into three groups and fed by oral gavage with vehicle (0.5% carboxymethyl cellulose), gemfibrozil (100 mg/kg) or fenofibrate (50 mg/kg; Sigma-Aldrich), another potent PPAR-α agonist (n=7/ group). All treatments were initiated at 2 h post-injury and continued daily for two weeks. The animals were sacrificed at 14 dpi.
Study IV
As a final attempt to determine if gemfibrozil has therapeutic value, we tested whether a higher dose begun after injury would be beneficial. Animals in this study were fed 300 mg/kg gemfibrozil by oral gavage starting 2 h post-injury and continuing once/day for 7 days post-injury (n=8). Beginning on day 8 post-injury, animals were given 200 mg/kg of gemfibrozil by oral gavage and 0.25% w/w of gemfibrozil in their chow. Control animals received vehicle (0.5% carboxymethyl cellulose) by gavage and were given ethanol treated chow throughout the second week post-injury. Animals were sacrificed on day 20 post-injury.
Behavioral testing
Locomotor testing was conducted using the Basso Mouse Scale (BMS) as a measure of functional recovery (Basso et al., 2006). Mice were tested at 1, 3, 7, 10, 14, 21 and 28 days post-injury (dpi) by investigators blind to the treatment groups. Locomotor scores and sub-scores were averaged for both hindlimbs and then analyzed using two-way ANOVA with repeated measures followed by Bonferroni post-hoc analysis.
Immunohistochemistry
At the appropriate time for each study, animals were anesthetized with an overdose of ketamine/xylazine (1.5× surgery dose) and intracardially perfused with 0.1 M PBS followed by 4% paraformaldehyde. The spinal cords were isolated and post-fixed for 2 h then transferred to 0.2 M phosphate buffer (PB) overnight. Spinal tissue was cryopreserved in 30% sucrose for 48 h after which cords were blocked into 1 cm pieces centered on the injury epicenter, frozen in OCT (Electron Microscopy Sciences, Hatfield, PA), then sectioned at 10 µm on a cryostat.
Standard laboratory immunohistochemical techniques were used for visualization of the different antibodies (Tripathi and McTigue, 2007). Cross-sections were first rinsed with 0.1 M PBS and incubated in the blocking solution BP+ (4% BSA/0.1% triton-x-100) for 1 h and then incubated overnight with the antibody of interest. The following antibodies were used at the given concentration: chicken antineurofilament (Aves, 1:1000) to label axons; rabbit anti-GFAP (Dako, 1:20,000) for astrocytes; rat anti-CD68 (Serotec, 1:1000) for activated microglia and macrophages; and rat anti-CD3 (Serotec, 1:400) for T cells. After overnight incubation, sections were rinsed in 0.1 M PBS and then overlaid with the respective secondary antibody at the appropriate dilution for 2 h followed by incubation with the avidin–biotin complex for 1 h. The sections were then developed using the chromogens diaminobenzamide (DAB, Vector) or SG substrate (Vector). Sections were rinsed in distilled water and dehydrated, and then the slides were coverslipped with Permount.
Lesion epicenters were identified as sections containing the least amount of white matter sparing using standard immunohistochemistry protocols to label myelin and axons (Tripathi and McTigue, 2007; McTigue et al., 2007).
All protocols included controls in which the primary or secondary antibodies were omitted. These controls consistently revealed no labeling. Digital plates were constructed using Adobe Photoshop (Adobe, San Jose, CA); brightness and contrast were enhanced when necessary on microscopic images to reproduce immunolabeling as viewed through the microscope.
Histological analysis
Tissue sparing analysis
This and all histological analyses were performed in a blinded fashion. A set of tissue double-labeled with eriochrome cyanine to label myelin and anti-neurofilament to label axons was used to quantify the area of spared white matter and spared gray matter stereologically. The Cavalieri method was used for this analysis on sections spanning from 0.75 mm rostral and caudal to the lesion epicenter as described previously by McTigue et al. (2007). The area measured in each region of interest was plotted against the distance from the lesion epicenter and was analyzed by two-way repeated measures ANOVA followed by Bonferroni post-hoc analysis.
CD68 measurements
Images of cross-sections from 3, 7 and 28 dpi spinal cords immunolabeled with anti-CD68 were digitized at 10× magnification. Using an image analysis system (MCID Elite), the area of CD68 immunoreactivity was measured on sections spanning the injury site by thresholding for CD68 signal. The groups were compared by two-way repeated measures ANOVA followed by Bonferroni post-hoc test.
T-cell measurements
The number of infiltrating T cells was quantified by immunolabeling sections with anti-CD3 antibody, a pan T cell marker. The number of T cells in the lesion or spared tissue on each section was counted manually at 40× magnification. Two-way ANOVA analysis was conducted on these counts followed by Bonferroni post-hoc tests.
Results
Study I: Gemfibrozil treatment initiated 3 days before SCI results in decreased locomotor function after injury
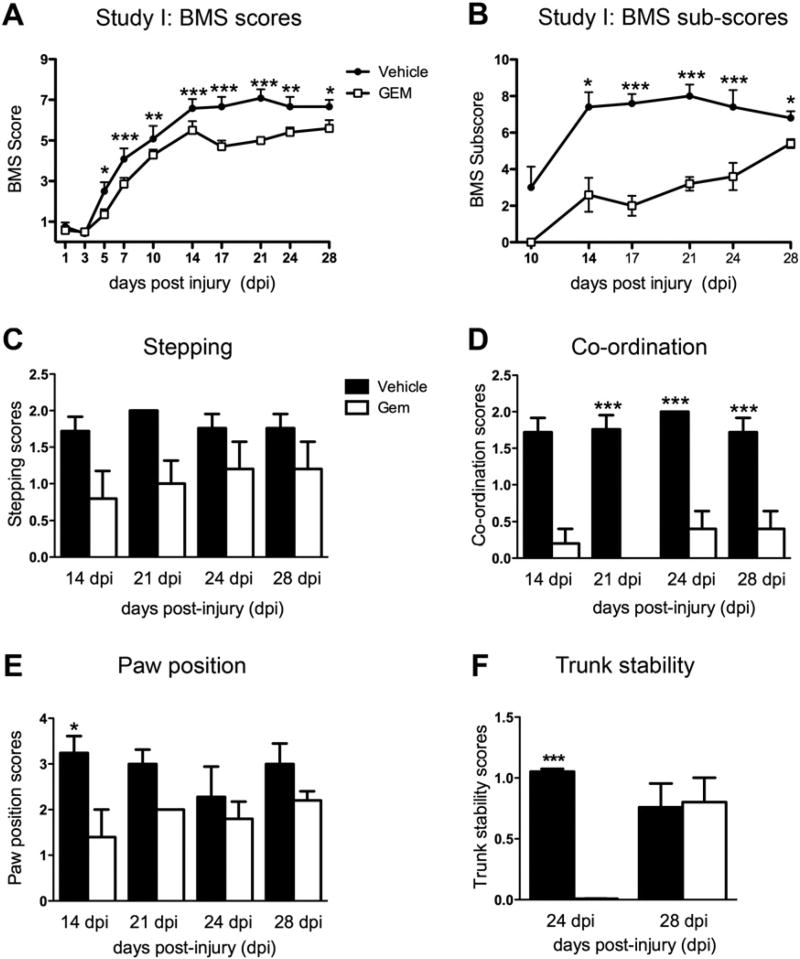
Open field locomotor function was tested using the Basso Mouse Scale (BMS) starting one day post-injury (dpi) through the end of the study. As shown in Fig. 1A, the gemfibrozil treated animals scored significantly lower compared to controls as early as 5 dpi on the BMS score and by 14 dpi on the BMS sub-score (Fig. 1B). The gemfibrozil animals were also worse at individual aspects comprising the sub-score, including forelimb–hindlimb coordination (Fig. 1D), paw position (Fig. 1E) and trunk stability (Fig. 1F). These differences were most striking at weeks 2–3 after injury.
Fig. 1.
Locomotor assessment of SCI animals treated with gemfibrozil (GEM) or vehicle in Study I. Mice were tested after SCI (75 kdyn) using the BMS locomotor scale starting at 1 day post-injury (dpi) until 28 dpi. Vehicle controls had significantly higher BMS scores starting at 5 dpi (A). BMS sub-scores were also significantly lower in the gemfibrozil treatment group (B). Specific behavioral categories were compared between groups. Gemfibrozil treated animals had a trend for reduced stepping (C), significantly reduced coordination at 21–28 dpi (D), worse paw position at 14 dpi (E), and reduced trunk stability at 24 dpi (F). *p<0.05, **p<0.01 and ***p<0.001.
Gemfibrozil treatment caused a trend for decreased white matter sparing after SCI
Spinal cords isolated at 28 dpi from animals in Study I were used to quantify the area of spared white and gray matter and frank lesion area (Figs. 2A, B). As shown in Figs. 2A and B, frank lesion was defined as the lesion core which did not include intact myelin or axons; this is illustrated by the dotted line in Figs. 2A and B. The three different areas were analyzed using the Cavalieri sampling technique (McTigue et al., 2007) for serial sections spanning from 0.75 mm rostral to caudal from the epicenter.
Fig. 2.
Spared tissue analysis of SCI animals in Study I. Spinal cord cross-sections spanning 0.75 mm rostral and caudal to the lesion epicenter (Epi) were stained for eriochrome cyanine to label myelin (blue) and neurofilament for axons (brown) (A, B). Black lines demarcate the border between intact spared tissue and pathological tissue. Stereologic analysis of spared white matter and gray matter revealed that gemfibrozil (GEM) treatment did not significantly change tissue sparing but did cause reduced white matter compared to vehicles at every distance examined (C, D).
Gemfibrozil treatment showed a trend for decreased area of spared white matter compared to the control mice (Fig. 2C). No differences were observed in the area of spared gray matter (Fig. 2D) and frank lesion area (data not shown).
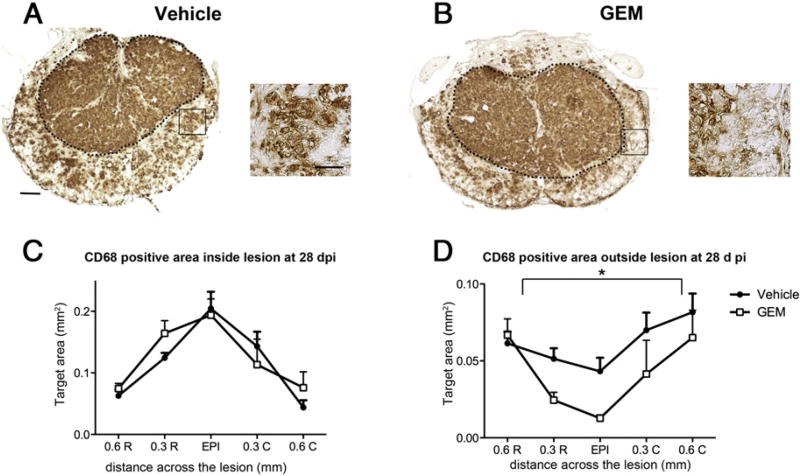
Gemfibrozil treatment decreased macrophage immunoreactivity but increased T cell infiltration into spared tissue
Previous work in the EAE model revealed that gemfibrozil treatment decreased the number of macrophages infiltrating the spinal cord (Lovett-Racke et al., 2004). Therefore, we examined the distribution of activated macrophages and microglia using the anti-CD68 antibody in tissue sections at and near the lesion epicenter. The core lesion (indicated by the black dotted line) and the spared tissue outside the lesion were examined separately (Figs. 3A, B). Gemfibrozil did not alter macrophage distribution at acute time points (3 dpi and 7 dpi) (data not shown). However, similar to the EAE study, gemfibrozil treatment decreased the number of activated macrophages in spared tissue outside the lesion at 28 dpi (p<0.05; Figs. 3C, D). Importantly, the total area outside the lesion core was not different between control and treated animals, which means that the reduced CD68 immunoreactivity was not due to measurement in a smaller area.
Fig. 3.
Analysis of the area of tissue occupied by activated microglia and macrophages in cross-sections from Study I. (A, B) Images of spinal cord sections immunolabeled for CD68 were used to outline the frank lesion core and tissue outside the lesion core (delineated by dotted line). Areas demarcated by black boxes are shown in high power adjacent to cross-sections. The area of immunoreactivity was measured separately for tissue inside (C) and outside (D) the lesion core at 28 dpi. Gemfibrozil (GEM) treated animals had significantly reduced CD68 immunoreactivity in tissue outside the lesion core (Two-way ANOVA with repeated measures, pb0.05). Low power image scale bar=100 µm, high power image scale bar=50 µm.
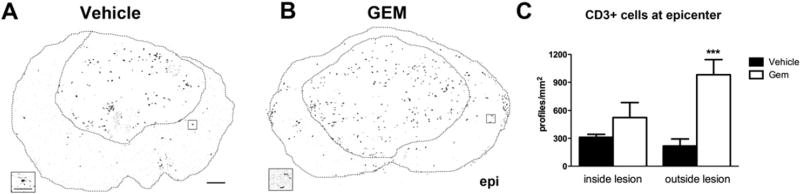
Since previous EAE studies also detected reduced T cell numbers in EAE spinal cords following gemfibrozil treatment (Lovett-Racke et al., 2004), we examined sections immunolabeled with a pan T cell marker to determine if T cell numbers or distribution were altered by gemfibrozil (Figs. 4A, B). Typically after SCI, infiltrating T cells localize to the frank lesion/scar area (Jones et al., 2005); therefore, T cells were counted separately in the lesion and in spared tissue at the epicenter. This revealed a significant interaction between the groups (p<0.05) and a significant effect of the drug treatment (p<0.001) based on two-way ANOVA analysis. T cells were present within the scar area of both groups, with slightly more present on average in the gemfibrozil animals compared to vehicle-treated mice (524 vs. 311, respectively; Fig. 4C). In tissue outside the scar, the vehicle treated animals had fewer T cells on average while gemfibrozil animals had significantly more T cells (p<0.001 vs. Veh; Fig. 4C). Thus, gemfibrozil treatment caused an unexpected and abnormally large accumulation of T cells in spared tissue after SCI.
Fig. 4.
Quantification of T cells inside and outside lesion core in Study I. Spinal cord sections were immunolabeled for CD3, a pan T cell marker (A, B), and the number of CD3 positive cells inside and outside the lesion was counted in vehicle and gemfibrozil (GEM) treated animals. Tissue sections and lesion cores are outlined. Areas in boxes are shown at high power below cross-sections. There were significantly more CD3+ cells outside the lesion in gemfibrozil treated mice compared to controls (C) (p<0.001). Low power image scale bar=100 µm, high power image scale bar=25 µm.
After spinal cord injury, astrocytes hypertrophy and upregulate the cytoskeletal protein GFAP. To determine if gemfibrozil altered astrocytic reactivity, cross-sections were immunolabeled for GFAP and the amount of positive staining was quantified. No difference in astrocyte reactivity was detected between the treatment groups (data not shown).
Study II: Deleterious effects of gemfibrozil were not reproduced
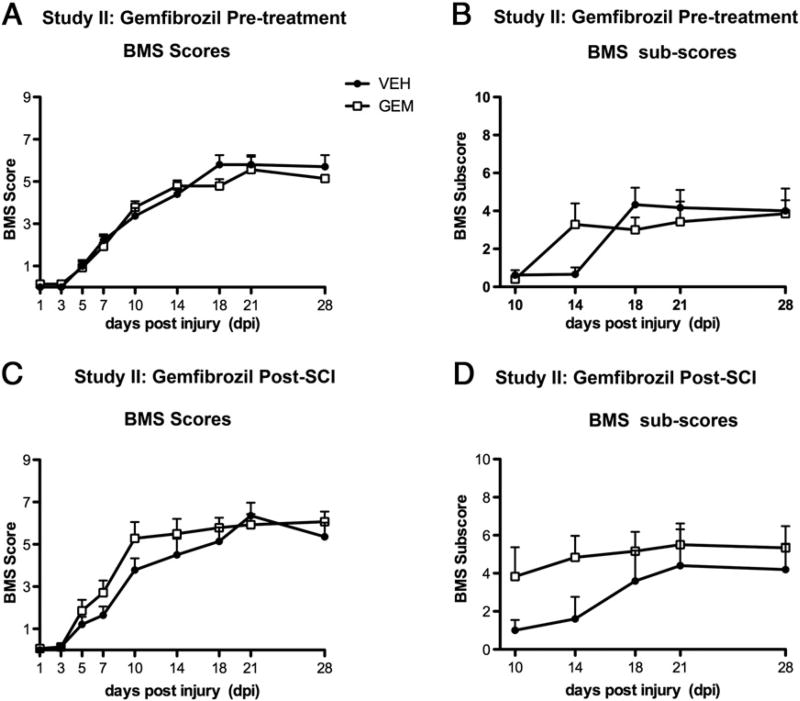
The results obtained in Study I were contrary to our hypothesis and contrasted with the neuroprotective role of gemfibrozil in other models of CNS injury such as TBI and stroke (Chen et al., 2007; Inoue et al., 2003). These conflicting results are important documentation of how the same drug could have opposite effects in different injury models. Thus, to verify these results, we performed a replication study. Unlike Study I, however, BMS scores and sub-scores were not different at any time post-injury between the gemfibrozil and vehicle groups (Figs. 5A, B). Histological analyses also revealed no differences in white matter or gray matter sparing or in T cell infiltration between the two groups (data not shown).
Fig. 5.
BMS locomotor analysis of mice in Study II that received gemfibrozil (GEM) or vehicle (VEH) beginning either before (A, B) or after SCI (C, D). Two-way repeated measures ANOVA revealed no differences between the groups for BMS scores or sub-scores.
To determine if initiating gemfibrozil treatment after SCI instead of before would be beneficial, animals were also included in this study that received chow containing gemfibrozil or vehicle in their recovery cages immediately after injury. Mice typically begin to consume chow that first evening so these animals would begin receiving drug (or vehicle) ~6–8 h post-injury. Gemfibrozil-treated animals in this group displayed a slightly accelerated rate of recovery on the BMS scale and a trend for higher BMS sub-scores (Figs. 5C, D). These results, however, were not significantly different from controls.
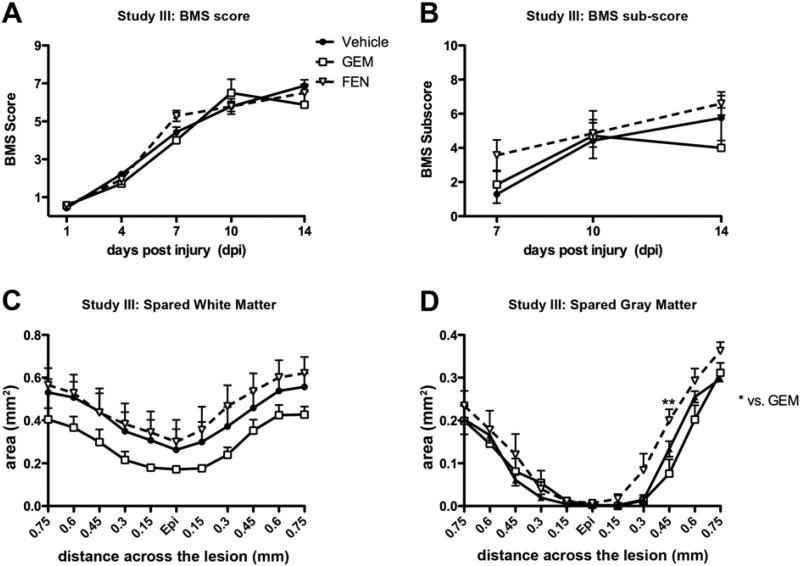
Study III: Fenofibrate and gemfibrozil showed no significant effects after SCI
Although Study II was designed to replicate Study I and the same injury parameters were used (75 kdyn force), the injuries in Study II appear to have been more severe based on the final average BMS scores of the control groups (7 vs. 5.7). Therefore, we speculated that gemfibrozil might have had a threshold effect that was only detectable in mildly injured mice. To address this, Study III was conducted using a milder injury severity (65 kD) and a more exact drug delivery route (daily oral gavage beginning 2 h post-injury). A separate group of mice was included that received similar oral gavage regimen of the more potent PPAR-α agonist fenofibrate (50 mg/kg).
BMS testing revealed no differences in locomotor recovery between PPAR-α agonist and control (Figs. 6A, B). In addition, spared white matter was not different between vehicle, gemfibrozil or fenofibrate groups (Fig. 6C), although a trend for decreased white matter at every distance examined was again noted in gemfibrozil animals. Interestingly, fenofibrate-treated mice had significantly more spared gray matter than gemfibrozil animals caudal to the injury epicenter (Fig. 6D). No differences were detected in the lesion area between the groups (data not shown). Thus, fenofibrate did not alter functional recovery after SCI; however, its marginal effect on tissue sparing may hold some promise. On the other hand, gemfibrozil had no significant positive or negative effects on locomotor or anatomical outcomes in this study.
Fig. 6.
Behavioral and histological analyses of mice in Study III that received gemfibrozil (GEM), fenofibrate (FEN) or vehicle beginning 2 h post-injury. (A, B) Locomotor recovery was tested using the BMS locomotor scale starting at 1 day post-injury (dpi). No differences were detected in the BMS scores or sub-scores. Stereological analysis of spared white matter (C) and gray matter (D) revealed no significant differences between vehicle and drug groups. However, the fenofibrate group had significantly more gray matter sparing at 0.45 mm caudal to epicenter (Epi) compared to gemfibrozil treated mice (p<0.01).
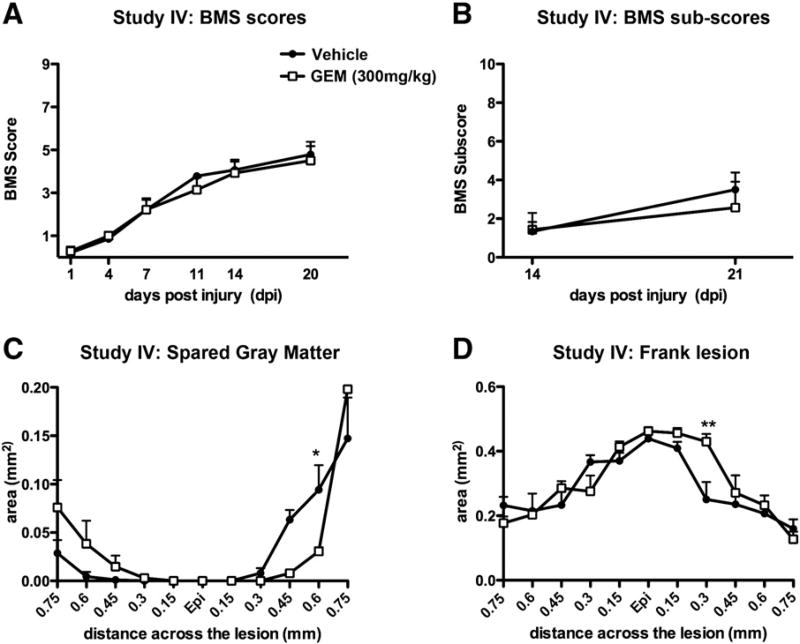
Study IV: Higher dose of gemfibrozil decreased tissue sparing after SCI
The dose of gemfibrozil tested so far in our studies was based on effectiveness in EAE studies. To verify that gemfibrozil is not beneficial after SCI, we next tested whether a higher dose (300 mg/kg) would be efficacious in SCI mice. Animals were given gemfibrozil by oral gavage at 2 h post-injury and then daily for the first week after injury, after which they received 200 mg/kg of drug through oral gavage supplemented with 0.25% w/w of gemfibrozil in their food (as in previous studies). This higher dose of gemfibrozil did not alter locomotor recovery compared to controls (Figs. 7A, B). Histological analysis revealed the gemfibrozil treatment caused a significant reduction in gray matter sparing and greater lesion size caudal to the lesion epicenter (Figs. 7C, D). Thus, not only did the higher dose of gemfibrozil fail to alter functional recovery, it also led to increased tissue pathology.
Fig. 7.
Behavioral and histological analyses of SCI animals in Study IV receiving post-SCI vehicle or a higher dose of gemfibrozil (GEM) compared Studies I–III. (A, B) The BMS locomotor scale was used starting at 1 day post-injury; no significant differences were detected between groups. (C, D) Stereological analysis of tissue sparing revealed gemfibrozil-treated mice had significantly less spared gray matter (C) and greater lesion size (D) caudal to the lesion epicenter (Epi). Two-way repeated measures ANOVA followed by Bonferroni post-hoc analysis, *p<0.05, **p<0.01.
Discussion
Several studies have shown beneficial effects of PPARα activation in models of multiple sclerosis, traumatic brain injury, stroke and SCI (Inoue et al., 2003; Besson et al., 2005; Chen et al., 2007; Genovese et al., 2009; Genovese et al., 2008; Lovett-Racke et al., 2004). Therefore, our goal in these studies was to test the hypothesis that gemfibrozil, a FDA approved PPARα agonist, would be beneficial in a rodent model of spinal contusion injury. Gemfibrozil is currently used clinically to treat hyperlipidemia and has not been tested to date in SCI models. This drug should have good penetration to the CNS as a study examining the distribution of gemfibrozil in rats consuming chow supplemented with drug for 3 days at a similar concentration as that used here (0.3% vs. 0.25% in our study) showed that the drug reaches the brain and activates PPAR response elements in the cortex (Sanguino et al., 2003). In addition, the disturbed blood–brain barrier present for several days post-injury will likely enhance drug delivery to the spinal cord. In our first study, we mimicked the design of successful EAE studies by administering gemfibrozil orally to mice before injury and continuing treatment throughout the study. Contrary to our hypothesis, gemfibrozil, at a dose that is efficacious in EAE, was deleterious to SCI mice, resulting in decreased locomotor recovery and greater histopathology compared to controls. A follow-up replication study, however, did not reproduce the adverse effects observed in Study I but also revealed no beneficial effect of the drug on locomotor function or tissue sparing.
The conflicting results from Studies I and II and the milder injury phenotype in Study II (refer to results) led us to postulate that the gemfibrozil effects are only manifested at certain injury thresholds. To test this, a milder injury severity was intentionally produced in Study III. To rule out any possibility of variable food intake, we used oral gavage for drug delivery in Study III. We also included a group of animals that received fenofibrate, a different and potent PPARα agonist, to determine if deleterious effects were specific to gemfibrozil. The results again revealed that gemfibrozil treatment had no impact on locomotor recovery and led to a trend for decreased tissue sparing. Fenofibrate treatment was also ineffective on locomotor recovery but did significantly increase gray matter sparing caudal to the lesion epicenter compared to gemfibrozil.
Because traumatic contusion injury is a much more rapid and severe injury in terms of tissue damage compared to EAE, it is feasible that gemfibrozil concentrations that are effective in EAE may be too low for SCI. Therefore, a final study was performed to determine if increased levels of gemfibrozil would improve recovery from SCI. In this study we tested a higher dose of gemfibrozil that was still well below the toxic range reported in rodents (Kurtz et al., 1976).Again, behavioral recovery in gemfibrozil animals was not different from controls. Furthermore, this concentration of gemfibrozil resulted in significantly increased lesion area and decreased gray matter sparing. Thus, four out of four studies resulted in either no change or worse behavioral and/or histological outcomes after gemfibrozil treatment in SCI mice, leading us to conclude that gemfibrozil is not neuroprotective and may in fact be injurious after SCI.
One feature of our studies that is consistent with others is the decrease in intraparenchymal activated microglia and macrophages following PPARα activation in Study I. In vitro work has shown that treating activated microglia with a PPARα agonist decreases NF-KB activity and production of nitric oxide and pro-inflammatory cytokines (Xu et al., 2005; Ramanan et al., 2008; Jana et al., 2007). Activation of PPARα also reduced microglial activation in the CNS in multiple models, including whole-brain irradiation, intracerebral injection of lipopolysaccharide, and in EAE spinal cords (Lovett-Racke et al., 2004; Ramanan et al., 2009; Wang and Namura, 2011). PPARα activation is reported to induce apoptosis of macrophages in the presence of TNF-α/ IFN-γ (Chinetti et al., 2001), perhaps contributing to the decreased number of activated macrophages detected in gemfibrozil-treated animals in the current study.
Despite the many studies showing reduced microglial activation and improved neurological recovery after activating the PPARα pathway, other studies have shown deleterious effects. For instance, cell death of stressed cerebellar neurons was exacerbated by addition of a PPARα agonist in vitro (Smith et al., 2001). Other in vitro studies revealed that both fenofibrate and gemfibrozil impaired mitochondrial function and, consequently, cellular respiration (Brunmair et al., 2004). Interestingly, several studies have documented a deleterious effect of PPARα activation on myocytes. PPARα-induced myotoxicity has been detected in both human and rat cells in vitro and in a primate in vivo model (Johnson et al., 2005; Liu et al., 2009; Liu et al., 2011; Zhao et al., 2010). Similar to the mice in Study I, primates treated with gemfibrozil displayed significant behavioral deficits. A potential mechanism for increased cell toxicity was decreased Akt phosphorylation, which would reduce signaling through intracellular cell survival pathways (Zhao et al., 2010).
Chronic use of gemfibrozil in some human patients has led to deleterious side effects, including gastrointestinal disturbances, nausea, depression, dizziness and allergy. In addition, a small proportion of patients developed rhabdomyolysis resulting in muscle weakness and kidney dysfunction, similar to the studies mentioned above (Roy and Pahan, 2009). Thus, in our first study, gemfibrozil treatment may have mediated systemic effects in the injured mice impinging on their overall health and affecting locomotor function. In fact, in Study I, recovery of bladder function in gemfibrozil-treated mice was noticeably delayed compared to the vehicle treated mice. The side effects of gemfibrozil can also be mediated by several reactive metabolites that can react with cellular proteins and DNA and lead to cellular dysfunction (Rubenstrunk et al., 2007).
PPARα activation also can potently alter T cell function. For instance, T cells treated with gemfibrozil show reduced proliferation and increased production of the anti-inflammatory cytokine IL-4, which favors differentiation of T cells into the Th2 type cells (anti-inflammatory) (Lovett-Racke et al., 2004). This accounts for the reduced T cell numbers and improved clinical scores in an EAE model (Lovett-Racke et al., 2004). Surprisingly, in Study I, gemfibrozil treatment led to a greater number of T cells distributing outside the lesion and into spared tissue, a region that usually contains few T cell after SCI. This elevation in T cells may have led to decreased function of white matter tracts, as T cell infiltration in a mouse model of MS was directly involved in motor impairment and axonal demyelination (Deb et al., 2010). In SCI, infiltration of myelin reactive T cells greatly exacerbates lesion size, tissue pathology and functional recovery (Popovich et al., 1997; Jones et al., 2002). In the Jones et al. (2002) study, enhanced T cell infiltration resulted in less white matter sparing and increased demyelination after SCI. Similarly, in Study I we detected a trend for reduced white matter sparing which correlated with a significant increase in the number of T cells in this region. Thus, it appears that gemfibrozil has the potential to change the lesion microenvironment after SCI such that activated macrophages are reduced yet T cell numbers are increased.
Our current work adds gemfibrozil and fenofibrate to the growing list of pharmaceutical agents tested in SCI studies. The commercial availability made gemfibrozil an attractive potential treatment for SCI; in addition, thousands of patients are currently using this medication to control hyperlipidemia. The long-term treatment of gemfibrozil in many chronic inflammatory conditions such as heart disease, rheumatoid arthritis, diabetes and murine EAE motivated us to test its effects on inflammation occurring post-SCI. However, in contrast to other studies, our work shows that gemfibrozil did not yield protective effects after SCI. Although effective in other disease models, gemfibrozil may in fact be harmful after SCI. Given that fenofibrate showed a trend for increased gray matter sparing in this study, it is possible that this or other PPAR-α agonists may be reparative in SCI although this still needs to be rigorously tested. Finally, our research may help design different treatment regimens for those researchers interested in pursuing PPAR-α agonists for SCI treatment.
Acknowledgments
The authors gratefully acknowledge Dr. Yuhong Yang for her demonstration of drug administration in food pellets. This work was funded by NINDS NS059776 and P30-NS045758.
References
- Ankeny DP, Guan Z, Popovich PG. B cells produce pathogenic antibodies and impair recovery after spinal cord injury in mice. J Clin Invest. 2009;119:2990–2999. doi: 10.1172/JCI39780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J. Neurotrauma. 2006;23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- Besson VC, Chen XR, Plotkine M, Marchand-Verrecchia C. Fenofibrate, a peroxisome proliferator-activated receptor alpha agonist, exerts neuroprotective effects in traumatic brain injury. Neurosci Lett. 2005;388:7–12. doi: 10.1016/j.neulet.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Bordet R, Ouk T, Petrault O, Gele P, Gautier S, Laprais M, Deplanque D, Duriez P, Staels B, Fruchart JC, Bastide M. PPAR: a new pharmacological target for neuroprotection in stroke and neurodegenerative diseases. Biochem. Soc. Trans. 2006;34:1341–1346. doi: 10.1042/BST0341341. [DOI] [PubMed] [Google Scholar]
- Brunmair B, Lest A, Staniek K, Gras F, Scharf N, Roden M, Nohl H, Waldhausl W, Furnsinn C. Fenofibrate impairs rat mitochondrial function by inhibition of respiratory complex I. J Pharmacol Exp Ther. 2004;311:109–114. doi: 10.1124/jpet.104.068312. [DOI] [PubMed] [Google Scholar]
- Chen XR, Besson VC, Palmier B, Garcia Y, Plotkine M, Marchand-Leroux C. Neurological recovery-promoting, anti-inflammatory, and anti-oxidative effects afforded by fenofibrate, a PPAR alpha agonist, in traumatic brain injury. J Neurotrauma. 2007;24:1119–1131. doi: 10.1089/neu.2006.0216. [DOI] [PubMed] [Google Scholar]
- Chinetti G, Lestavel S, Bocher V, Remaley AT, Neve B, Torra IP, Teissier E, Minnich A, Jaye M, Duverger N, Brewer HB, Fruchart JC, Clavey V, Staels B. PPAR-alpha and PPAR-gamma activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat. Med. 2001;7:53–58. doi: 10.1038/83348. [DOI] [PubMed] [Google Scholar]
- Combs CK, Johnson DE, Karlo JC, Cannady SB, Landreth GE. Inflammatory mechanisms in Alzheimer’s disease: inhibition of beta-amyloid-stimulated proinflammatory responses and neurotoxicity by PPARgamma agonists. J. Neurosci. 2000;20:558–567. doi: 10.1523/JNEUROSCI.20-02-00558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta S, Roy A, Jana M, Hartley DM, Pahan K. Gemfibrozil ameliorates relapsing–remitting experimental autoimmune encephalomyelitis independent of peroxisome proliferator-activated receptor-alpha. Mol Pharmacol. 2007;72:934–946. doi: 10.1124/mol.106.033787. [DOI] [PubMed] [Google Scholar]
- Deb C, Lafrance-Corey RG, Schmalstieg WF, Sauer BM, Wang H, German CL, Windebank AJ, Rodriguez M, Howe CL. CD8+ T cells cause disability and axon loss in a mouse model of multiple sclerosis. PLoS One. 2010;5:e12478. doi: 10.1371/journal.pone.0012478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deplanque D, Gele P, Petrault O, Six I, Furman C, Bouly M, Nion S, Dupuis B, Leys D, Fruchart JC, Cecchelli R, Staels B, Duriez P, Bordet R. Peroxisome proliferator-activated receptor-alpha activation as a mechanism of preventive neuroprotection induced by chronic fenofibrate treatment. J Neurosci. 2003;23:6264–6271. doi: 10.1523/JNEUROSCI.23-15-06264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2008;209:378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming JC, Norenberg MD, Ramsay DA, Dekaban GA, Marcillo AE, Saenz AD, Pasquale-Styles M, Dietrich WD, Weaver LC. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129:3249–3269. doi: 10.1093/brain/awl296. [DOI] [PubMed] [Google Scholar]
- Genovese T, Esposito E, Mazzon E, Di Paola R, Meli R, Bramanti P, Piomelli D, Calignano A, Cuzzocrea S. Effects of palmitoylethanolamide on signaling pathways implicated in the development of spinal cord injury. J Pharmacol Exp Ther. 2008;326:12–23. doi: 10.1124/jpet.108.136903. [DOI] [PubMed] [Google Scholar]
- Genovese T, Esposito E, Mazzon E, Crisafulli C, Paterniti I, Di Paola R, Galuppo M, Bramanti P, Cuzzocrea S. PPAR-alpha modulate the anti-inflammatory effect of glucocorticoids in the secondary damage in experimental spinal cord trauma. Pharmacol Res. 2009;59:338–350. doi: 10.1016/j.phrs.2009.01.018. [DOI] [PubMed] [Google Scholar]
- Gocke AR, Hussain RZ, Yang Y, Peng H, Weiner J, Ben LH, Drew PD, Stuve O, Lovett-Racke AE, Racke MK. Transcriptional modulation of the immune response by peroxisome proliferator-activated receptor-{alpha} agonists in autoimmune disease. J Immunol. 2009;182:4479–4487. doi: 10.4049/jimmunol.0713927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulbert RJ, Hamilton MG. Methylprednisolone for acute spinal cord injury: 5-year practice reversal. Can J Neurol Sci. 2008;35:41–45. doi: 10.1017/s031716710000754x. [DOI] [PubMed] [Google Scholar]
- Inoue H, Jiang XF, Katayama T, Osada S, Umesono K, Namura S. Brain protection by resveratrol and fenofibrate against stroke requires peroxisome proliferator-activated receptor alpha in mice. Neurosci Lett. 2003;352:203–206. doi: 10.1016/j.neulet.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Ito H, Sugimoto Y, Tomioka M, Kai N, Tanaka M. Does high dose methylprednisolone sodium succinate really improve neurological status in patient with acute cervical cord injury? Spine. 2009;34:2121–2124. doi: 10.1097/BRS.0b013e3181b613c7. [DOI] [PubMed] [Google Scholar]
- Jana M, Jana A, Liu X, Ghosh S, Pahan K. Involvement of phosphatidylinositol 3-kinase-mediated up-regulation of I kappa B alpha in anti-inflammatory effect of gemfibrozil in microglia. J Immunol. 2007;179:4142–4152. doi: 10.4049/jimmunol.179.6.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TE, Zhang X, Shi S, Umbenhauer DR. Statins and PPARalpha agonists induce myotoxicity in differentiated rat skeletal muscle cultures but do not exhibit synergy with co-treatment. Toxicol Appl Pharmacol. 2005;208:210–221. doi: 10.1016/j.taap.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Jones TB, Basso DM, Sodhi A, Pan JZ, Hart RP, MacCallum RC, Lee S, Whitacre CC, Popovich PG. Pathological CNS autoimmune disease triggered by traumatic spinal cord injury: implications for autoimmune vaccine therapy. J. Neurosci. 2002;22:2690–2700. doi: 10.1523/JNEUROSCI.22-07-02690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TB, Hart RP, Popovich PG. Molecular control of physiological and pathological T-cell recruitment after mouse spinal cord injury. J. Neurosci. 2005;25:6576–6583. doi: 10.1523/JNEUROSCI.0305-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz SM, Fitzgerald JE, Fisken RA, Schardein JL, Reutner TH, Lucas JA. Toxicological studies on gemfibrozil. Proc R Soc Med. 1976;69(Suppl 2):15–23. doi: 10.1177/00359157760690S205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Xie S, Sun H, Gonzalez FJ, Wei X, Dai R. Myotoxicity of gemfibrozil in cynomolgus monkey model and its relationship to pharmacokinetic properties. Toxicol Appl Pharmacol. 2009;235:287–295. doi: 10.1016/j.taap.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Yang J, Gonzalez FJ, Cheng GQ, Dai R. Biphasic regulation of intracellular calcium by gemfibrozil contributes to inhibiting L6 myoblast differentiation: implications for clinical myotoxicity. Chem Res Toxicol. 2011;24:229–237. doi: 10.1021/tx100312h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett-Racke AE, Hussain RZ, Northrop S, Choy J, Rocchini A, Matthes L, Chavis JA, Diab A, Drew PD, Racke MK. Peroxisome proliferator-activated receptor alpha agonists as therapy for autoimmune disease. J Immunol. 2004;172:5790–5798. doi: 10.4049/jimmunol.172.9.5790. [DOI] [PubMed] [Google Scholar]
- McTigue DM, Tripathi RB, Wei P, Lash AT. The PPAR gamma agonist Pioglitazone improves anatomical and locomotor recovery after rodent spinal cord injury. Exp. Neurol. 2007;205:396–406. doi: 10.1016/j.expneurol.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J. Comp Neurol. 1997;377:443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Guan Z, Wei P, Huitinga I, van Rooijen N, Stokes BT. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp. Neurol. 1999;158:351–365. doi: 10.1006/exnr.1999.7118. [DOI] [PubMed] [Google Scholar]
- Profyris C, Cheema SS, Zang D, Azari MF, Boyle K, Petratos S. Degenerative and regenerative mechanisms governing spinal cord injury. Neurobiol. Dis. 2004;15:415–436. doi: 10.1016/j.nbd.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Pyper SR, Viswakarma N, Yu S, Reddy JK. PPARalpha: energy combustion, hypolipidemia, inflammation and cancer. Nucl Recept Signal. 2010;8:e002. doi: 10.1621/nrs.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanan S, Kooshki M, Zhao W, Hsu FC, Robbins ME. PPARalpha ligands inhibit radiation-induced microglial inflammatory responses by negatively regulating NF-kappaB and AP-1 pathways. Free Radic Biol Med. 2008;45:1695–1704. doi: 10.1016/j.freeradbiomed.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanan S, Kooshki M, Zhao W, Hsu FC, Riddle DR, Robbins ME. The PPARalpha agonist fenofibrate preserves hippocampal neurogenesis and inhibits microglial activation after whole-brain irradiation. Int J Radiat Oncol Biol Phys. 2009;75:870–877. doi: 10.1016/j.ijrobp.2009.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Pahan K. Gemfibrozil, stretching arms beyond lipid lowering. Immunopharmacol Immunotoxicol. 2009;31:339–351. doi: 10.1080/08923970902785253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstrunk A, Hanf R, Hum DW, Fruchart JC, Staels B. Safety issues and prospects for future generations of PPAR modulators. Biochim Biophys Acta. 2007;1771:1065–1081. doi: 10.1016/j.bbalip.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Sanguino E, Ramon M, Roglans N, Alegret M, Sanchez RM, Vazquez-Carrera M, Laguna JC. Gemfibrozil increased the specific binding of rat-cortex nuclear extracts to a PPRE probe. Life Sci. 2003;73:2927–2937. doi: 10.1016/j.lfs.2003.04.001. [DOI] [PubMed] [Google Scholar]
- Smith SA, May FJ, Monteith GR, Roberts-Thomson SJ. Activation of the peroxisome proliferator-activated receptor-alpha enhances cell death in cultured cerebellar granule cells. J Neurosci Res. 2001;66:236–241. doi: 10.1002/jnr.1216. [DOI] [PubMed] [Google Scholar]
- Tripathi R, McTigue DM. Prominent oligodendrocyte genesis along the border of spinal contusion lesions. Glia. 2007;55:698–711. doi: 10.1002/glia.20491. [DOI] [PubMed] [Google Scholar]
- Wang G, Namura S. Effects of chronic systemic treatment with peroxisome proliferator-activated receptor alpha activators on neuroinflammation induced by intracerebral injection of lipopolysaccharide in adult mice. Neurosci Res. 2011;70:230–237. doi: 10.1016/j.neures.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Storer PD, Chavis JA, Racke MK, Drew PD. Agonists for the peroxisome proliferator-activated receptor-alpha and the retinoid X receptor inhibit inflammatory responses of microglia. J Neurosci Res. 2005;81:403–411. doi: 10.1002/jnr.20518. [DOI] [PubMed] [Google Scholar]
- Yang Y, Gocke AR, Lovett-Racke A, Drew PD, Racke MK. PPAR alpha regulation of the immune response and autoimmune encephalomyelitis. PPAR Res. 2008;2008:546753. doi: 10.1155/2008/546753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Okuyama M, Hashimoto H, Tagawa Y, Jomori T, Yang B. Bezafibrate induces myotoxicity in human rhabdomyosarcoma cells via peroxisome proliferator-activated receptor alpha signaling. Toxicol In Vitro. 2010;24:154–159. doi: 10.1016/j.tiv.2009.08.001. [DOI] [PubMed] [Google Scholar]