Abstract
High energy trauma to cartilage causes surface fissures and microstructural damage, but the degree to which this damage renders the tissue more susceptible to wear and contributes to the progression of post-traumatic osteoarthritis (PTOA) is unknown. Additionally, no treatments are currently available to strengthen cartilage after joint trauma and to protect the tissue from subsequent degradation and wear. The purposes of this study were to investigate the role of mechanical damage in the degradation and wear of cartilage, to evaluate the effects of impact and subsequent genipin crosslinking on the changes in the viscoelastic parameters of articular cartilage, and to test the hypothesis that genipin crosslinking is an effective treatment to enhance the resistance to biochemical degradation and mechanical wear. Results demonstrate that cartilage stiffness decreases after impact loading, likely due to the formation of fissures and microarchitectural damage, and is partially or fully restored by crosslinking. The wear resistance of impacted articular cartilage was diminished compared to undamaged cartilage, suggesting that mechanical damage that is directly induced by the impact may contribute to the progression of PTOA. However, the decrease in wear resistance was completely reversed by the crosslinking treatments. Additionally, the crosslinking treatments improved the resistance to collagenase digestion at the impact-damaged articular surface. These results highlight the potential therapeutic value of collagen crosslinking via genipin in the prevention of cartilage degeneration after traumatic injury.
Keywords: post-traumatic osteoarthritis, articular cartilage, collagen, crosslinking, genipin
Of the estimated 21 million Americans that suffer from osteoarthritis (OA), approximately 12% of those cases are traumatic in origin.1,2 High energy trauma to a synovial joint causes an array of mechanical, cellular, and biochemical responses that can ultimately lead to posttraumatic osteoarthritis (PTOA). A single traumatic event can result in an increased expression of pro-inflammatory cytokines that are thought to activate degradative enzymes in the cartilage and lead to reduced mechanical properties and ultimately wear resistance.3–6 Additionally, mechanical cracks or fissures have been observed at the injury sites on the cartilage surface immediately following trauma, extending down at approximately a 45 degree angle into superficial, middle, or deep zones.3,7–13 Even without evidence of fissures, microstructural damage can occur at the articular surface at the site of injury.14 In spite of the prevalence of this acute damage after a traumatic overload to the joint, its effect on mechanical wear of cartilage and the development of PTOA is unknown.
PTOA often progresses to the point where the joint needs to be replaced, and total joint replacements are widely successful procedures. However, PTOA affects many younger individuals for whom arthroplasty is a poor option due to the limited lifespan of the implants.15–17 Current surgical treatments at the time of injury, such as anatomic reduction of intra-articular fractures, ligament repair, joint stabilization, and osteotomies, treat intra-articular fractures or improve joint instability or incongruity,2,18–22 but do not address the mechanical damage to the articular surface or intervene in the post-traumatic cellular response. Recent studies have demonstrated that biological therapies can limit chondrocyte damage caused by a mechanical overload and impair the subsequent activation of catabolic pathways. For example, D’Lima et al. demonstrated that a caspase inhibitor reduces chondrocyte aptoposis23 while Haut and coworkers have shown that a surfactant decreases cellular necrosis after a mechanical insult to cartilage tissue.24,25 Martin et al. have reported that antioxidants are effective at reducing chondrocyte death and concomitant matrix degradation.26–28 Ding et al. investigated another biological target, mitogen activated protein kinases, which can be inhibited to reduce injury-related chondrocyte death and proteoglycan loss.29 In spite of the promise of these biologic treatments, they do not address the mechanical damage at the articular surface, or restore the mechanical integrity of the tissue.
Our previous work investigated collagen crosslinking of cartilage using genipin, a natural plant extract, as a potential therapeutic treatment. Both 2 and 10 mM genipin crosslinking treatments improved the wear resistance of healthy, intact cartilage in vitro. These treatments increased the stiffness via indentation and significantly protected the tissue from collagenase digestion. The 2 mM genipin treatment was non-toxic to chondrocytes, indicating its potential as a clinical treatment to prevent osteoarthritis.30 However, the effect of crosslinking on cartilage that has been damaged by a traumatic overload has not been investigated. The goal of this study was to investigate the effect of mechanical damage and genipin crosslinking on cartilage’s viscoelastic parameters, wear resistance, coefficient of friction, and biochemical enzymatic degradation.
METHODS
Impact Damage
Bovine stifles from approximately 1-year old animals were obtained from a local abattoir (Martins Meats, Wakarusa, IN) and were stored frozen at −23°C until use. Osteochondral specimens with a 9.5 mm diameter and approximately 25 mm in length were cored from the condyles such that the articular surface was perpendicular to the coring axis, with no more than two specimens taken per condyle. The specimens were placed with the articular surface facing upwards into the loading chamber of a custom designed drop tower, which was similar to previously reported devices (Fig. S1).31–33 Preliminary testing determined that cartilage was visually damaged in unconfined compression using a spherical impact head that was 3.2 cm in diameter, dropped from a height of 25 cm with a total impactor mass of 499 g. Specimens that resulted in bone fracture or delamination of the cartilage from the bone were discarded. The drop tower was instrumented with an accelerometer (Kistler 8743A5; Novi, MI) and a load cell (Kistler 9712B5000) for the collection of impact data at 100 kHz (n = 8). The accelerometer data was integrated twice with respect to time to determine the velocity and displacement using a custom MATLAB script. The maximum and average contact pressures at peak impact were calculated from the load and displacement data using Hertz contact theory.34 The impact energy was evaluated by integrating the load with respect to displacement. Average strain through the depth of the tissue was calculated by dividing the tissue displacement by thickness, and the average strain rate was calculated from the maximum average strain divided by the time duration of the impact to that point. A range of strains and strain rates were calculated for cartilage thicknesses from 1.2 to 1.5 mm, based on our previous observation that 8–10 sections of 150 μm thickness can be taken through the depth of our samples.
Genipin Crosslinking
Following impact, cartilage specimens were incubated in 0, 2, or 10 mM genipin (Challenge Bioproducts, Taiwan) solutions in phosphate-buffered saline (PBS) at 37°C for 15 min in a shaking water bath. After incubation in the genipin, specimens were transferred to PBS and incubated at 37°C to bring the total incubation time to 24 h.30
Histology and Immunohistochemistry
Alcian blue staining was performed to visualize cartilage damage and glycosaminoglycan (GAG) levels post-impact. Specimens were impacted as described above and then incubated in PBS at 37°C for 24 h. After incubation, the specimens were cut to 6 mm in length and were fixed in paraformaldehyde, decalcified in a formic acid solution, and embedded in OCT. Cryosections (7 μm) taken perpendicular to the cartilage surface were mounted to glass microscope slides, stained with Alcian Blue solution (Sigma Aldrich, St. Louis, MO) and counterstained with Nuclear Fast Red (Electron Microscopy Sciences, Hatfield, PA). Images were taken using an optical microscope (Nikon ME1600, Nikon Instruments Inc.; Melville, NY) connected to a digital camera (Optronics; Goleta, CA).
Immunohistochemistry was performed to assess damaged collagen using antibody COL2-3/4M, and collagenase cleavage using antibody COL2-3/4Cshort (both from Ibex Technologies, Inc, Mont-Royal, Canada). COL2-3/4M nonspecifically targets an epitope within type 2 collagen’s triple-helix which is exposed in the damaged molecule. Since both mechanical overloading and collagenase cleavage can result in collagen damage, no distinction can be made between these two forms of damage with this antibody alone. Therefore, COL2-3/4Cshort, which is specifically an indicator of collagenase cleavage, was used in conjunction with COL2-3/4M to visualize the damage that was exclusively due to mechanical disruption. Immunohistochemistry using COL2-3/4M and COL2-3/4Cshort was performed on 7 μm cartilage cryosections that had been removed from the subchondral bone. The sections underwent a mild fixation in 95% ethanol before they were subjected to antigen retrieval with 0.5% hyaluronidase in PBS for 20 min at 37°C. Nonspecific binding sites and endogenous biotin were blocked at room temperature with goat serum, 0.01% Avidin (Pierce Biotechnology, Rockford, IL), and 0.001% Biotin (Sigma Aldrich). Sections were then incubated in 1:400 dilutions of primary antibodies, either COL2-3/4M or COL2-3/4Cshort, for 1 h at 37°C. Biotinylated secondary antibodies directed against the appropriate species (Vector Laboratories, Burlingame, CA; 1:100) were applied for 1 h at 37°C. Only the secondary antibody was applied for the negative control. Sections were incubated in avidin-biotin complex according to the manufacturer’s instruction (Vectastain Elite ABC Kit; Vector Laboratories) and developed with DAB peroxidase (Vector Laboratories) for 10 min at 37°C. Slides were rinsed between each step.
SGAG Release From Impacted Specimens
A modified dimethylmethylene blue (DMMB) assay was performed to determine the effect of the 24 h incubation on sulfated glycosaminoglycan (sGAG) loss from impacted and control specimens (n = 8). Osteochondral specimens were impacted then immediately incubated in 1 ml of PBS at 37°C for 24 h, as in the 0 mM condition. Specimens that were not impacted but were incubated in PBS at 37°C for 24 h acted as controls. Briefly, aliquots of the incubation solution were mixed with a dye solution consisting of 80 μM DMMB, 1% ethanol, 40 mM guanidine-HCl, 315 μM formic acid, and 25 μM sodium hydroxide at a pH of 3.5 for 30 min and then centrifuged. The supernatant was removed and the remaining pellet was resuspended in a dissociation buffer of 10% isopropanol and 4M guanidine-HCl. The resultant solution was measured colorimetrically at 600 nm and the amount of sGAG was estimated from chondroitin sulfate standards.
Viscoelastic Parameters From Indentation
Stress-relaxation tests were performed on separate 9.5 mm diameter osteochondral specimens with a Hysitron TI950 TriboIndenter (Minneapolis, MN) equipped with a 3-D OmniProbe® transducer and a 750 μm diameter flat punch probe with a 20 μm edge radius.35 The load function consisted of a 20-sec ramp to peak displacement, followed by a 50-sec hold at peak displacement and a 1-sec unloading segment. Testing was carried out with a 0.5 mN preload followed by a 67 μm peak indentation depth. This protocol was previously found to produce repeatable measurements of the unloading stiffness that were sensitive to changes due to crosslinking.36 Indentations were performed in quadruplicate at three locations selected at random on each specimen. Immediately following indentation testing, the articular surface of the cartilage was impacted via drop tower, using the previously described protocol. After impact the specimens underwent indentation again at the same locations. Following the post-impact indentation testing, specimens were crosslinked in genipin, as described above, equilibrated in PBS at room temperature for an additional 2 h, and then indentation testing was repeated at the same three locations on four specimens per condition (n = 12). The relaxation portion of the indentation stress relaxation data was analyzed with a standard linear solid model (SLS) as previous described.30 The changes in instantaneous stiffness, equilibrium stiffness, and relaxation time constant were reported. The unloading stiffness was evaluated as the slope from a linear fit of the top 10% of the unloading curve.37 The unloading stiffness is directly proportional to the elastic modulus of the tissue for a flat punch indenter.38 Values for each apparent parameter were normalized by the corresponding pre-impact value.
Collagenase Digestion
In additional experiments, 9.5 mm diameter osteochondral specimens were impacted and then 5.9 mm diameter cylinders were cored from the center of the specimens such that the articular surface was perpendicular to the coring axis. The smaller diameter was used to ensure flatness of the articular surface so that uniform sections could be taken. The specimens were cut to a length of 6 mm using an Isomet Low Speed Saw (Buehler, Lake Bluff, IL). Specimens were incubated in 0, 2, or 10 mM solutions of genipin in PBS for 15 min and then in genipin-free PBS for the remaining 24 h, as above (n = 4). Specimens that were not impacted and were incubated in PBS for 24 h acted as controls. Using a sledge microtome (HM 450 Richard Allan, Kalamazoo, MI) equipped with a freezing stage (Physitemp, Clifton, NJ) set at −25°C, 150 μm sections were taken through the depth of the articular cartilage as has previously been described.30 Individual sections were incubated for 45 min at 37°C in 0.5 ml of a 2 mg/ml solution of type I collagenase from Clostridium histolyticum in 50 mM Trizma® buffer at pH 7.42 containing 10 mM CaCl2 (all from Sigma Aldrich). To quantify the collagen that had been digested, the samples of the digest solution were hydrolyzed at 100°C for 18 h in concentrated HCl (38%) and assessed for hydroxyproline with a chloromine-T assay. Hydroxyproline is an amino acid constituent that is found almost exclusively in collagen. As impact damage and subsequent wear occur primarily at the articular surface, the quantity of collagen that had been digested at the articular surface was reported in addition to data taken throughout depth of the tissue.
Friction and Wear Testing
Friction testing was conducted on 9.5 mm diameter osteochondral specimens that had been impacted then immediately incubated in either 0 or 10 mM genipin solution in PBS as described above. Specimens that were not impacted and were incubated in 0 mM genipin acted as controls. The coefficient of friction (COF) between cartilage and stainless steel (T316; Ra = 0.016 ± 0.004 μm) was measured in a hydrating solution consisting of 0.15 M NaCl with protease inhibitors (1 mM ethylenediaminetetraacetic acid, 5 mM benzamadine, and 10 mM n-ethylmaleimide). To provide insight into the subsequent wear test, reciprocal sliding motion was carried out using a Universal Micro-Tribometer (Bruker, Inc., Campbell, CA) under a constant normal load of 70 N (approximately 1.6 MPa contact pressure, or greater than the average pressure in the knee but below measured peak pressures39) for 30 min at a sliding speed of 4 mm/sec, with each back and forth portion being 18 mm long (n = 4). The friction and normal forces were averaged over each cycle of reciprocal motion, the COF was obtained from the ratio of these values, and the initial value from the first reciprocal cycle was reported. Between tests, the stainless steel was thoroughly cleaned with 70% ethanol, followed by a distilled water rinse.
To perform wear testing, specimens were impacted then crosslinked in 0, 2, or 10 mM genipin solutions. Specimens that were not impacted and were incubated in 0 mM genipin served as controls. Cartilage was worn against 316L stainless steel discs (Ra= 0.015 ± 0.002 μm) as previously described (n = 6 per condition).40 Briefly, specimens were loaded into a pin-on-disk tribometer (OrthoPOD from AMTI; Watertown, MA) with the hydrating fluid and tested in cycles consisting of four 18 mm strokes in a square path with a sliding velocity of 4 mm/sec. A load of 70 N was applied at a rate of 150 N/sec and removed for the final 45% (~8 mm) of each stroke to permit specimen rehydration. Testing was conducted at room temperature for a total of 9,600 cycles and a wear distance of 384 m. The load and cycle number were chosen based on preliminary studies that determined these conditions resulted in consistent wear on impacted, non-crosslinked specimens.
To quantify cartilage wear, the amount of hydroxyproline that was released to the hydrating saline solution was assessed. The hydrating baths were lyophilized, re-suspended in papain digest solution, and incubated at 60°-C overnight. Samples of the digest solution were hydrolyzed and assessed with a chloromine-T assay, as above. Additionally, verification of wear was performed with India ink staining the articular surface; the areas that were stained after wiping the surface with a damp cloth indicated damage based on the adherence of India ink to fibrillated cartilage.40
Statistics
Differences between groups that had been impacted and treated with the different genipin concentrations were determined using a one-way ANOVA with Tukey’s post-hoc test with significance set at p < 0.05 (Graphpad Prism Software, La Jolla, CA). A one sample t-test was used to determine the difference of unloading stiffness, instantaneous stiffness, equilibrium stiffness, and relaxation time constant normalized to corresponding initial values from 1.0. A two-way ANOVA with Tukey post-hoc test was performed to determine the significance of the different groups and the cartilage depth on the amount of hydroxyproline released during collagenase digestion. Data are presented as mean ± standard deviation.
RESULTS
Impact Damage
The impact protocol resulted in a maximum contact pressure at peak displacement of 52.8 ± 17.0 MPa, with an average contact pressure of 35.2 ± 11.4 MPa at the same time point. The average impact energy and velocity were 0.89 ± 0.30 J and 1.8 ± 0.35 m/sec, respectively. Assuming that only the cartilage deformed, the maximum strains were 0.903 ± 0.312 and 0.723 ± 0.250 for cartilage thicknesses of 1.2 and 1.5 mm, respectively, while the mean strain rates were 1370 ± 580 and 1100 ± 460 sec−1, respectively. Alcian blue staining of impacted cartilage demonstrated fissure formation at the articular surface, but also revealed that proteoglycans (sGAGs) were retained by the bulk of the cartilage post-impact (Fig. 1A). Immunohistochemistry indicated that collagen became damaged under the impact in the superficial zone (Fig. 1B), while staining for collagenase cleavage was much fainter and more diffuse (Fig. 1C). Specimens that did not receive an impact did not show evidence of damaged collagen (Fig. S2A), nor was non-specific binding observed (Fig. S2B). SGAG loss due to the 24 h incubation was 59.2 ± 38.7 and 93.8 ± 47.5 μg in the control and impacted specimens, respectively. No significant differences were detected between the two groups (p = 0.133).
Figure 1.

Histology (A) and immunohistochemistry (B and C) of articular cartilage after a single impact. (A) Alcian blue staining. (B) Damaged collagen. (C) Collagenase-cleaved collagen. Scale bar = 200 μm.
Viscoelastic Parameters From Indentation
The average instantaneous, equilibrium, and unloading stiffness all decreased with impact by 52.1, 47.6, and 40.2%, respectively (Fig. 2A–C) as compared to pre-impact values. Additionally, all the stiffness measurements decreased further for the untreated (0 mM genipin) specimens after the 24 h incubation, with the equilibrium stiffness exhibiting the greatest change of 84%. However, treatment with genipin reversed this effect of the impact. At 2 mM, genipin treatment tended to restore the stiffness, and the 10 mM genipin treatment significantly increased all stiffness measurements compared to the untreated (0 mM genipin) specimens. The impact load did not affect the relaxation time constant, but crosslinking with either the 2 or 10 mM genipin treatments caused a significant decrease (Fig. 2D).
Figure 2.

Viscoelastic parameters of articular cartilage. Indentation testing was performed pre-impact, immediately after single impact, and after treatment with the designated concentrations of genipin, all at the same locations of the cartilage surface. (A) Instantaneous stiffness; (B) Equilibrium stiffness; (C) Unloading stiffness; (D) Relaxation time constant. Data represent the mean ± SD of ratio to pre-impact data. ** and ***: different from 1.0 (**p < 0.01, ***p < 0.001). Different letters indicate statistical significance between groups (p < 0.05).
Collagenase Digestion
There were no significant differences between the non-impacted and impacted 0 mM groups in the amount of hydroxyproline released to solution during collagenase digestion at any depth within the cartilage (Fig. 3A). Throughout the depth of the cartilage, treatment with 10 mM genipin tended to decrease hydroxyproline released compared to the other treatment groups. In the surface cartilage sections, collagenase digestion was equivalent in both the non-impacted and impacted 0 mM genipin groups (Fig. 3B). The amount of digestion decreased in the 2 mM genipin group in the surface sections, but only compared to the non-impacted controls. Treatment with 10 mM genipin significantly decreased collagenase digestion compared to both the non-impacted control and untreated (0 mM genipin) groups in the surface sections.
Figure 3.
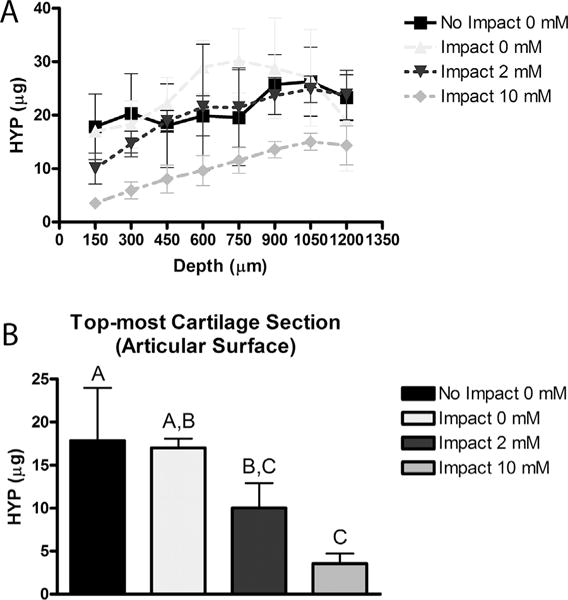
Hydroxyproline (HYP) released by collagenase digestion. Articular cartilage was either un-injured (No Impact), or subjected to a single impact. Specimens were then treated with the designated concentrations of genipin and sliced into 150 μm sections starting at the cartilage surface. (A) Hydroxyproline released from 150 μm thick sections from the designated cartilage depth. (B) Hydroxyproline released from the surface section of the cartilage. Data is a subset of data in A (150 on x-axis). Data represent mean ± SD of hydroxyproline content per section. Different letters indicate statistical significance between groups (p < 0.05).
Friction and Wear
Impact loading did not alter the COF of the articular cartilage when loaded to 70 N. Treatment with 10 mM genipin after impact loading also had no effect on the COF (Fig. 4). India ink staining suggested that impacted specimens sustained more wear than the non-impacted specimens in the absence of genipin treatment, but that wear was reduced by crosslinking the tissue in 2 and 10 mM genipin (Fig. 5A). Quantitative testing of wear confirmed that the impacted specimens without treatment released significantly more collagen during the wear test than those that had not been impacted, and that the 2 and 10 mM genipin crosslinking treatments reduced the wear of the impacted specimens to levels that were comparable to that of the non-impacted controls (Fig. 5B).
Figure 4.

Top: Representative coefficient of friction (COF) over the 30 min test. Bottom: The initial COF of articular cartilage surfaces. Articular cartilage was either un-injured (No Impact), or subjected to a single impact. Specimens were then treated with the designated concentrations of genipin before COF measurement. Data represent mean ± SD of COF.
Figure 5.

Wear testing of articular cartilage. (A) India ink staining of articular cartilage subjected to the designated impact and genipin treatments. Images were obtained before and after wear testing and represent the maximum wear for each condition. (B) Hydroxyproline (HYP) released from articular cartilage during wear testing following the designated impact and genipin treatments. Data represent mean ± SD of hydroxyproline content per sample. (*p < 0.05, **p < 0.01).
DISCUSSION
This study investigated the effect of a single, blunt impact on the mechanical behavior and biochemical degradation of articular cartilage. In addition, genipin crosslinking was investigated as a potential therapeutic treatment to slow the degeneration of impact-damaged cartilage and potentially the progression of PTOA. The results indicate that the acute effects of an injurious impact load to articular cartilage leads to decreases in material stiffness but that treatment with genipin partially or fully restored the viscoelastic parameters of the tissue. The wear resistance of impacted articular cartilage was also diminished compared to undamaged cartilage, suggesting that the mechanical damage that is directly induced by the impact may contribute to the development of PTOA. However, the wear resistance of the damaged tissue was fully restored by the crosslinking treatments. The crosslinking treatments also improved the resistance to collagenase digestion at the impact-damaged articular surface. Taken together, these results demonstrate the potential therapeutic value of collagen crosslinking, and highlight genipin as a promising approach to the prevention of cartilage degeneration after traumatic injury.
Impact loading significantly decreased all cartilage stiffness measurements. This is consistent with the fissure formation at the articular surface observed by histology and the microarchitectural damage to the collagen network in the superficial zone that was observed via immunohistochemistry. Another factor may have been the 58.7% average increase in sGAGs released from the impacted specimens during the 24 h incubation, though the difference was not significant. Genipin treatment tended to restore the stiffness of the tissue up to a point. However, the restoration of stiffness by crosslinking was likely limited due to the formation of the fissures, which the crosslinking treatments were unable to repair. Impact did not change the stress relaxation time constant, but it was decreased by both genipin crosslinking treatments, similar to what was seen previously with healthy, intact cartilage.30 It should be noted that the change in viscoelastic parameters were measured via 70 μm indentation and may not reflect the change in properties through the depth of the tissue.
There was no significant difference in collagenase digestion between the non-impacted and impacted 0 mM genipin groups anywhere through the depth, indicating that the acute mechanical damage induced by impact does not lead to enhanced susceptibility of cartilage to collagenase. Regardless, levels of degradative enzymes are elevated in joints after a trauma; a single impact load to cartilage causes an adverse cellular response, including an increased expression of pro-inflammatory cytokines such as interleukin-1 (IL-1),6 which are thought to activate degradative enzymes4 that reduce the mechanical properties of the cartilage over time.5,11 Weakening of the tissue through this catabolic pathway is one mechanism by which a traumatic injury progresses to PTOA. A therapeutic treatment that protects cartilage from the degradative post-injury environment may slow the development of PTOA. The present data indicate that crosslinking the cartilage in 2 mM genipin decreased collagenase digestion at the articular surface compared to healthy cartilage, while the tissue that received the 10 mM treatment was even more resistant to collagenase. These results indicate that collagen crosslinking enhances the resistance to biochemical degradation at the articular surface of impact-damaged cartilage, and may preserve cartilage after trauma.
The increased wear observed in response to impaction injury is likely due to the microarchitectural damage and fissure formation that occurred at the articular surface and may contribute to the progression of PTOA. The wear data show that the cartilage that had been damaged and then treated with either concentration of genipin did not differ from non-impacted controls, indicating that both genipin treatments restored the wear resistance that was lost after the traumatic impact. As crosslinking did not alter the COF, the improved wear resistance is likely due to strengthening of the tissue.
Limitations of this study include the fact that it is difficult to directly compare the impact from the current study to those in whole joints. The impact load that was imparted by the drop tower is designed to mimic physiologic joint trauma from automobile accidents, sports injuries, or military combat injuries. This model successfully generates standardized, reproducible, cartilage damage, including cartilage fissures at the articular surface and microarchitectural damage, which are hallmarks of joint trauma. Similarly, it is difficult to compare impact parameters such as peak stress and strain between experimental studies because of differences in specimen geometry and anatomic location. Previous experimental work found that stresses above 20–30 MPa applied at strain rates of 500–1,000 sec−1 are necessary to cause chondrocyte death and fissure formation,31 consistent with the loading applied here. One estimation that we made in our analysis of the impact load was that only cartilage deformed during our impact protocol and not bone; although cartilage is much more compliant, the deformation would have been distributed to both tissues. Another estimate was the thickness of the cartilage, which was assumed to be in the range of 1.2–1.5 mm, but was not directly measured. The study was performed using approximately 1-year old, skeletally immature bovine tissue, and the measured results may not be indicative of those from cartilage with a mature morphology. Finally, we note that the specimens were frozen before use, and that the matrix of all the specimens would have been exposed to degradative enzymes from the ruptured cells. A degraded matrix may respond differently to impact and cross-linking than healthy tissue, and future studies are planned to investigate whether crosslinking strengthens cartilage that has been enzymatically degraded as in the post-traumatic environment.
Current treatments to prevent the development of PTOA aim to improve joint instability or incongruity,2,18–22 and are necessary to restore normal joint function. Recent studies have investigated biological treatments that impair aspects of the post-trauma cellular response.23–29,41 These treatments aim to decrease the risk of OA after an injury by limiting damage to the chondrocytes. However, none of these therapies address the diminished material properties of the damaged cartilage tissue, or protect the articular surface from subsequent mechanical wear or biochemical degradation. The results of this study suggest that a collagen crosslinking agent such as genipin may be of therapeutic value either independently or as a complement to therapies that alter cell behavior. In this study, the beneficial effects of genipin were observed even at the lower concentration of 2 mM. Previous research has demonstrated that this genipin crosslinking treatment is non-toxic to chondrocytes, though toxicity is observed at higher concentrations.30 Genipin treatments may be ideal for clinical applications where the joint is open, as intra-articular injection would crosslink all the tissues of the joint, including the ligaments and synovium. Alternately, it may be possible to devise a method to deliver genipin locally via an arthroscopic instrument that includes a genipin-soaked sponge and joint distension with gas rather than fluid. Further experimental work will be necessary to assess the safety and efficacy of genipin in vivo, as well as to determine how to best achieve the potential benefits of collagen crosslinking as a treatment for PTOA.
Supplementary Material
Acknowledgments
We thank Huseyin Arman for performing a portion of the immunohistochemistry in this study. The research was supported by the US Army Medical Research and Materiel Command W81XWH-07-066 (DRW and TCO) and the Wendell F. Bueche Fellowship in Engineering (MEM), NIH grant AR047702 (SBT), and Department of Veterans’ Affairs merit award BX000447(SBT). This publication was made possible in part by grants 1563721 from the NSF and AR069657 from the NIH (DRW).
Grant sponsor: US Army Medical Research and Materiel Command; Grant number: W81XWH-07-066; Grant sponsor: Wendell F. Bueche Fellowship in Engineering; Grant sponsor: NIH; Grant numbers: AR047702, AR069657; Grant sponsor: Department of Veterans’ Affairs Merit Award; Grant number: BX000447; Grant sponsor: NSF; Grant number: 1563721.
Footnotes
AUTHORS’ CONTRIBUTIONS
CMB contributed to the study design, performed experiments, collected data, analyzed and interpreted the data, and drafted the manuscript. MEM contributed to the study design, performed experiments, and analyzed and interpreted the data. MJS contributed to the study design and the collection and analysis of the data. TCO was involved in the design of the study, the critical review of the manuscript and in acquiring funding for the study. SBT participated in the conception of the study, the interpretation of the data, and contributed to the manuscript. DRW contributed to the study conception and design, the analysis and interpretation of the data, manuscript preparation, and in acquiring funding for the study. All authors approved the final version of the manuscript.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article at the publisher’s website.
Conflicts of interest: None.
References
- 1.Buckwalter JA, Martin JA. Sports and osteoarthritis. Curr Opin Rheumatol. 2004;16:634. doi: 10.1097/01.bor.0000132647.55056.a9. [DOI] [PubMed] [Google Scholar]
- 2.Buckwalter JA, Brown TD. Joint injury, repair, and remodeling: roles in post-traumatic osteoarthritis. Clin Orthop Relat Res. 2004;423:7. [PubMed] [Google Scholar]
- 3.Haut RC, Ide TM, De Camp CE. Mechanical responses of the rabbit patello-femoral joint to blunt impact. J Biomech Eng. 1995;117:402. doi: 10.1115/1.2794199. [DOI] [PubMed] [Google Scholar]
- 4.Backus JD, Furman BD, Swimmer T, et al. Cartilage viability and catabolism in the intact porcine knee following transarticular impact loading with and without articular fracture. J Orthop Res. 2010;29:501–510. doi: 10.1002/jor.21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newberry WN, Mackenzie CD, Haut RC. Blunt impact causes changes in bone and cartilage in a regularly exercised animal model. J Orthop Res. 1998;16:348–354. doi: 10.1002/jor.1100160311. [DOI] [PubMed] [Google Scholar]
- 6.Lotz M. Cytokines in cartilage injury and repair. Clin Orthop Relat Res. 2001;391:S108–S115. doi: 10.1097/00003086-200110001-00011. [DOI] [PubMed] [Google Scholar]
- 7.Repo RU, Finlay JB. Survival of articular cartilage after controlled impact. J Bone Joint Surg. 1977;59:1068. [PubMed] [Google Scholar]
- 8.Thompson RC, Oegema TR, Lewis JL, et al. Osteoarthrotic changes after acute transarticular load. An animal model. J Bone Joint Surg. 1991;73:990. [PubMed] [Google Scholar]
- 9.Atkinson TS, Haut RC, Altiero NJ. Impact-induced fissuring of articular cartilage: an investigation of failure criteria. J Biomech Eng. 1998;120:181. doi: 10.1115/1.2798300. [DOI] [PubMed] [Google Scholar]
- 10.Newberry WN, Zukosky DK, Haut RC. Subfracture insult to a knee joint causes alterations in the bone and in the functional stiffness of overlying cartilage. J Orthop Res. 1997;15:450–455. doi: 10.1002/jor.1100150319. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Haut RC, Altiero NJ. An analytical model to study blunt impact response of the rabbit PF joint. J Biomech Eng. 1995;117:485. doi: 10.1115/1.2794212. [DOI] [PubMed] [Google Scholar]
- 12.Thompson RC, Jr, Vener MJ, Griffiths HJ, et al. Scanning electron-microscopic and magnetic resonance-imaging studies of injuries to the patellofemoral joint after acute transarticular loading. J Bone Joint Surg Am. 1993;75:704–713. doi: 10.2106/00004623-199305000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Tomatsu T, Imai N, Takeuchi N, et al. Experimentally produced fractures of articular cartilage and bone. The effects of shear forces on the pig knee. J Bone Joint Surg Br. 1992;74:457–462. doi: 10.1302/0301-620X.74B3.1587902. [DOI] [PubMed] [Google Scholar]
- 14.Wilson W, van Burken C, van Donkelaar C, et al. Causes of mechanically induced collagen damage in articular cartilage. J Orthop Res. 2006;24:220–228. doi: 10.1002/jor.20027. [DOI] [PubMed] [Google Scholar]
- 15.Gelber AC, Hochberg MC, Mead LA, et al. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann Intern Med. 2000;133:321–328. doi: 10.7326/0003-4819-133-5-200009050-00007. [DOI] [PubMed] [Google Scholar]
- 16.Roos EM. Joint injury causes knee osteoarthritis in young adults. Curr Opin Rheumatol. 2005;17:195–200. doi: 10.1097/01.bor.0000151406.64393.00. [DOI] [PubMed] [Google Scholar]
- 17.Lotz MK, Kraus VB. New developments in osteoarthritis. Posttraumatic osteoarthritis: pathogenesis and pharmacological treatment options. Arthritis Res Ther. 2010;12:211. doi: 10.1186/ar3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kramer WC, Hendricks KJ, Wang J. Pathogenetic mechanisms of posttraumatic osteoarthritis: opportunities for early intervention. Int J Clin Exp Med. 2011;4:285–298. [PMC free article] [PubMed] [Google Scholar]
- 19.Buckwalter JA. Articular cartilage: injuries and potential for healing. J Orthop Sports Phys Ther. 1998;28:192–202. doi: 10.2519/jospt.1998.28.4.192. [DOI] [PubMed] [Google Scholar]
- 20.Buckwalter JA, Martin JA. Osteoarthritis. Adv Drug Deliv Rev. 2006;58:150–167. doi: 10.1016/j.addr.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Schulze-Tanzil G. Activation and dedifferentiation of chondrocytes: implications in cartilage injury and repair. Ann Anat. 2009;191:325–338. doi: 10.1016/j.aanat.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Nelson F, Billinghurst RC, Pidoux RT, et al. Early post-traumatic osteoarthritis-like changes in human articular cartilage following rupture of the anterior cruciate ligament. Osteoarthr Cartil. 2006;14:114–119. doi: 10.1016/j.joca.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 23.D’Lima DD, Hashimoto S, Chen PC, et al. Prevention of chondrocyte apoptosis. J Bone Joint Surg. 2001;83:S25–S26. doi: 10.2106/00004623-200100021-00006. [DOI] [PubMed] [Google Scholar]
- 24.Phillips DM, Haut RC. The use of a non-ionic surfactant (P188) to save chondrocytes from necrosis following impact loading of chondral explants. J Orthop Res. 2004;22:1135–1142. doi: 10.1016/j.orthres.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Rundell SA, Baars DC, Phillips DM, et al. The limitation of acute necrosis in retro-patellar cartilage after a severe blunt impact to the in vivo rabbit patello-femoral joint. J Orthop Res. 2005;23:1363–1369. doi: 10.1016/j.orthres.2005.06.001.1100230618. [DOI] [PubMed] [Google Scholar]
- 26.Martin JA, Buckwalter JA. Post-traumatic osteoarthritis: the role of stress induced chondrocyte damage. Biorheology. 2006;43:517–521. [PubMed] [Google Scholar]
- 27.Ramakrishnan P, Hecht BA, Pedersen DR, et al. Oxidant conditioning protects cartilage from mechanically induced damage. J Orthop Res. 2010;28:914–920. doi: 10.1002/jor.21072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin JA, McCabe D, Walter M, et al. N-acetylcysteine inhibits post-impact chondrocyte death in osteochondral explants. J Bone Joint Surg Am. 2009;91:1890–1897. doi: 10.2106/JBJS.H.00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ding L, Heying E, Nicholson N, et al. Mechanical impact induces cartilage degradation via mitogen activated protein kinases. Osteoarthr Cartil. 2010;18:1509–1517. doi: 10.1016/j.joca.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGann ME, Bonitsky CM, Jackson ML, et al. Genipin crosslinking of cartilage enhances resistance to biochemical degradation and mechanical wear. J Orthop Res. 2015;33:1571–1579. doi: 10.1002/jor.22939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finlay JB, Repo RU. Impact characteristics of articular cartilage. ISA Trans. 1978;17:29–34. [PubMed] [Google Scholar]
- 32.Burgin LV, Aspden RM. A drop tower for controlled impact testing of biological tissues. Med Eng Phys. 2007;29:525–530. doi: 10.1016/j.medengphy.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Scott CC, Athanasiou KA. Design, validation, and utilization of an articular cartilage impact instrument. Proc Ins Mech Eng H. 2006;220:845–855. doi: 10.1243/09544119JEIM97. [DOI] [PubMed] [Google Scholar]
- 34.Johnson KL. Contact mechanics. Cambridge, UK: Cambridge University Press; 1985. pp. 92–95. [Google Scholar]
- 35.Liu K, VanLandingham MR, Ovaert TC. Mechanical characterization of soft viscoelastic gels via indentation and optimization-based inverse finite element analysis. J Mech Behav Biomed Mater. 2009;2:355–363. doi: 10.1016/j.jmbbm.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 36.McGann ME, Bonitsky CM, Ovaert TC, et al. The effect of collagen crosslinking on the biphasic poroviscoelastic cartilage properties determined from a semi-automated microindentation protocol for stress relaxation. J Mech Behav Biomed Mater. 2014;34:264–272. doi: 10.1016/j.jmbbm.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Sneddon IN. The relation between load and penetration in the axisymmetric boussinesq problem for a punch of arbitrary profile. Int J Eng Sci. 1965;3:47–57. [Google Scholar]
- 38.Oliver WC, Pharr GM. Improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J Mater Res. 1992;7:1564–1583. [Google Scholar]
- 39.Cottrell JM, Scholten P, Wanich T, et al. A new technique to measure the dynamic contact pressures on the Tibial Plateau. J Biomech. 2008;41:2324–2329. doi: 10.1016/j.jbiomech.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 40.McGann ME, Vahdati A, Wagner DR. Methods to assess in vitro wear of articular cartilage. Proc Inst Mech Eng H. 2012;226:612–622. doi: 10.1177/0954411912447014. [DOI] [PubMed] [Google Scholar]
- 41.Anderson DD, Chubinskaya S, Guilak F, et al. Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. J Orthop Res. 2011;29:802–809. doi: 10.1002/jor.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


