Abstract
Animal model research has shown that the central features of tinnitus, the perception of sound without an acoustic correlate, include elevated spontaneous and stimulus-driven activity, enhanced burst-mode firing, decreased variance of inter-spike intervals, and distortion of tonotopic frequency representation. Less well documented are cell-specific correlates of tinnitus. Unipolar brush cell (UBC) alterations in animals with psychophysical evidence of tinnitus has recently been reported. UBCs are glutamatergic interneurons that appear to function as local-circuit signal amplifiers. UBCs are abundant in the dorsal cochlear nucleus (DCN) and very abundant in the flocculus (FL) and paraflocculus (PFL) of the cerebellum. In the present research, two indicators of UBC structure and function were examined: Doublecortin (DCX) and epidermal growth factor receptor substrate 8 (Eps8). DCX is a protein that binds to microtubules where it can modify their assembly and growth. Eps8 is a cell-surface tyrosine kinase receptor mediating the response to epidermal growth factor; it appears to have a role in actin polymerization as well as cytoskeletal protein interactions. Both functions could contribute to synaptic remodeling. In the present research UBC Eps8 and DCX immunoreactivity (IR) were determined in 4 groups of rats distinguished by their exposure to high-level sound and psychophysical performance: Unexposed, exposed to high-level sound with behavioral evidence of tinnitus, and two exposed groups without behavioral evidence of tinnitus. Compared to unexposed controls, exposed animals with tinnitus had Eps8 IR elevated in their PFL; other structures were not affected, nor was DCX IR affected. This was interpreted as UBC upregulation in animals with tinnitus. Exposure that failed to produce tinnitus did not increase either Eps8 or DCX IR. Rather Eps8 IR was decreased in the FL and DCN of one subgroup (Least-Tinnitus), while DCX IR decreased in the FL of the other subgroup (No-Tinnitus). Neuron degeneration was also documented in the cochlear nucleus and PFL of exposed animals, both with and without tinnitus. Degeneration was not found in unexposed animals. Implications for tinnitus neuropathy are discussed in the context of synaptic remodeling and cerebellar sensory modulation.
Keywords: tinnitus, cerebellum, unipolar brush cell, doublecortin, Eps8, auditory afferent degeneration, animal model
Introduction
Chronic subjective tinnitus is the persistent perception of sound (“ringing in the ears”) in the absence of objective sound. Across a range of definitions, survey methods, and societies, between 10 and 15 percent of the adult population report having chronic subjective tinnitus (Hoffman et al., 2004). Of those, 10 to 15 percent seek medical advice or treatment for the condition (Tunkel et al., 2014). Although a standard of care has been established, actionable recommendations focus on counseling and acceptance because there is no broadly effective treatment for tinnitus (Tunkel et al., 2014). Lack of generally effective treatment may in part be attributed to incomplete knowledge and the unexpected complexity of tinnitus pathophysiology (Roberts et al., 2010). Following high-level sound exposure, central auditory changes are evident long afterward (Bauer et al., 2007). Homeostatic mechanisms appear to compensate and in some instances to overcompensate for peripheral deficits (Norena, 2011). Overcompensation may produce the sensation of sound without an objective physical correlate, i.e., tinnitus. Mechanisms that underpin compensatory processes are poorly understood. They may involve down-regulation of inhibitory neurotransmission mediated by γ-amino butyric acid (GABA) (Wang et al., 2011), and/or up-regulation of excitatory neurotransmission mediated by glutamic acid (Glu) (Brozoski et al., 2013; Tzounopoulos et al., 2004; Zeng et al., 2009), or modulation by other transmitter systems, such as acetylcholine (Deng et al., 2015; Godfrey et al., 2013; Schofield et al., 2011). Current research suggests that tinnitus dysfunction is distributed in the central auditory system (Knipper et al., 2013; Rauschecker, 1999; Roberts et al., 2010) and that additionally it involves areas outside of the traditionally-defined auditory pathway (Langguth et al., 2007; Lanting et al., 2009).
System-level tinnitus alterations have been extensively investigated in the dorsal cochlear nucleus (DCN) (Kaltenbach, 2006; Kaltenbach et al., 2008), inferior colliculus (Bauer et al., 2008; Holt et al., 2010; Jastreboff et al., 1986; Melcher et al., 2000), auditory thalamus (Kalappa et al., 2014; Richardson et al., 2011), and auditory cortex (Deng et al., 2015; Engineer et al., 2011; Langers et al., 2012; Norena et al., 2006; Tan et al., 2007; Zhang et al., 2011). Nevertheless specific cellular mechanisms remain unclear. Cellular mechanisms that have been described include loss of cochlear spiral ganglion cells (Bauer et al., 2007), DCN fusiform cell elevated excitation (Brozoski et al., 2002), glutamatergic plastic alterations in the auditory brainstem (Middleton et al., 2011; Shore et al., 2008), and medial geniculate neuron bursting (Sametsky et al., 2015). Using an animal tinnitus model, we recently described the potential role of unipolar brush cells (UBCs) in the DCN as well as in the flocculus (FL) and paraflocculus (PFL) of the cerebellum (Bauer et al., 2013b). Rats with tinnitus had immunohistochemical evidence of UBC up regulation when compared to rats without tinnitus.
While plastic alterations in the DCN and other structures have for some time been recognized as correlates of tinnitus and sequelae to high-level sound exposure (Kaltenbach et al., 2008; Middleton et al., 2011), cerebellar involvement is somewhat novel (Bauer et al., 2013a; Brozoski et al., 2013; Shulman et al., 1999). Auditory afferents are known to pass through, and skirt around the DCN as they enter the PFL (Huang et al., 1982; Morest et al., 1997; Rasmussen, 1990), while cerebellar connections to other auditory areas, such as the auditory cortex, have also been described (Azizi et al., 1985). The cerebellum and DCN are also known to share a very similar cytoarchitecture, suggestive of similar functions such as signal timing and gain control (Bell et al., 2008; Oertel et al., 2004). While the cerebellum historically is considered to be a motor, or motor-accessory area (Ito, 1984), research in recent decades indicates a much broader role in sensory, perceptual, and perhaps cognitive function (Baumann et al., 2015; Bell et al., 2008; Ito, 2008). The crystalline cytoarchitecture of the cerebellum suggests that it serves as a fast-acting comparator and temporal processing engine (Ito, 2006). Since the cerebellum receives afferents from, and sends efferents to, many cortical systems (Baumann et al., 2015), it could be used as a shared resource for temporal integration and stabilization of sensory images (Gao et al., 1996). In summary, evidence has been accumulating that a major role of the cerebellum appears to be perceptual optimization (Roth et al., 2013), i.e., tempering perception through the windows of context and experience.
The FL, with its dense UBC innervation, is generally accepted as a locus of gain-control, well-studied in the context of the vestibulo-ocular reflex (Broussard et al., 2011). UBCs fit into this scheme as elements of a distributed gain-control network. UBCs have been described as tunable feed-forward amplifiers that “provide an anatomic substrate for the powerful feedforward excitation of a large neuronal ensemble by a single synaptic input” (Dino et al., 2000). It is notable that UBC gain control circuits function in the DCN as well as in the cerebellum (Bell et al., 2008; Dino et al., 2008). Recently it has been shown that putative UBCs in the PFL respond directly to auditory stimuli (Voelker et al., 2016). The specialized dendritic brush of UBCs encapsulates a “giant excitatory synapse from a single MF [mossy fiber]” or another UBC, and displays various ionotropic and metabotropic glutamate receptors (Rousseau et al., 2012). The dendritic brushes also encapsulate inhibitory GABAergic and glycinergic boutons of Golgi cell axons (Rousseau et al., 2012).
UBCs are effectively, although not equivalently, immunostained by doublecortin (DCX) and epidermal growth factor receptor substrate 8 (Eps8). DCX, a microtubule associated protein (MAP), is a component of the microtubule cytoskeleton. It stabilizes microtubules and promotes their polymerization, although its structure is not homologous to other MAPs. When DCX binds to microtubules it can modify their assembly and growth (Moores et al., 2004). Eps8 is a cell-surface tyrosine kinase receptor that, among other things, mediates the cell's response to epidermal growth factor (EGF). Eps8 also appears to have a role in actin polymerization, as well as facilitating cytoskeleton protein-protein interactions. As such, Eps8 elevation could mark cells engaged in plastic dendritic remodeling. The remodeling process could be triggered by glutamate via N-methyl d-aspartate glutamatergic receptors (NMDA-R). Eps8 is found at high levels in the cerebellar granule cell layer, where it is associated with post-synaptic NMDA-R, but not α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPA-R) (Offenhauser et al., 2006). Eps8 has also been shown to regulate actin-cytoskeleton dynamics, particularly in the context of stress, where NMDA-R activation has been shown to cause rapid actin remodeling in the dendritic spines of hippocampal and cerebellar granule neurons (Fischer et al., 2000; Furuyashiki et al., 2002; Halpain et al., 1998; Shiraishi et al., 2003; Star et al., 2002). In contrast, elevated DCX could mark cells with upregulated cellular processes directed at maintaining or re-establishing homeostatic equilibrium. The homeostatic mechanism could be MAP2-K2p-mediated membrane voltage stabilization. Two-pore potassium channels (K2p), sometimes called K-leak channels, are “passive” K-channels that stabilize membrane potential (Feliciangeli et al., 2015). Cells that have been disrupted, for example from over stimulation, may attempt to regain homeostatic equilibrium using a MAP2–K2p mechanism (Renigunta et al., 2015). A key link is that DCX overexpression has been shown, in vitro, to enhance MAP2 (Santra et al., 2006). Conversely, decreased DCX could indicate destabilization, or loss of homeostatic equilibrium.
In the present study the working hypothesis was that cerebellar and cochlear nucleus UBCs are imbalanced in tinnitus animals because of the role they play in compensating for damage- or age-related decreased peripheral input. A compensatory mechanism could involve NMDA-R-mediated restructuring of dendrites, a process that involves Eps8 upregulation. It was further hypothesized that there could be a coincident braking mechanism mediated by MAP2 and K2p, and that this process might be reflected by DCX upregulation. A derivative hypothesis would be that rats not developing tinnitus after acoustic trauma would have greater DCX upregulation, reflecting a more pronounced braking effect on UBCs.
Method
The experimental protocol was approved by the Laboratory Animal Care and Use Committee of Southern Illinois University School of Medicine (protocol #149-12-009).
Subjects
25 male Long-Evans rats (Harlan, Indianapolis, IN, USA) 90 days old at the outset of the study, were individually housed and maintained at 25° C with a 12/12 hr reversed light/dark schedule. While undergoing behavioral testing, food access was restricted so as to maintain body weight between 80 and 95 percent of free-feeding weight. Water access was unrestricted.
Tinnitus induction and auditory brainstem evoked potential (ABR) hearing assessment
Between 104 and 107 days of age, the animals were anesthetized with a 1.7 percent isoflurane / O2 mixture, placed in a head holder, and a speaker driver (FT17H, Fostex, Tokyo, Japan) attached to 2 cm length of flexible tubing was positioned at the entrance of the right ear canal. Sixteen rats were randomly selected and unilaterally exposed once to band-limited noise for 1 hr, with a peak level of 120 dB (SPL) centered at 16 kHz, falling to ambient levels at 8 kHz and 24 kHz. ABR levels, in μV root mean square (ABRrms), were obtained before and after exposure for pure tones at 8, 10, 12, 16, 20, 24, and 32 kHz, each presented in 10 dB decrements between 95 and 5 dB (SPL re 20 μPa). The nine unexposed animals had their hearing levels determined without high-level sound exposure. The sound exposures and/or hearing level determinations preceded behavioral training. During exposure the contralateral canal was obstructed by the canal-fitted plastic tube from the contralateral speaker. Details of the sound exposure have been previously published (Bauer et al., 2001; Bauer et al., 2006).
ABR measurements were obtained using either an IHS 4964 high frequency system (Intelligent Hearing Systems, Miami, FL, USA) or a customized TDT System 3 (Tucker Davis Technologies, Alachua, FL, USA). Stainless steel needle electrodes were placed subcutaneously at the vertex and bullae. Hearing levels and thresholds were obtained for 5 msec duration tone bursts presented at a rate of 20/sec. Tone bursts were gated using a cosine envelope (2.5 msec rise/decay, 0 msec plateau) and were presented in decreasing intensity series. ABRrms levels were determined for the peri-stimulus period encompassing the 5 msec tone bursts, plus an additional 5 msec post-stimulus period. Evoked potentials (X 100,000) were averaged over 256 sweeps, digitized with 4 μsec resolution, and imported to spreadsheets (Excel 2007, Microsoft, Redmond, WA, USA) for statistical analysis. Custom spreadsheet algorithms determined RMS levels as well as thresholds.
Sound calibration
Sound levels were calibrated with a Bruel & Kjaer Pulse sound measurement system (Pulse 13.1 software), equipped with a 3560C high-frequency module. Exposure levels and ABR levels were determined using a 4138 pressure-field microphone (Bruel & Kjaer, Norcross, GA, USA) coupled to the output transducers using rubber tubing with internal dimensions approximately matching that of an adult rat external auditory canal. Sound level determination was possible between 5 and 140 dB (SPL re 20 μPa), and from 5 Hz to 104 kHz. Sound levels in the behavioral test chambers were calibrated using a B&K 4191 open-field microphone (frequency range 3.1 Hz to 40 kHz). All levels reported are unweighted.
Tinnitus assessment
Tinnitus was determined using a behavioral procedure demonstrated to be sensitive to tinnitus, and described elsewhere in detail (Bauer et al., 2001; Brozoski et al., 2015). Briefly, an operant conditioned-suppression procedure was used to characterize the animals’ perception of tones and silent periods presented in the context of ambient 60 dB (SPL) broad-band noise (BBN). The animals were required to discriminate between the presence and absence of sound when tested with a variety of sounds of different composition, frequency, and level. While the features of tinnitus in rats (and other species) cannot be directly known, by definition tinnitus cannot sound like silence. Animals were tested daily in operant test chambers (Lafayette Instruments, Mod. 80001, Lafayette, IN, USA) equipped with lid-mounted speakers. Speaker-off periods (i.e., silence) acquired significance for the animals because lever pressing during the silent periods led to a foot shock at the end of the period (0.5 mA, 1 sec duration). Foot shock could be avoided by not lever pressing (i.e., suppression). Outside of silent periods, lever pressing never led to foot shocks. The behavior of interest was lever pressing during randomly inserted test sounds (1 min duration) that substituted for some of the speaker-off presentations (also 1 min duration). Each one hour test session contained 10 randomly inserted, non-contiguous presentations of test sounds or silence; background BBN was off during test-sound presentations. Two of the ten presentations were always silent (i.e., speaker off) periods. The remaining 8 were randomly-selected tones or noise, with different levels in each presentation. The decision task for the animals was to discriminate between the test-sound presentations and speaker off. Lever pressing was quantified using a relative rate measure, the suppression ratio (R). R was determined as a running measure for successive 1-min segments of each session using the formula R = B/(A+B), where A was the number of lever presses in the preceding 1-min segment and B the number of lever presses in the current 1-min segment. R can vary between 0 and 1. A value of 0 is attained when lever pressing in the current minute is 0, a value of 0.5 when lever pressing in the current minute is equal to that of the previous minute, and a value of 1 when lever pressing in the previous minute is zero. R provided a running index of behavior, in 1-min epochs, and enabled a quantitative comparison between subjects as well as unbiased compilation of group data. R is a useful index of perceptual performance since it is sensitive to short-term behavioral effects, such as those produced by sensory events, but it is insensitive to gradual behavioral effects, such as those produced by changes in motivational status, for example, satiation. In the context of the present procedure, it was expected that exposed rats with tinnitus would have lower R-values than unexposed rats, when tested with stimuli that resembled their tinnitus (the conditioned signal for potential foot shock). Typically a minimum of 5 sessions for each animal for each sound condition (e.g., BBN, 20 kHz tones, etc.) were averaged to derive individual and group psychophysical functions.
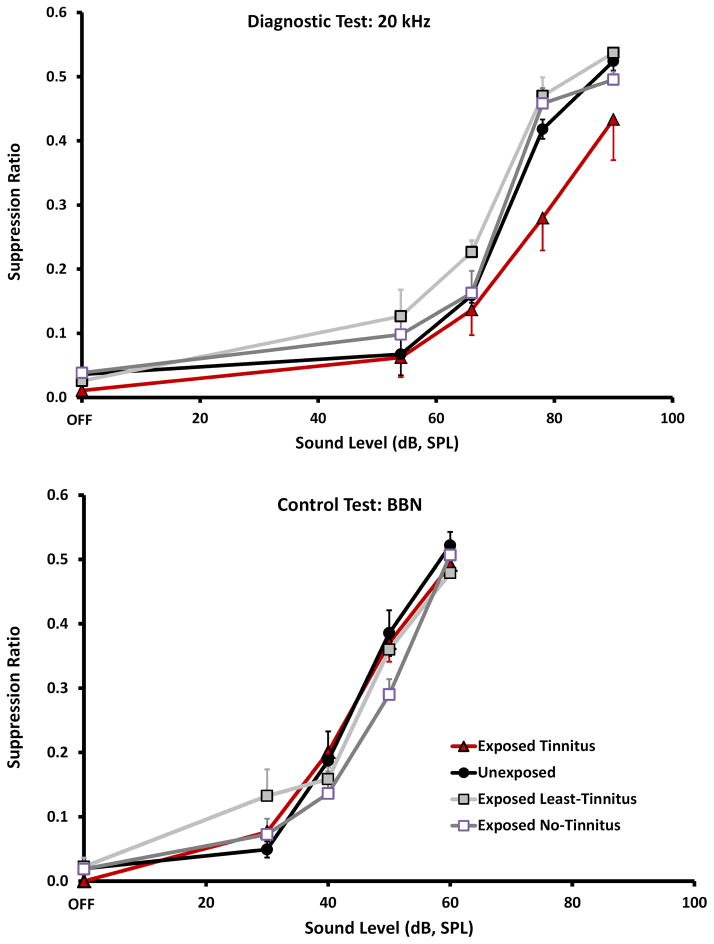
Psychophysical performance was analyzed by comparing group discrimination functions (Fig. 1) using a two-factor mixed ANOVA: Experimental groups were considered the independent treatment dimension while sound level comprised the repeated-measures dimension. Post-hoc tests were used to examine the pairwise difference between the sound-exposed groups and unexposed control.
Figure 1.
Psychophysical performance of animals (n = 4 / group) selected for immunoreactive (IR) quantification. Top panel: 20 kHz tone presentations, used as the tinnitus diagnostic. The Exposed Tinnitus group function with dark triangular data points, was significantly down shifted (p = 0.021) from the Unexposed Control group, dark function with round data points. The Exposed No-Tinnitus group, unfilled square data points, was not significantly different than Unexposed (p = 0.482), and the Least-Tinnitus group, shaded square data points, was significantly up shifted from the Unexposed (p = 0.009). Bottom panel: Broad-band noise (BBN) presentations, used as a diagnostic for hearing loss; BBN testing showed no significant difference (p = 0.479) between groups. In both panels the X-axis shows the level of test sound presentations and the Y-axis (Suppression Ratio) shows bar pressing rate relative to background-sound bar pressing. Error bars show the standard error of the mean.
Immunohistochemistry
At the conclusion of psychophysical testing and final ABR testing, the rats received a lethal dose of anesthetic (Euthasol, Virbac, Ft. Worth, TX, U.S.A.) and were transcardially perfused with physiological saline followed by 4% paraformaldehyde (Cat#19202, Electron Microscopy Sciences, Hatfield, PA, U.S.A.) in 100 mM phosphate buffer saline (PBS, pH 7.2). The brains were coded to designate treatment groups, thus experimentally blinding experimenters involved in tissue processing, microscopy, and image analysis. The identification code was not broken until statistical analyses were complete. Excised brains were post fixed in 4% paraformaldehyde (in 100 mM PBS, pH 7.2) for 24 hr. After post fixation, the brains were blocked, punched unilaterally to mark the left side using a blunt 20 ga hypodermic needle, and stored at 4°C until equilibrated in 30% sucrose-PBS solution. Blocked brains were mounted for cutting using O.C.T. compound (Sakura Finetek USA, Inc., Torrance, CA, USA). Serial 40 μm-thick frozen (-20°C) coronal sections were cut (Leica CM1850, Leica Microsystems, Wetzlar, Germany) from tissue blocks containing the complete brainstem and cerebellum. Section order was maintained by storing four sequential sections per well in a multi-well plate. The sections were processed in 12-well cell culture plates with polyester mesh sieve inserts (#3478, Corning Lifesciences, Tewksbury, MA, USA) containing 100 mM PBS plus 0.05% Tween (CalBiochem, 655204, EMD Biosciences, Inc., La Jolla, CA, USA) (PBS-T), and gently agitated. Prior to the primary antibody exposure, sections were rinsed for 120 min in 0.3% H2O2 in 50 mM tris-buffered saline with 0.1% Tween (TBS-T). Sections not immediately stained were stored for later use at -22° C in cryopreservative (30% ethylene glycol - Fisher Scientific E178-500, 30% glycerol - Acros Organics 332030010, in 100mM PBS).
Nonspecific binding was blocked using 100 mM PBS with 1% bovine serum albumin (Sigma A7906-100G), 0.5% Triton X-100, and 150 μL normal serum (1.5%; Vector ABC Elite kit - horse normal serum for Eps8, PK-6102; and rabbit normal serum for DCX; PK-6105; Vector Laboratories, Inc., Burlingame, CA, U.S.A.) for 30 min at 21°C. Sections were then placed in primary antibody (DCX 1:500 or Eps8 1:2000, from their respective Vector Kits) in blocking solution, or in vehicle only (non-stained control) for 24 hours on an orbital shaker (40 rpm) at 4°C. Between antibody exposures the sections were rinsed four times in PBS-T, TBS-T, or distilled water with 0.1% Tween (DW-T). Rinse durations matched the duration of the antibody exposure, up to a maximum 2 hrs. Sections were then incubated for 1 hr at 21°C with the appropriate secondary antibody (goat IgG-DCX, 1:200 or mouse IgG-Eps8, 1:200) in 50 mM TBS-T. These sections were then rinsed four times and incubated for 1 hr at 21°C in the avidin-biotin-complex (ABC) reaction solution provided in the Vector kit. The tissue was rinsed four more times and then stained using 3,3’-diaminobenzidine tetrahydrocholride (DAB) (Sigma D5905-100TAB, U.S.A.). A second control was exposed only to ABC and DAB, without primary antibody or secondary antibody. A third control was exposed only to DAB.
Stained sections were mounted on glass slides, dried overnight and then further dehydrated using a series of ethanol and xylene solutions. The slides were coverslipped using Permount (Fisher Scientific, Waltham, MA, USA), cured overnight, and examined using bright-field light microscopy (Zeiss, Photomicroscope III, Carl Zeiss, Jena, Germany). Images of sections containing the areas of interest (AOI) were digitized using a laser scanner (Nikon Super Coolscan 5000, www.nikon.com) and saved as TIFF images with a resolution of 500 dpi. High-magnification images used to depict UBC staining were obtained using digital microscope system (Zeiss Axio Imager M2, Hamamatsu Mod. C10600-BB-H camera; MBF Bioscience, Williston, VT, USA).
Immunostaining quantification
Brain sections of 4 representative animals from each treatment group were selected by an unblinded experimenter (KW) not involved in quantification, and passed on to blinded experimenters for IR quantification. Group size (n = 4) was chosen to reduce the serial-section IR quantification to a manageable task, while establishing equal group size and optimizing the psychophysical features of each group, e.g., the Tinnitus group was composed of the 4 animals with the best evidence of tinnitus, as further described. Selection criteria were applied to individual 20 kHz psychophysical performance functions (i.e., the tinnitus-diagnostic stimulus condition): exposed animals with tinnitus were those with the greatest downshift in their performance function (Fig. 1, Exposed-Tinnitus, top panel, dark triangular data points); exposed animals with no tinnitus (Fig. 1, Exposed No-Tinnitus, top panel, open square data points) had performance equivalent to that of unexposed control animals (Fig. 1, Unexposed, top panel, circular dark data points); exposed animals with the least tinnitus (Fig. 1, Exposed Least-Tinnitus, top panel, filled square data points) were defined by performance functions upshifted from that of unexposed controls. The Unexposed group was composed of animals from that condition with median-level performance.
Digital images of the DCN, FL, and PFL, extending from the structures’ most rostral to most caudal aspect, were imported into Image J (ver.1.49, http://imagej.nih.gov/ij). Each was converted to a 32-bit grayscale image and dimensionally scaled (pixels/mm) using a calibration scale bar. The perimeter of each AOI was manually outlined using ImageJ’s polygon tool. This permitted area determination. After AOI area determination, image brightness threshold was adjusted to identify stained areas, which showed as black on a white background. Threshold level was optimized individually for each AOI by directly comparing thresholded to non-thresholded views. For large AOI, such as the PFL, often sub-areas had to be independently thresholded, and assembled into a montage for quantifying the entire AOI. Although the images were derived from laser scans with constant beam illumination, staining intensity was never perfectly homogenous, either within brain slices, or from one slice to another. Neither automated nor fixed-criterion thresholding adequately compensated for subtle stain variations. As in previous work (Bauer et al., 2013b), IR was quantified as the above-threshold area stained, rather than cell counts. Cell counts (e.g., particles, quantified using the ImageJ Particle Analysis Tool) would substantially underestimate IR level because the irregularly-shaped UBCs frequently touched or overlapped in the image plane (Figs. 2 & 3). That is to say, a cluster of several overlapping stained cells would be counted as one. Quantifying above-threshold stained area had the additional advantage of distinguishing patchy or lightly stained cells from those with denser more uniform staining. That is to say, a cell stained throughout would provide a larger number than one only partially stained. Numeric data from ImageJ were copied to spreadsheets, from which IR was determined for each AOI. Statistical analyses were performed on relative AOI stained volume (stained volume / total AOI volume). Two independent, treatment-blinded experimenters quantified staining, and their combined results were used to determine IR. Correspondence between the complete data sets of each experimenter was high (r = 0.739, p < 0.0001).
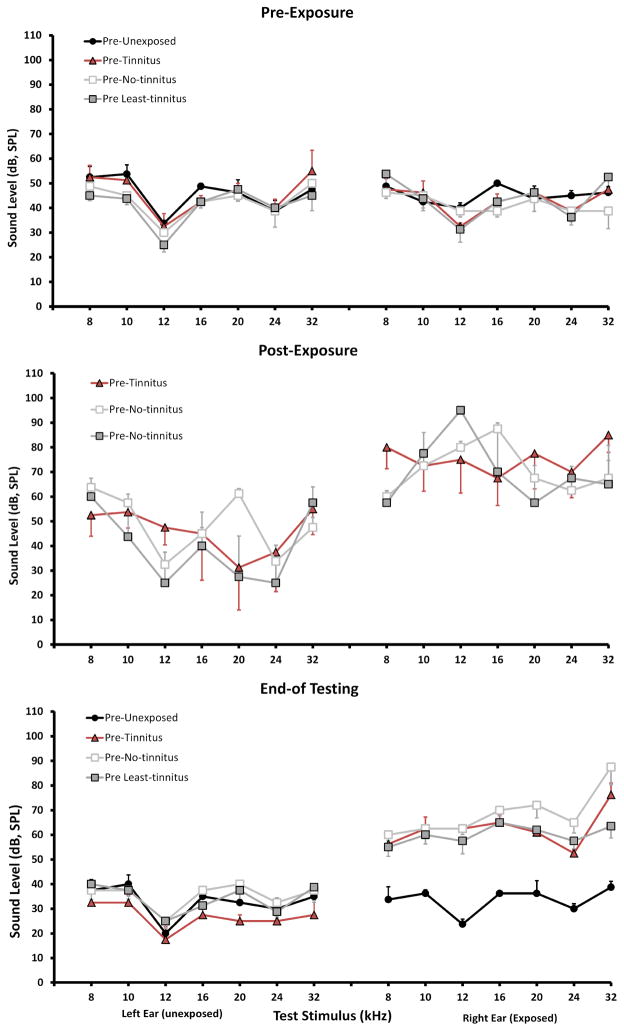
Figure 2.
Auditory brainstem response (ABR) pure-tone thresholds (8 – 32 kHz) of the four experimental groups (same animals, as depicted in Figure 1). Top panel: pre-exposure thresholds; mid panel: immediate post-exposure thresholds; lower panel: end-of-behavioral testing thresholds. Only the right ear was exposed for 1 hr; n = 4/group; error bars show the standard error of the mean.
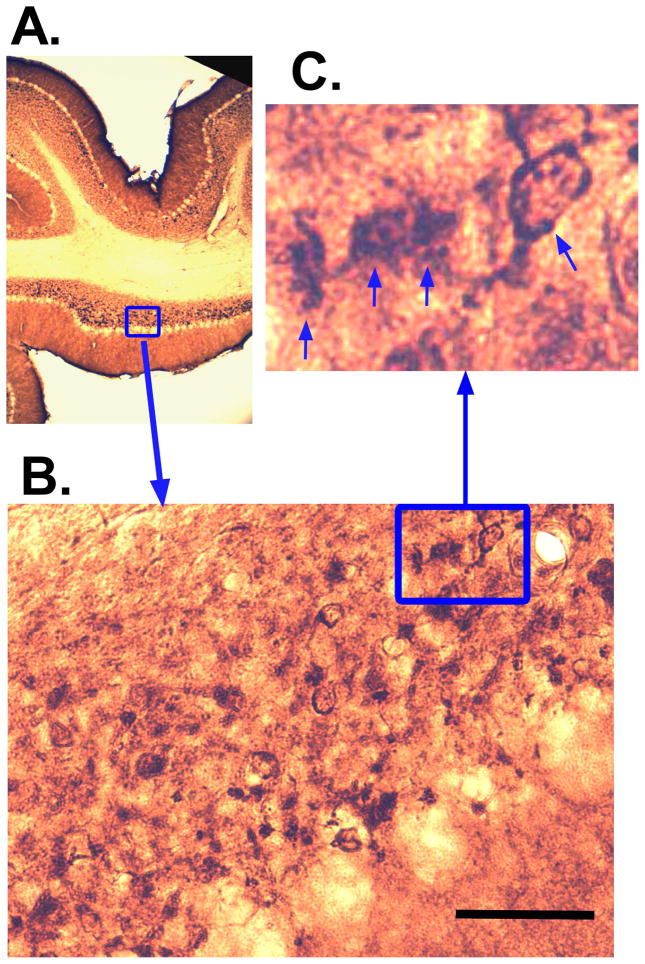
Figure 3.
Representative epidermal growth factor 8 (Eps8) IR in the paraflocculus (PFL) of an exposed animal with tinnitus. Panes A – C show successively higher magnified views of a unipolar brush cell (UBC) configuration with brush contact between two adjacent UBCs (arrows in C). Calibration bar = 50 μm.
IR comparisons were quantified using mixed two-factor ANOVAs. Experimental group (Unexposed, Tinnitus, No-Tinnitus, Least-Tinnitus) was considered the independent variable dimension while brain section was the repeated measures dimension. AOI (DCN, FL, PFL), and IR epitope (Eps8, DCX), were considered to be statistically independent. Significance levels for post-hoc pair-wise IR comparisons between the Unexposed and the exposed groups (Tinnitus, No-Tinnitus, Least-Tinnitus) were corrected for significance inflation using the Bonferroni procedure. Individual IR data distributions were checked for normality by direct comparison to unit normal distributions with equal mean and variance. There were no significant differences between any of the empirical IR distributions and unit normal distributions (significance levels from t = 1.65, df = 39, p = 0.108, to t = 0.05, df = 37, p = 0.961).
Neural degeneration staining and quantification
Brain sections not used for IR were examined for evidence of neural degeneration. Sections were prepared following the manufacturer’s directions for the FD NeuroSilver™ Kit II (User Manual PK 301/301A, Version 2012-02; FD NeuroTechnologies, Inc., Ellicott City, MD, USA). Briefly, cryopreserved sections were washed in double-distilled water using a rocker plate and mild agitation. Two 5-min washes preceded two 10-min incubations in approximately 40 ml of solution mixture AB. Next, tissue was placed in 32 ml of solution mixture ABE for 10 min followed by two 2-min incubations in 50 ml of solution mixture CF and then placed in 50 ml of DF mixture for 5 min. Tissue was then washed twice for 3 min. While completely protected from light, the sections were floated in solution G (44 ml) for 10 minutes followed by a second incubation in a fresh solution of reagent G for 90 min (44 ml). Tissue was then mounted directly on microscope slides from solution G, dried overnight, and dehydrated using an ethanol / xylene solution series. Afterward, the slides were coverslipped, air cured overnight, then photographed and quantified using a Digital Microscope System running Neurolucida Explorer software (version 9, MBF Bioscience, Williston, VT, USA). Degeneration was quantified over the 8 sequential sections that spanned the rostral-caudal axis of the entire cochlear nucleus (CN) for each subject. Sections were viewed under 10x magnification and areas of degeneration, defined by visible silver stain, were outlined using the Neurolucida XYZ tool. Total volume of the CN was similarly determined using the XYZ tool. Degeneration volume relative to CN volume was then determined. Group comparisons were made using the mean and range of each group.
Results
Hearing
Sound exposure immediately increased hearing thresholds in exposed ears from 30 to 40 dB (SPL re 20 μPa) (Fig 2, mid panel). This loss was significant above 12 kHz (p = 0.0003 – 0.0135, across frequencies). Hearing thresholds of unexposed ears were not significantly affected. Levels were retested at the conclusion of psychophysical tinnitus testing (Fig. 2, lower panel). ABR thresholds, from 8 to 32 kHz of unexposed ears were equivalent for all groups (30 – 40 dB, SPL re 20 μPa, ± 1.6 – 4.2 SEM). Compared to unexposed animals, exposed-ear ABR thresholds from 8 to 32 kHz were elevated by 13 to 27 dB (SPL, re 20 μPa) for Tinnitus animals, and 20 to 37 dB (SPL re 20 μPa) for Least-Tinnitus animals. Therefore neither exposed-ear nor unexposed-ear thresholds predicted psychophysically-defined tinnitus.
Tinnitus
For purposes of analysis, as previously described, 16 optimal animals (4 groups of 4 animals each) were selected from the 25 in the study, based on their 20 kHz psychophysical performance. The four groups were: one exposed with tinnitus (Tinnitus), two exposed without tinnitus, and one unexposed (Unexposed). The two groups without tinnitus consisted of one group with performance identical to that of unexposed controls (No-Tinnitus), and one group with the least evidence of tinnitus (Least-Tinnitus). The Least-Tinnitus group was empirically defined by an upshifted 20 kHz psychophysical function. Psychophysical performance is summarized in Fig. 1. The top panel shows performance when tested with 20 kHz tones, the diagnostic for tinnitus in the present model (Bauer et al., 2001). Tinnitus was indicated by a downshifted function because performance is affected by sound-off conditioning. Sensations resembling those of sound-off periods produce more suppression. For all animals, irrespective of treatment or test frequency, suppression was greater (i.e., low lever press rates) at low sound levels. Tinnitus animals, however, showed greater suppression to mid-to-upper level sounds at specific frequencies (resembling their tinnitus), when compared to either Unexposed (F1,24 = 6.12; p = 0.021) or exposed No-Tinnitus animals (F1,24 = 7.87; p = 0.010). This mid-to-upper-level suppression was evident in Tinnitus animals when tested with 20 kHz tones (Fig. 1 top panel, triangular data points). The psychophysical performance of exposed No-Tinnitus animals (Fig. 1, top panel, open square data points) was identical to that of Unexposed (F1,24 = 0.509; p = 0.482), while the function upshift of exposed Least-Tinnitus animals (Fig. 1, top panel, filled square data points; F1,24 = 8.20; p = 0.009) was possibly indicative of hyperacusis. BBN discrimination functions, as shown in the bottom panel of Fig. 1, were identical for all groups (F 3,48 = 0.84; p = 0.479). It was concluded that the Tinnitus group had evidence of 20 kHz tonal tinnitus, while free-field hearing, as indicated by the BBN functions, was equivalent for all groups.
Immunohistochemistry
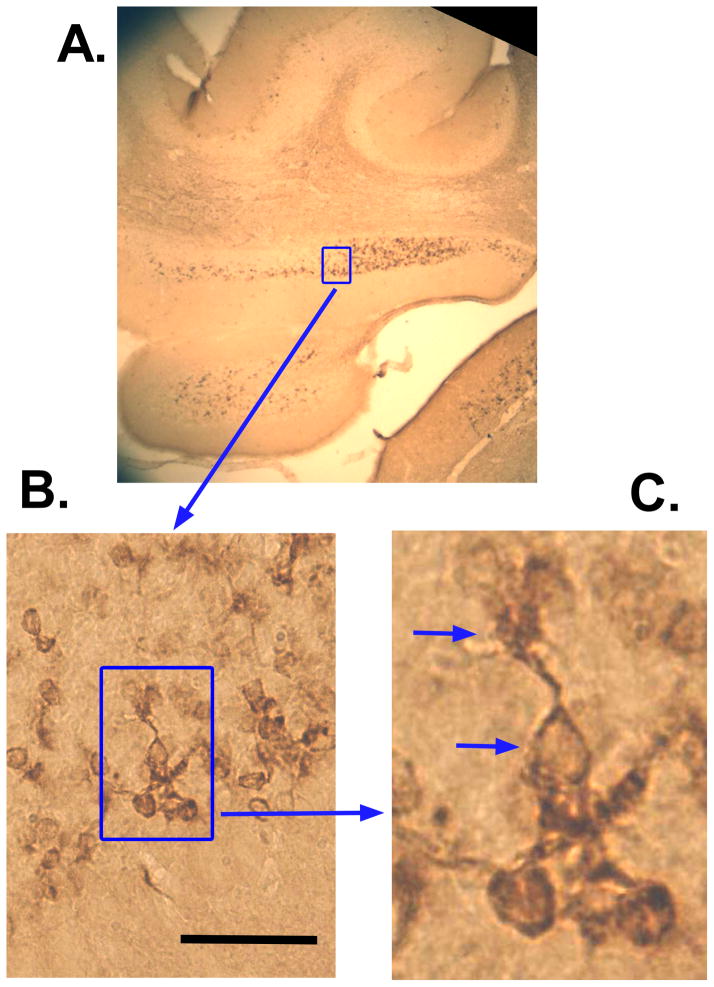
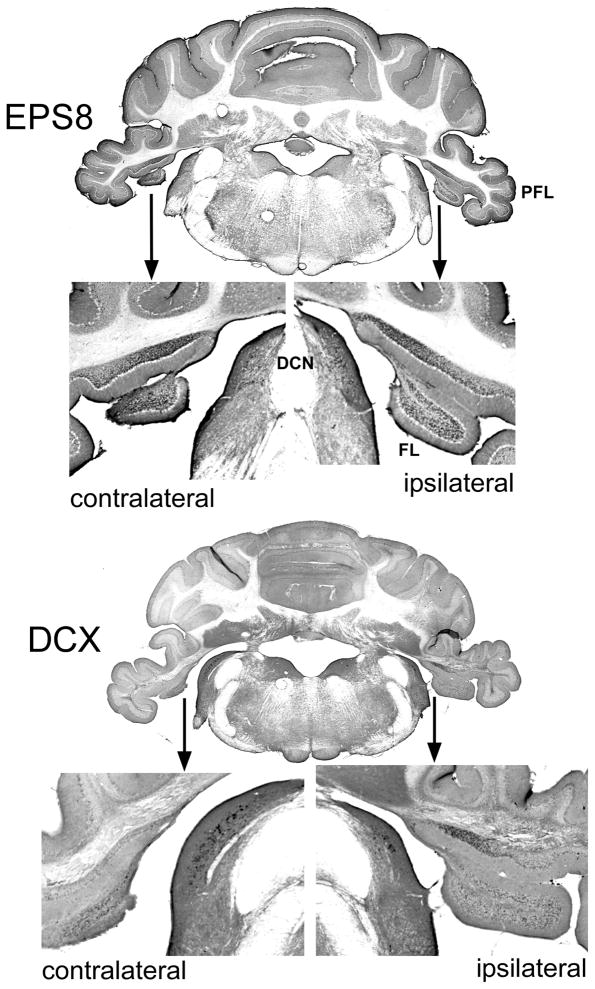
Examples of Eps8 and DCX IR appear in Figs. 3 and 4, respectively, taken from the PFL of a Tinnitus animal. Examples of cells that conform to morphological descriptions of UBCs (Mugnaini et al., 1997) are indicated with arrows in the high-magnification panes of Figs. 3 and 4 (see Discussion). The unipolar brush-like appendage of IR-marked cells is clearly visible in Figs. 3C and 4C. Also evident is the serial connection of UBCs, as shown in Fig. 3C. This configuration is thought to contribute to feed forward amplification in the UBC network, since UBCs release glutamate and harbor glutamatergic receptors. Bilateral macroscopic examples of Eps8 and DCX IR are shown in Fig. 5. For all animals Eps8 IR was greater than DCX IR. This was particularly evident in the FL and medial aspects of the PFL granule cell zone (Fig. 5).
Figure 4.
Representative doublecortin (DCX) IR in the PFL of an exposed animal with tinnitus. Panels A – C show successively higher magnified views of a typical UBC region. Arrows in C indicate a cell body and unipolar brush. Calibration bar = 50 μm.
Figure 5.
Macroscopic bilateral view of Eps8 (top panes) and DCX (lower panes) IR in the animal depicted in Fig. 3. DCN, dorsal cochlear nucleus; FL, flocculus; PFL, paraflocculus. Eps8 and DCX IR puncta are evident the PFL, FL, and DCN.
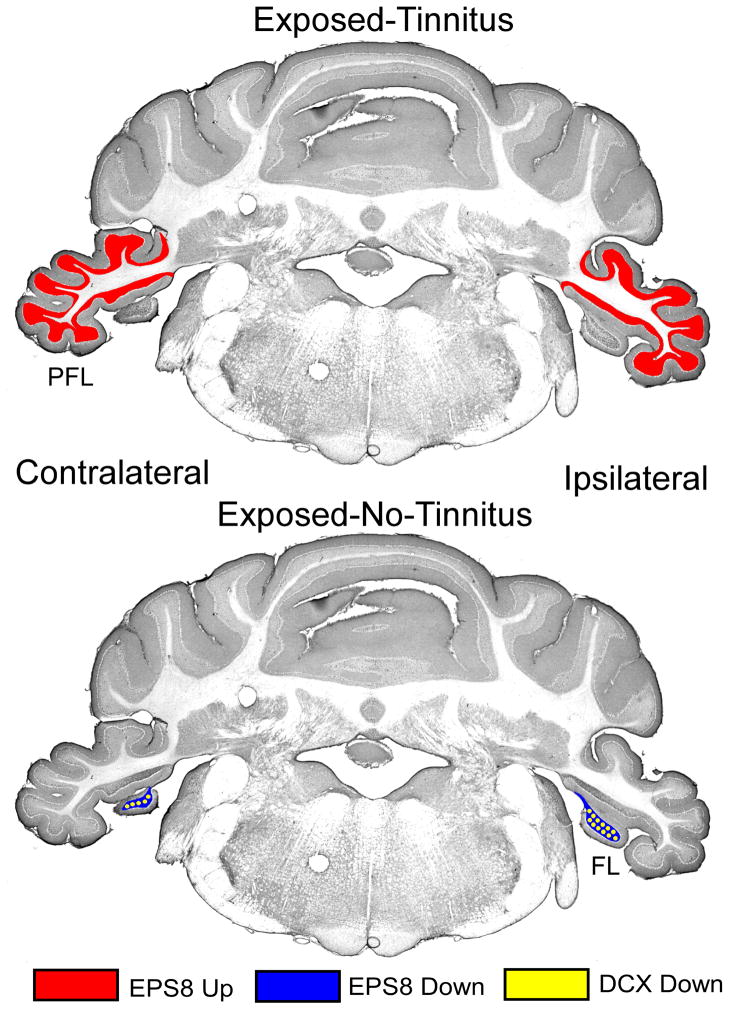
Equivalent sound exposure produced different IR profiles, depending upon the presence or absence of tinnitus, as summarized in Table 1 and Fig. 6. For statistical analysis, IR was quantified bilaterally. This was done not only to improve statistical power but because ipsilateral - contralateral differences were not significant for either Eps8 (p = 0.984 to 0.179) or DCX (p = 0.651 to 0.502), for any of the AOI. Tinnitus animals (compared to Unexposed) were unique in that only they showed an Eps8 IR elevation. This elevation (effect size 0.81) was evident in their PFL (Unexposed IR, 1.4% vs Tinnitus IR, 2.8%; F1,60 = 8.409, p = 0.0156), but not elsewhere (Table 1). It suggests a tinnitus-related UBC upregulation in PFL. In contrast, exposed No-Tinnitus animals (Table 1, bottom row) had bilaterally decreased Eps8 (Unexposed IR, 9.1% vs No-Tinnitus IR, 2.8%; F1,48 = 151.04, p < 0.0001), as well as decreased DCX, in their FL (Unexposed IR, 2.8% vs No-Tinnitus IR, 2.1%; F1,48 = 10.83, p = 0.0056). Least-Tinnitus animals (Table 1, third row) also showed decreased Eps8 IR in their FL (Unexposed IR, 9.1% vs Least-Tinnitus IR, 5.4%; F1,48 = 39.69, p < 0.0001) as well as in their DCN (Unexposed IR, 5.4% vs Least-Tinnitus IR, 3.9%; F1,48 = 7.55, p = 0.025), but no changes in DCX IR in any AOI. In summary, Tinnitus animals showed a unique PFL UBC-localized Eps8 increase. In contrast, exposed animals without tinnitus showed a pattern of decreased UBC-localized Eps8 and DCX IR distributed across the FL and DCN (numerically summarized in Table 1 and graphically depicted in Fig. 6). These results confirm that high-level sound exposure leading to tinnitus affects UBC function differently than high-level sound exposure not producing tinnitus. Eps8 elevation in UBCs of Tinnitus animals could indicate an upregulation or remodeling of synaptic function, as later discussed.
Table 1.
Percent immunoreactivity of the areas of interest (AOI). Comparisons were made between the exposed groups (Exp-Tinn; Exp-Least-Tinn; Exp-No-Tinn) and the Unexposed, for each AOI (DCN, FL, PFL). Differences are indicated by directional arrows and significance levels by bold print and (*). Probability levels (p’) were corrected for repeated-test inflation using the Bonferroni correction (k = 3). Effect size (Cohen’s d) is shown in brackets [ ]
| EPS8 | DCX | |||||
|---|---|---|---|---|---|---|
| Group | DCN | FL | PFL | DCN | FL | PFL |
| Unexposed | 5.4% | 9.1% | 1.4% | 3.2% | 2.8% | 1.2% |
| Exp-Tinn | 5.8% | 7.4% |
2.8%*
[0.81]

|
3.3% | 2.3% | 1.9% |
| Exp-Least-Tinn |
3.9%*
[0.64]

|
5.4%***
[1.69]

|
1.7% | 2.5% | 2.8% | 1.4% |
| Exp-No-Tinn | 4.7% |
2.8%***
[3.68]

|
1.3% | 2.4% |
2.1%**
[0.86]

|
1.0% |
-
DCN: Eps8 decreased in Exposed Least-Tinnitus (Exp-Least-Tinn).DCX not altered.
-
FL: Eps8 decreased in Exp-Least-Tinn and Exposed No-Tinnitus (Exp-No-Tinn).DCX decreased in Exp-No-Tinn.
-
PFL: Eps8 elevated in Exp-Tinn.DCX not altered.
- DCN: Dorsal cochlear nucleus.
- FL: Flocculus of the cerebellum.
- PFL: Paraflocculus of the cerebellum.
- EPS8: Epidermal growth factor substrate receptor 8, a cell-surface tyrosine kinase receptor that, mediates cellular response to epidermal growth factor.
- DCX: Doublecortin, a micro-tubule associated protein.
p < 0.05
p < 0.01
p < 0.001
Figure 6.
Graphic summary of significant (p < 0.05) group IR differences between Unexposed Controls and Exposed Tinnitus, and Exposed No-Tinnitus. In the Tinnitus group (top) Eps8 was increased in the PFL. In the No-Tinnitus group (bottom) both EPS8 and DCX were decreased in the FL.
Rostro-caudal gradients
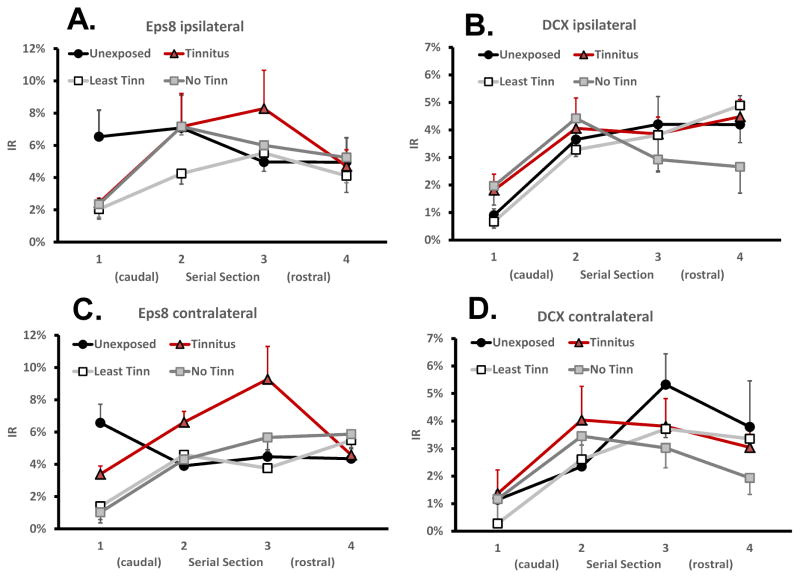
Contiguous coronal serial sections, extending from the most caudal to the most rostral aspect of each AOI, enabled IR rostro-caudal gradients to be determined. Rostro-caudal IR gradients were not significant in the FL and PFL (not shown), but were present bilaterally in the DCN for both DCX and Eps8 (Fig. 7). Rostral DCX IR was significantly higher than caudal (F3,84 = 17.14; p < 0.00001), and was present in all groups, with no significant difference between groups (F7,96 = 0.83; p = 0.565; Fig. 7B & 7D). A similar, but smaller rostro-caudal gradient was evident in exposed animals’ DCN for Eps8, with rostral levels, once again higher (F3,96 = 9.70; p = 0.00001; Fig. 7A & 7D). The functional significance of these gradients is unknown, but the methodological significance is that non-systematic sampling across coronal sections, i.e., along the rostro-caudal transect, could indicate spurious IR treatment group differences. Spurious group differences could be more evident for DCX because of its more pronounced rostro-caudal gradient.
Figure 7.
DCN Rostro-caudal DCX and Eps8 IR gradients in the four treatment groups (Unexposed, Exposed Tinnitus, Exposed No-Tinnitus, Exposed Least-Tinnitus). DCX IR (right panels) was higher in rostral sections than in caudal (F3,96 = 17.14; p < 0.00001), with no significant difference between groups (F7,96 = 0.83; p = 0.686). Eps8 also showed a high-rostral low-caudal gradient, but it was significant only in the three exposed groups (F3,84 = 18.91; p < 0.0001), and not in the Unexposed group (F3,28 = 1.10; p = 0.366).
Neural degeneration
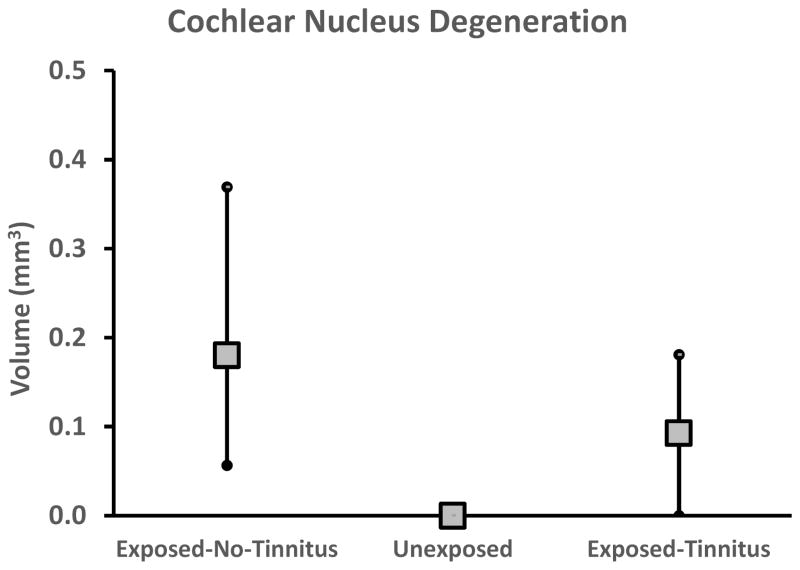
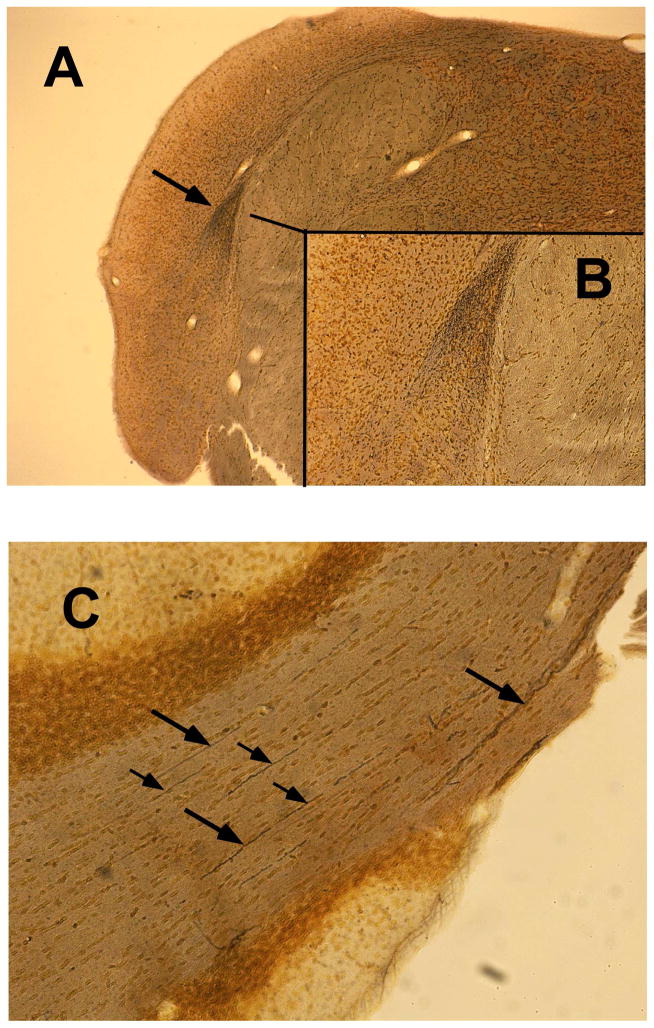
The pathological cascade, from cochlear dysfunction to CNS sequelae responsible for tinnitus, is not well understood. In an attempt to reveal features of this cascade, neurodegeneration was analyzed in the CN, FL, and PFL. Evidence of neuron fiber degeneration was found in the CN of sound-exposed animals, both with and without evidence of tinnitus, but not in unexposed animals (Fig. 8). Brain tissue was taken from the animals 3 months after exposure, when they were 6.3 months old. Degeneration of primary auditory nerve fibers was observed from the root entry zone extending into the ventral and dorsal cochlear nuclei. Fine diffuse degeneration was present in the dorsal acoustic stria, with more pronounced degeneration in the intermediate acoustic stria (Fig. 9A & 9B), the ventral cochlear nucleus and the granule cell region of the subpeduncular corner. Degeneration appeared exclusively in exposed animals, irrespective of tinnitus status, and was somewhat variable, as indicated by the range of variation depicted in Fig. 8. There was little evidence of degeneration in the DCN, outside of the dorsal acoustic stria, and minimal degeneration in the FL (not shown). Degeneration in the PFL was evident from the transition zone at the cerebellar peduncle extending to the lateral aspects of the PFL folia. PFL degeneration was visible in numerous parallel fibers coursing through the folia white matter (Fig. 9C). CN and PFL degeneration appear to reflect features of central pathology caused by auditory trauma, but because it was evident in both tinnitus and non-tinnitus animals, it suggests that tinnitus-specific processes occur downstream of the degeneration.
Figure 8.
Cochlear nucleus degeneration volume evident in Exposed Tinnitus, Exposed No-Tinnitus, and Unexposed groups. Error bars indicate total range and data points the group mean.
Figure 9.
Representative cochlear nucleus (A & B) and PFL (C) degeneration in an Exposed Tinnitus animal. Diffuse degeneration was evident in the dorsal and intermediate acoustic stria as well as in the ventral cochlear nucleus (A. arrow and B. inset) medial to the DCN. Fiber tract degeneration (C. arrows) was also evident in the PFL.
Discussion
The overarching hypothesis in the present research was that DCN and cerebellar circuits temper auditory perception using contextual information and historical events (Roth et al., 2013). Acute catastrophic events, such as system-damaging sound exposure, or chronic slow events, such as age-related neural loss, could lead to circuit destabilization and disruption of steady-state as well as dynamic processing. Previous research suggested that tinnitus-inducing sound exposure affected cerebellar and cochlear nucleus UBCs and that those effects could be evaluated using DCX as a marker (Bauer et al., 2013b). UBCs might be particularly susceptible because as tunable local circuit amplifiers (Dino et al., 2000) they modulate sensory afferent levels, perhaps comprising an automatic gain control circuit. In tinnitus animals it was hypothesized that UBCs could participate in compensation for damage-related loss of peripheral sensitivity. The present experiment examined both DCX and Eps8 as indicators of UBC plasticity. A PFL-specific UBC compensatory mechanism for noise damage could involve restructuring dendritic connections to improve signal transmission from mossy fibers to granule cells, a process potentially reflected by Eps8 elevation. It was further hypothesized that there might be an associated braking mechanism mediated by MAP2 and K2p upregulation. This process could be mediated by increased DCX levels. If this were the case, individuals not developing tinnitus after acoustic trauma would show greater DCX elevation, reflecting a more pronounced braking effect.
The obtained results supported the first hypothesis but not the second. Animals with psychophysical evidence had increased Eps8 IR in the cerebellar PFL. This could indicate enhanced gain mediated by glutamateric local-circuit synapses involving UBCs. Animals not developing tinnitus after exposure to identical sound, did not show the hypothesized elevated DCX IR, i.e., increased braking. Rather they showed decreased Eps8 IR in their DCN and FL, i.e., an apparent loss of gain rather than increased braking. No-Tinnitus animals also showed lower DCX IR in their FL, again contrary to the DCX-mediated braking hypothesis. Setting the braking hypothesis aside, loss of tonic inhibition has been hypothesized for some time as one of tinnitus’ pathophysiological mechanisms (Eggermont et al., 2004; Kraus et al., 2011; Moller, 2007; Wang et al., 2011; Yang et al., 2011). Although animals in the present experiment with decreased floccular DCX did not have tinnitus, they did have a concomitant floccular Eps8 decrease. This floccular decrement clearly set them apart from Tinnitus animals. If UBC upregulation, as marked by Eps8, is necessary for tinnitus, then disruption of that process could prevent the emergence of tinnitus. Further understanding UBC circuit dynamics will be necessary to fully explain this outcome.
The putative function of MAP2 is very similar to that ascribed to DCX and Eps8, i.e., assembly and polymerization of actin-rich microtubules. Microtubule mechanics can affect cytoskeletal structure as well as molecular transport (Sekerkova et al., 2007). “It has been suggested that the rich actin/Eps8 complement of UBC brushes is involved in plastic rearrangements reflecting the activity of the mossy fiber synapse” (Mugnaini et al., 2011). The post-synaptic brush of UBCs contains an unusual density of actin microfilaments perpendicularly inserted into the post-synaptic density. Associated is a rich complement of Eps8 receptors, which are also found in granule cell dendrites, although at a much lower concentration (Sekerkova et al., 2007). The functional significance of this design could be to mediate synaptic remodeling and plasticity.
UBC’s are local-circuit neurons of irregular shape with dendrites that terminate in large (20 – 40 μm) densely stained club-like “brush” structures (Mugnaini et al., 1997). To quote Mugnaini et al. (Mugnaini et al., 2011) : “Most of the UBC neurons had a single, randomly orientated dendrite of varying length that ended with a conspicuous brush formation that embraced a mossy fiber terminal.” These features of UBC morphology can be seen in the right-hand panes of Figures 3 and 4, and are indicated by marker arrows. The significance of the brush apparatus is that it provides a large contact area for a single glutamatergic input and potentially several inhibitory contacts from golgi cells (Rousseau et al., 2012).
Previously we reported that DCX IR was bilaterally elevated in the DCN and PFL of animals with tinnitus, compared to unexposed controls (Bauer et al., 2013b). DCX IR elevation was not found in the present experiment in Tinnitus animals, although Eps8 IR was elevated. A critical difference between the present and the previous experiment was that continuous serial sections were used in the present study, but not the previous. This was relevant because a large rostro-caudal DCX gradient was found the DCN (Fig. 7B & 7D). DCN rostro-caudal sampling bias in the previous experiment could account for the reported group differences in DCX IR.
Neuron fiber degeneration was evident in the CN and PFL of exposed animals, with and without tinnitus, but not in unexposed animals. This suggests that while afferent degeneration may constitute an initial step in the pathophysiological events leading to tinnitus, it is not a specific indicator of tinnitus, at least in the brainstem and cerebellum. The critical events leading to tinnitus are likely to appear elsewhere in the physiological cascade. The argument advanced here is that one of those critical events could be UBC synaptic remodeling in the PFL. The events connecting afferent degeneration to UBC activation remain to be described, as do potential pre-dispositional factors that place some individuals at greater risk.
It should be noted that the pattern and amount of degeneration reported in the present study probably underestimated the total population of degenerated fibers, since the tissue used in the present study was obtained from animals at just one time point, three months after acoustic trauma. Kim et al. (Kim et al., 2004) noted that waves of neural degeneration involving large and small diameter fibers occurred for months after cochlear injury. They concluded that de novo degeneration was emergent for an indefinite period after a single cochlear injury. If their observations are correct, complete quantification of degeneration would not be possible in the present or similarly designed studies where animals are sacrificed at a single time point after exposure. However, even if the presently-measured degeneration did not reflect the total population of lost neurons, the observed degeneration suggests that the pathophysiological events specifically responsible for tinnitus are different than degeneration per se. This, because high-level sound-driven degeneration was observed in both tinnitus and non-tinnitus animals. These results appear to be at odds with other research suggesting a functional relationship between aspects of cochlear damage and tinnitus (Bauer et al., 2007). The issue will have to be resolved by future experiments. However it is not surprising that degeneration was present in the CN. Furman et al., reported loss of high-threshold primary afferents in guinea pigs two weeks after high-level sound exposure (2 hrs), along with a 30 percent loss of inner hair cell ribbon synapses (Furman et al., 2013). Loss of cochlear spiral ganglion cells has been documented in rats with tinnitus (Bauer et al., 2007). The degeneration observed in the present study could be fibers from those neurons. Identifying the origin or targets of the degenerated fibers was beyond the scope of the present experiment.
Glutamatergically driven local amplifier circuits in the PFL, composed of UBCs, could be a necessary link in the pathophysiological cascade leading to chronic tinnitus. In the present study, animals with psychophysically defined tinnitus had enhanced Eps8 IR which could indicate synaptic remodeling in the PFL. Synaptic remodeling could enhance the efficiency of mossy fiber to granule cell transmission via UBC interneurons, and further elevate tonic excitation, since UBCs in vivo have high levels of spontaneous activity (Ruigrok et al., 2011). Research by Borges-Merjane and Trussell suggest an additional potentially important dimension. Their in vitro experiments (Borges-Merjane et al., 2015), using brain slices from presumably normal-hearing mice, indicate that UBCs, both in the DCN and vestibulocerebellum, fall into two groupings when driven by either application of glutamate or stimulation of extrinsic mossy fibers: ON type units and OFF type units. ON units, comprising about 60 percent of the cells studied, exhibited a prolonged depolarization and spiking, mediated primarily by AMPA sensitive metabotropic GluR1α receptors. OFF type units, comprising about 40 percent of the cells studied, in contrast exhibited a prolonged hyperpolarization and cessation of driven or spontaneous spiking, when similarly stimulated. In the cerebellum the OFF response was mediated by AMPA sensitive metabotropic GluR2 receptors. This added complexity of a bimodal population of UBCs might represent an additional dimension of flexibility in response to environmental events. It seems plausible that the ratio of ON to OFF units might shift when there is a chronic loss of afferent drive. An imbalance of ON units could push the PFL into a tonically elevated level of activity. Additionally it has been shown in the in vitro research of Rousseau et al. (Rousseau et al., 2012) that cerebellar UBCs receive inhibitory input from Golgi cells that is mediated by a fast GABAergic component as well as a slow glycinergic component. This dual-function inhibition appears at UBC dendrites that harbor metabotropic GluR1 receptors, while single-function slow inhibition is mediated by glycinergic receptors located on dendrites with metabotropic GluR2 receptors. Synthesizing the results of these two studies, ON UBCs (mGluR1) appear to have fast GABAaR mediated inhibitory modulation while OFF UBCs (mGluR2) are modulated by slow glycinergic inhibition. The dual ON/OFF features of UBC output, in combination with dual fast/slow inhibitory modulation, shows that UBCs are not simple excitatory relays, but rather comprise a dynamic signal transmission network between mossy fibers and granule cells. A derivative prediction would be that facilitating GABAergic transmission at the level of the PFL would attenuate tinnitus, while facilitating glycinergic transmission would exacerbate tinnitus.
A final question raised by the present research is how cerebellar output factors into tinnitus pathology. That tinnitus reflects alterations in neural function distributed across multiple brain systems is now generally accepted (Knipper et al., 2013; Roberts et al., 2010; Shore et al., 2016). GABAergic axons of Purkinje cells comprise the singular output of the cerebellum (Ito, 2006). Therefore the cerebellum exerts its influence largely or entirely through inhibition. Consistent with this, Vogler et al. have shown that surgical removal of the PFL, either 2 or 13 weeks after high-level sound exposure, exacerbates exposure-related elevated spontaneous activity of inferior colliculus central nucleus neurons (Vogler et al., 2016). Superficially it would appear that elevated PFL output would dampen activity at other CNS sites and attenuate that neurophysiological substrate of tinnitus. But this might not be the case. Systemic taurine has consistently been shown to decrease cerebellar Purkinje cell output (Frederickson et al., 1978; Okamoto et al., 1983; Sakai et al., 1985). Following the preceding logic, taurine induced reduction of cerebellar inhibitory output would predict increased tinnitus. Yet the opposite has been shown. Systemic taurine reversibly abolishes tinnitus in an animal model (Brozoski et al., 2010). Inhibitory neurons can inhibit other inhibitory neurons, with the net effect being downstream excitation. At the level of auditory thalamus, in vitro experiments have shown that increased inhibition is associated with behavioral evidence of tinnitus in an animal model (Sametsky et al., 2015). Understanding the complete central circuitry of tinnitus awaits further study.
Highlights.
Unipolar brush cells (UBC) may serve as a cellular substrate of chronic tinnitus
Tinnitus elevated a synaptic remodeling indicator, Eps8, in cerebellar UBCs
Eps8 was not elevated, but decreased, in UBCs of non-tinnitus rats
Synaptic remodeling in a UBC gain-modulation circuit could underpin chronic tinnitus
Sound exposure caused neural degeneration, but was not exclusive to tinnitus
Acknowledgments
Funding: This work was supported by the National Institutes of Health [grant # 1R01DC009669-01], and internal grants from Southern Illinois University School of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azizi SA, Burne RA, Woodward DJ. The auditory corticopontocerebellar projection in the rat: inputs to the paraflocculus and midvermis. An anatomical and physiological study. Exp Brain Res. 1985;59:36–49. doi: 10.1007/BF00237663. [DOI] [PubMed] [Google Scholar]
- Bauer CA, Brozoski TJ. Assessing tinnitus and prospective tinnitus therapeutics using a psychophysical animal model. J of the Assoc for Res in Otolaryngol. 2001;2:54–64. doi: 10.1007/s101620010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CA, Ciobanu L, Brozoski TJ. Central neural activity in rats with tinnitus evaluated with MEMRI. International Society for Magnetic Resonance Imaging; Seattle, Washington: 2006. [DOI] [PubMed] [Google Scholar]
- Bauer CA, Brozoski TJ, Myers K. Primary afferent dendrite degeneration as a cause of tinnitus. J Neurosci Res. 2007;85:1489–98. doi: 10.1002/jnr.21259. [DOI] [PubMed] [Google Scholar]
- Bauer CA, Kurt W, Sybert LT, Brozoski TJ. The cerebellum as a novel tinnitus generator. Hear Res. 2013a;295:130–9. doi: 10.1016/j.heares.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CA, Wisner KW, Baizer JS, Brozoski TJ. Tinnitus, unipolar brush cells, and cerebellar glutamatergic function in an animal model. PLoS One. 2013b;8:e64726. doi: 10.1371/journal.pone.0064726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CA, Turner JG, Caspary DM, Myers KS, Brozoski TJ. Tinnitus and inferior colliculus activity in chinchillas related to three distinct patterns of cochlear trauma. J Neurosci Res. 2008;86:2564–78. doi: 10.1002/jnr.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann O, Borra RJ, Bower JM, Cullen KE, Habas C, Ivry RB, Leggio M, Mattingley JB, Molinari M, Moulton EA, Paulin MG, Pavlova MA, Schmahmann JD, Sokolov AA. Consensus paper: the role of the cerebellum in perceptual processes. Cerebellum. 2015;14:197–220. doi: 10.1007/s12311-014-0627-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell CC, Han V, Sawtell NB. Cerebellum-like structures and their implications for cerebellar function. Annu Rev Neurosci. 2008;31:1–24. doi: 10.1146/annurev.neuro.30.051606.094225. [DOI] [PubMed] [Google Scholar]
- Borges-Merjane C, Trussell LO. ON and OFF unipolar brush cells transform multisensory inputs to the auditory system. Neuron. 2015;85:1029–42. doi: 10.1016/j.neuron.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard DM, Titley HK, Antflick J, Hampson DR. Motor learning in the VOR: the cerebellar component. Exp Brain Res. 2011;210:451–63. doi: 10.1007/s00221-011-2589-z. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Bauer CA. Animal models of tinnitus. Hear Res. 2015 doi: 10.1016/j.heares.2015.10.011. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Bauer CA, Caspary DM. Elevated fusiform cell activity in the dorsal cochlear nucleus of chinchillas with psychophysical evidence of tinnitus. J Neurosci. 2002;22:2383–90. doi: 10.1523/JNEUROSCI.22-06-02383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, Caspary DM, Bauer CA, Richardson BD. The effect of supplemental dietary taurine on tinnitus and auditory discrimination in an animal model. Hear Res. 2010;270:71–80. doi: 10.1016/j.heares.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, Wisner KW, Odintsov B, Bauer CA. Local NMDA receptor blockade attenuates chronic tinnitus and associated brain activity in an animal model. PLoS One. 2013;8:e77674. doi: 10.1371/journal.pone.0077674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng A, Liang X, Sun Y, Xiang Y, Yang J, Yan J, Sun W. Scopolamine attenuates auditory cortex response. Acta Otolaryngol. 2015;135:1132–7. doi: 10.3109/00016489.2015.1064996. [DOI] [PubMed] [Google Scholar]
- Dino MR, Mugnaini E. Distribution and phenotypes of unipolar brush cells in relation to the granule cell system of the rat cochlear nucleus. Neuroscience. 2008;154:29–50. doi: 10.1016/j.neuroscience.2008.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dino MR, Schuerger RJ, Liu Y, Slater NT, Mugnaini E. Unipolar brush cell: a potential feedforward excitatory interneuron of the cerebellum. Neuroscience. 2000;98:625–36. doi: 10.1016/s0306-4522(00)00123-8. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends Neurosci. 2004;27:676–82. doi: 10.1016/j.tins.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, Borland MS, Kilgard MP. Reversing pathological neural activity using targeted plasticity. Nature. 2011;470:101–4. doi: 10.1038/nature09656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliciangeli S, Chatelain FC, Bichet D, Lesage F. The family of K2P channels: salient structural and functional properties. J Physiol. 2015;593:2587–603. doi: 10.1113/jphysiol.2014.287268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M, Kaech S, Wagner U, Brinkhaus H, Matus A. Glutamate receptors regulate actin-based plasticity in dendritic spines. Nat Neurosci. 2000;3:887–94. doi: 10.1038/78791. [DOI] [PubMed] [Google Scholar]
- Frederickson RC, Neuss M, Morzorati SL, McBride WJ. A comparison of the inhibitory effects of taurine and GABA on identified Purkinje cells and other neurons in the cerebellar cortex of the rat. Brain Res. 1978;145:117–26. doi: 10.1016/0006-8993(78)90800-4. [DOI] [PubMed] [Google Scholar]
- Furman AC, Kujawa SG, Liberman MC. Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J Neurophysiol. 2013;110:577–86. doi: 10.1152/jn.00164.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuyashiki T, Arakawa Y, Takemoto-Kimura S, Bito H, Narumiya S. Multiple spatiotemporal modes of actin reorganization by NMDA receptors and voltage-gated Ca2+ channels. Proc Natl Acad Sci U S A. 2002;99:14458–63. doi: 10.1073/pnas.212148999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao JH, Parsons LM, Bower JM, Xiong J, Li J, Fox PT. Cerebellum implicated in sensory acquisition and discrimination rather than motor control. Science. 1996;272:545–7. doi: 10.1126/science.272.5261.545. [DOI] [PubMed] [Google Scholar]
- Godfrey DA, Kaltenbach JA, Chen K, Ilyas O. Choline acetyltransferase activity in the hamster central auditory system and long-term effects of intense tone exposure. J Neurosci Res. 2013;91:987–96. doi: 10.1002/jnr.23227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpain S, Hipolito A, Saffer L. Regulation of F-actin stability in dendritic spines by glutamate receptors and calcineurin. J Neurosci. 1998;18:9835–44. doi: 10.1523/JNEUROSCI.18-23-09835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman HJ, Reed GW. Epidemiology of Tinnitus. In: Snow JB Jr, editor. Tinnitus: Theory and Management. B.C. Decker; Hamilton, Ont: 2004. pp. 16–42. [Google Scholar]
- Holt AG, Bissig D, Mirza N, Rajah G, Berkowitz B. Evidence of key tinnitus-related brain regions documented by a unique combination of manganese-enhanced MRI and acoustic startle reflex testing. PLoS One. 2010;5:e14260. doi: 10.1371/journal.pone.0014260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CM, Liu G, Huang R. Projections from the cochlear nucleus to the cerebellum. Brain Res. 1982;244:1–8. doi: 10.1016/0006-8993(82)90897-6. [DOI] [PubMed] [Google Scholar]
- Ito M. The cerebellum and neural control. Raven Press; New York: 1984. [Google Scholar]
- Ito M. Cerebellar circuitry as a neuronal machine. Prog Neurobiol. 2006;78:272–303. doi: 10.1016/j.pneurobio.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Ito M. Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci. 2008;9:304–13. doi: 10.1038/nrn2332. [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ, Sasaki CT. Salicylate-induced changes in spontaneous activity of single units in the inferior colliculus of the guinea pig. J Acoust Soc Am. 1986;80:1384–91. doi: 10.1121/1.394391. [DOI] [PubMed] [Google Scholar]
- Kalappa BI, Brozoski TJ, Turner JG, Caspary DM. Single unit hyperactivity and bursting in the auditory thalamus of awake rats directly correlates with behavioural evidence of tinnitus. J Physiol. 2014;592:5065–78. doi: 10.1113/jphysiol.2014.278572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach JA. Summary of evidence pointing to a role of the dorsal cochlear nucleus in the etiology of tinnitus. Acta Otolaryngol Suppl. 2006:20–6. doi: 10.1080/03655230600895309. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Godfrey DA. Dorsal cochlear nucleus hyperactivity and tinnitus: are they related? Am J Audiol. 2008;17:S148–61. doi: 10.1044/1059-0889(2008/08-0004). [DOI] [PubMed] [Google Scholar]
- Kim JJ, Gross J, Potashner SJ, Morest DK. Fine structure of degeneration in the cochlear nucleus of the chinchilla after acoustic overstimulation. J Neurosci Res. 2004;77:798–816. doi: 10.1002/jnr.20213. [DOI] [PubMed] [Google Scholar]
- Knipper M, Van Dijk P, Nunes I, Ruttiger L, Zimmermann U. Advances in the neurobiology of hearing disorders: recent developments regarding the basis of tinnitus and hyperacusis. Prog Neurobiol. 2013;111:17–33. doi: 10.1016/j.pneurobio.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Kraus KS, Ding D, Jiang H, Lobarinas E, Sun W, Salvi RJ. Relationship between noise-induced hearing-loss, persistent tinnitus and growth-associated protein-43 expression in the rat cochlear nucleus: does synaptic plasticity in ventral cochlear nucleus suppress tinnitus? Neuroscience. 2011;194:309–25. doi: 10.1016/j.neuroscience.2011.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langers DR, de Kleine E, van Dijk P. Tinnitus does not require macroscopic tonotopic map reorganization. Front Syst Neurosci. 2012;6:2. doi: 10.3389/fnsys.2012.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langguth B, Goodey R, Azevedo A, Bjorne A, Cacace A, Crocetti A, Del Bo L, De Ridder D, Diges I, Elbert T, Flor H, Herraiz C, Ganz Sanchez T, Eichhammer P, Figueiredo R, Hajak G, Kleinjung T, Landgrebe M, Londero A, Lainez MJ, Mazzoli M, Meikle MB, Melcher J, Rauschecker JP, Sand PG, Struve M, Van de Heyning P, Van Dijk P, Vergara R. Consensus for tinnitus patient assessment and treatment outcome measurement: Tinnitus Research Initiative meeting, Regensburg, July 2006. Progress in brain research. 2007;166:525–36. doi: 10.1016/S0079-6123(07)66050-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanting CP, de Kleine E, van Dijk P. Neural activity underlying tinnitus generation: results from PET and fMRI. Hear Res. 2009;255:1–13. doi: 10.1016/j.heares.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Melcher JR, Sigalovsky IS, Guinan JJ, Jr, Levine RA. Lateralized tinnitus studied with functional magnetic resonance imaging: abnormal inferior colliculus activation. J Neurophysiol. 2000;83:1058–72. doi: 10.1152/jn.2000.83.2.1058. [DOI] [PubMed] [Google Scholar]
- Middleton JW, Kiritani T, Pedersen C, Turner JG, Shepherd GM, Tzounopoulos T. Mice with behavioral evidence of tinnitus exhibit dorsal cochlear nucleus hyperactivity because of decreased GABAergic inhibition. Proc Natl Acad Sci U S A. 2011;108:7601–6. doi: 10.1073/pnas.1100223108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller AR. The role of neural plasticity in tinnitus. Progress in brain research. 2007;166:37–45. doi: 10.1016/S0079-6123(07)66003-8. [DOI] [PubMed] [Google Scholar]
- Moores CA, Perderiset M, Francis F, Chelly J, Houdusse A, Milligan RA. Mechanism of microtubule stabilization by doublecortin. Mol Cell. 2004;14:833–9. doi: 10.1016/j.molcel.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Morest DK, Kim J, Bohne BA. Neuronal and transneuronal degeneration of auditory axons in the brainstem after cochlear lesions in the chinchilla: cochleotopic and non-cochleotopic patterns. Hear Res. 1997;103:151–68. doi: 10.1016/s0378-5955(96)00172-4. [DOI] [PubMed] [Google Scholar]
- Mugnaini E, Dino MR, Jaarsma D. The unipolar brush cells of the mammalian cerebellum and cochlear nucleus: cytology and microcircuitry. Progress in brain research. 1997;114:131–50. doi: 10.1016/s0079-6123(08)63362-2. [DOI] [PubMed] [Google Scholar]
- Mugnaini E, Sekerkova G, Martina M. The unipolar brush cell: a remarkable neuron finally receiving deserved attention. Brain Res Rev. 2011;66:220–45. doi: 10.1016/j.brainresrev.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norena AJ. An integrative model of tinnitus based on a central gain controlling neural sensitivity. Neurosci Biobehav Rev. 2011;35:1089–109. doi: 10.1016/j.neubiorev.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Norena AJ, Eggermont JJ. Enriched acoustic environment after noise trauma abolishes neural signs of tinnitus. Neuroreport. 2006;17:559–63. doi: 10.1097/00001756-200604240-00001. [DOI] [PubMed] [Google Scholar]
- Oertel D, Young ED. What's a cerebellar circuit doing in the auditory system? Trends Neurosci. 2004;27:104–10. doi: 10.1016/j.tins.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Offenhauser N, Castelletti D, Mapelli L, Soppo BE, Regondi MC, Rossi P, D'Angelo E, Frassoni C, Amadeo A, Tocchetti A, Pozzi B, Disanza A, Guarnieri D, Betsholtz C, Scita G, Heberlein U, Di Fiore PP. Increased ethanol resistance and consumption in Eps8 knockout mice correlates with altered actin dynamics. Cell. 2006;127:213–26. doi: 10.1016/j.cell.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Kimura H, Sakai Y. Taurine-induced increase of the Cl-conductance of cerebellar Purkinje cell dendrites in vitro. Brain Res. 1983;259:319–23. doi: 10.1016/0006-8993(83)91266-0. [DOI] [PubMed] [Google Scholar]
- Rasmussen G. Remarks on the cochleo-cerebellar connections. Research Notebooks, History of Medicine Division, National Library of Medicine; Washington D.C: 1990. pp. 1–42. [Google Scholar]
- Rauschecker JP. Auditory cortical plasticity: a comparison with other sensory systems. Trends Neurosci. 1999;22:74–80. doi: 10.1016/s0166-2236(98)01303-4. [DOI] [PubMed] [Google Scholar]
- Renigunta V, Schlichthorl G, Daut J. Much more than a leak: structure and function of K(2)p- channels. Pflugers Arch. 2015;467:867–94. doi: 10.1007/s00424-015-1703-7. [DOI] [PubMed] [Google Scholar]
- Richardson BD, Ling LL, Uteshev VV, Caspary DM. Extrasynaptic GABA(A) receptors and tonic inhibition in rat auditory thalamus. PLoS One. 2011;6:e16508. doi: 10.1371/journal.pone.0016508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LE, Eggermont JJ, Caspary DM, Shore SE, Melcher JR, Kaltenbach JA. Ringing ears: the neuroscience of tinnitus. J Neurosci. 2010;30:14972–9. doi: 10.1523/JNEUROSCI.4028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth MJ, Synofzik M, Lindner A. The cerebellum optimizes perceptual predictions about external sensory events. Curr Biol. 2013;23:930–5. doi: 10.1016/j.cub.2013.04.027. [DOI] [PubMed] [Google Scholar]
- Rousseau CV, Dugue GP, Dumoulin A, Mugnaini E, Dieudonne S, Diana MA. Mixed inhibitory synaptic balance correlates with glutamatergic synaptic phenotype in cerebellar unipolar brush cells. J Neurosci. 2012;32:4632–44. doi: 10.1523/JNEUROSCI.5122-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruigrok TJ, Hensbroek RA, Simpson JI. Spontaneous activity signatures of morphologically identified interneurons in the vestibulocerebellum. J Neurosci. 2011;31:712–24. doi: 10.1523/JNEUROSCI.1959-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai Y, Okamoto K, Kimura H. Pharmacological evidence for taurine as an inhibitory neurotransmitter in the cerebellum. Prog Clin Biol Res. 1985;179:313–9. [PubMed] [Google Scholar]
- Sametsky EA, Turner JG, Larsen D, Ling L, Caspary DM. Enhanced GABAA-Mediated Tonic Inhibition in Auditory Thalamus of Rats with Behavioral Evidence of Tinnitus. J Neurosci. 2015;35:9369–80. doi: 10.1523/JNEUROSCI.5054-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santra M, Liu XS, Santra S, Zhang J, Zhang RL, Zhang ZG, Chopp M. Ectopic expression of doublecortin protects adult rat progenitor cells and human glioma cells from severe oxygen and glucose deprivation. Neuroscience. 2006;142:739–52. doi: 10.1016/j.neuroscience.2006.06.065. [DOI] [PubMed] [Google Scholar]
- Schofield BR, Motts SD, Mellott JG. Cholinergic cells of the pontomesencephalic tegmentum: connections with auditory structures from cochlear nucleus to cortex. Hear Res. 2011;279:85–95. doi: 10.1016/j.heares.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekerkova G, Dino MR, Ilijic E, Russo M, Zheng L, Bartles JR, Mugnaini E. Postsynaptic enrichment of Eps8 at dendritic shaft synapses of unipolar brush cells in rat cerebellum. Neuroscience. 2007;145:116–29. doi: 10.1016/j.neuroscience.2006.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi Y, Mizutani A, Yuasa S, Mikoshiba K, Furuichi T. Glutamate-induced declustering of post-synaptic adaptor protein Cupidin (Homer 2/vesl-2) in cultured cerebellar granule cells. J Neurochem. 2003;87:364–76. doi: 10.1046/j.1471-4159.2003.02003.x. [DOI] [PubMed] [Google Scholar]
- Shore SE, Roberts LE, Langguth B. Maladaptive plasticity in tinnitus--triggers, mechanisms and treatment. Nat Rev Neurol. 2016;12:150–60. doi: 10.1038/nrneurol.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore SE, Koehler S, Oldakowski M, Hughes LF, Syed S. Dorsal cochlear nucleus responses to somatosensory stimulation are enhanced after noise-induced hearing loss. Eur J Neurosci. 2008;27:155–68. doi: 10.1111/j.1460-9568.2007.05983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman A, Strashun A. Descending auditory system/cerebellum/tinnitus. Int Tinnitus J. 1999;5:92–106. [PubMed] [Google Scholar]
- Star EN, Kwiatkowski DJ, Murthy VN. Rapid turnover of actin in dendritic spines and its regulation by activity. Nat Neurosci. 2002;5:239–46. doi: 10.1038/nn811. [DOI] [PubMed] [Google Scholar]
- Tan J, Ruttiger L, Panford-Walsh R, Singer W, Schulze H, Kilian SB, Hadjab S, Zimmermann U, Kopschall I, Rohbock K, Knipper M. Tinnitus behavior and hearing function correlate with the reciprocal expression patterns of BDNF and Arg3.1/arc in auditory neurons following acoustic trauma. Neuroscience. 2007;145:715–26. doi: 10.1016/j.neuroscience.2006.11.067. [DOI] [PubMed] [Google Scholar]
- Tunkel DE, Bauer CA, Sun GH, Rosenfeld RM, Chandrasekhar SS, Cunningham ER, Jr, Archer SM, Blakley BW, Carter JM, Granieri EC, Henry JA, Hollingsworth D, Khan FA, Mitchell S, Monfared A, Newman CW, Omole FS, Phillips CD, Robinson SK, Taw MB, Tyler RS, Waguespack R, Whamond EJ. Clinical practice guideline: tinnitus. Otolaryngol Head Neck Surg. 2014;151:S1–S40. doi: 10.1177/0194599814545325. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T, Kim Y, Oertel D, Trussell LO. Cell-specific, spike timing-dependent plasticities in the dorsal cochlear nucleus. Nat Neurosci. 2004;7:719–25. doi: 10.1038/nn1272. [DOI] [PubMed] [Google Scholar]
- Voelker C, Sekerkova G, Martina M, Richter CP. Cells Responding to Auditory Stimulus in the Paraflocculus of the Cerebellum. Vol. 39. Association for Research in Otolaryngology; San Diego, CA: 2016. pp. 183–184. ARO Abstracts. [Google Scholar]
- Vogler DP, Robertson D, Mulders WH. Influence of the paraflocculus on normal and abnormal spontaneous firing rates in the inferior colliculus. Hear Res. 2016;333:1–7. doi: 10.1016/j.heares.2015.12.021. [DOI] [PubMed] [Google Scholar]
- Wang H, Brozoski TJ, Caspary DM. Inhibitory neurotransmission in animal models of tinnitus: maladaptive plasticity. Hear Res. 2011;279:111–7. doi: 10.1016/j.heares.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Weiner BD, Zhang LS, Cho SJ, Bao S. Homeostatic plasticity drives tinnitus perception in an animal model. Proc Natl Acad Sci U S A. 2011;108:14974–9. doi: 10.1073/pnas.1107998108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C, Nannapaneni N, Zhou J, Hughes LF, Shore S. Cochlear damage changes the distribution of vesicular glutamate transporters associated with auditory and nonauditory inputs to the cochlear nucleus. J Neurosci. 2009;29:4210–7. doi: 10.1523/JNEUROSCI.0208-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhang Y, Zhang X. Auditory cortex electrical stimulation suppresses tinnitus in rats. J Assoc Res Otolaryngol. 2011;12:185–201. doi: 10.1007/s10162-010-0246-z. [DOI] [PMC free article] [PubMed] [Google Scholar]