Abstract
BACKGROUND. Programmed death 1 (PD-1) inhibition activates partially exhausted cytotoxic T lymphocytes (peCTLs) and induces tumor regression. We previously showed that the peCTL fraction predicts response to anti–PD-1 monotherapy. Here, we sought to correlate peCTL and regulatory T lymphocyte (Treg) levels with response to combination immunotherapy, and with demographic/disease characteristics, in metastatic melanoma patients.
METHODS. Pretreatment melanoma samples underwent multiparameter flow cytometric analysis. Patients were treated with anti–PD-1 monotherapy or combination therapy, and responses determined by Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1) criteria. peCTL and Treg levels across demographic/disease variables were compared. Low versus high peCTL (≤20% vs. >20%) were defined from a previous study.
RESULTS. One hundred and two melanoma patients were identified. The peCTL fraction was higher in responders than nonresponders. Low peCTL correlated with female sex and liver metastasis, but not with lactate dehydrogenase (LDH), tumor stage, or age. While overall response rates (ORRs) to anti–PD-1 monotherapy and combination therapy were similar in high-peCTL patients, low-peCTL patients given combination therapy demonstrated higher ORRs than those who received monotherapy. Treg levels were not associated with these factors nor with response.
CONCLUSION. In melanoma, pretreatment peCTL fraction is reduced in women and in patients with liver metastasis. In low-peCTL patients, anti–PD-1 combination therapy is associated with significantly higher ORR than anti–PD-1 monotherapy. Fewer tumor-infiltrating peCTLs may be required to achieve response to combination immunotherapy.
TRIAL REGISTRATION. UCSF IRB Protocol 138510
FUNDING. NIH DP2-AR068130, K08-AR062064, AR066821, and Burroughs Wellcome CAMS-1010934 (M.D.R.). Amoroso and Cook Fund, and the Parker Institute for Cancer Immunotherapy (A.I.D.).
Keywords: Dermatology, Oncology
Levels of partially exhausted cytotoxic lymphocytes within the tumor microenvironment can determine response to combination checkpoint blockade.
Introduction
Immune regulation in the tumor microenvironment is a complex and multifaceted phenomenon (1). Chronic antigen exposure in the setting of tumor growth and metastasis leads to anergy and dysfunction (exhaustion) of the adaptive immune response, in part through increased engagment of the programmed death 1 (PD-1) receptor with its ligand (PD-L1) (2). The net result of signaling through this axis is an attenuation of the cytotoxic and cytokine-producing capacity of tumor-infiltrating lymphocytes, leading to ineffective antitumor immune responses (2). Inhibition of the PD-1 pathway has proven to be effective in many solid and hematological tumors (3–5). Understanding the host and tumor microenvironment factors that mediate response to PD-1 blockade can provide critical insights into the host antitumor immune response (6, 7). These factors include tumor mutation burden (6–8), host microbiome (9, 10), disease stage (11), lactate dehydrogenase (LDH) levels (11), baseline tumor size (11), and the presence of liver metastasis (12–14). These factors have been referred to as the cancer immunogram (15) or the cancer immune set point (16), and include both tumor intrinsic and extrinsic factors (17).
We have recently shown that the fraction of partially exhausted cytotoxic T lymphocytes (peCTLs, tumor-infiltrating CD8+ T cells expressing high levels of cytotoxic T lymphocyte–associated antigen 4 [CTLA-4] and PD-1) strongly correlates with response to anti–PD-1 monotherapy (18). Functionally, these cells may contain tumor-antigen-specific T cells (19, 20), and they demonstrate a partially exhausted phenotype which is hypothesized to be activated by treatment with anti–PD-1 (18, 21). Here, we examine how the relative abundance of peCTLs correlates with specific host factors, as well as response to immune checkpoint monotherapy and combination therapy.
Results
A total of 112 unique melanoma tumors were analyzed. While age was not correlated, sex significantly correlated with the percentage of peCTLs, with males having a larger proportion of these cells compared with females (males, 25.95% vs. females 17.5%, P = 0.041, Mann-Whitney test) (Figure 1, A and C). In contrast, the percentage of tumor-infiltrating regulatory T lymphocytes (Tregs, as defined by CD45+CD3+CD4+Foxp3+CD25+CTLA-4hi cells within the CD45+CD3+CD4+ gate) did not correlate with age or sex (Figure 1, B and D).
Figure 1. Percentage of partially exhausted cytotoxic T lymphocytes (peCTLs, CTLA-4hiPD-1hi) and regulatory T lymphocytes (Tregs, CD25+FoxP3+CD4+) in relation to patient sex and age.
Flow cytometric data from metastatic tumors taken and pregated on live CD45+CD3+CD8+ cells. (A) peCTLs expressed as a percentage of total CD8+ T cells, with sex on the x axis (n = 102). (B) Tregs expressed as a percentage of total CD4+ T cell population, with sex on the x axis (n = 93). (C) peCTLs as a percentage of total CD8+ T cells, with age on the x axis (n = 102). (D) Percentage of Tregs in the total CD4+ T cell population, with age on the x axis (n = 93). Statistical significance was determined by the Mann-Whitney test; P value is shown. NS, not significant.
Given prior data regarding the reduced response to anti–PD-1 therapy in patients with elevated LDH and liver metastasis (11, 22), we examined these and other disease characteristics, including American Joint Committee on Cancer (AJCC) stage (stage III vs. IV). Disease stage is known to be correlated with survival (23), while the presence of liver metastasis has been reported to inversely correlate with response to checkpoint inhibitors in melanoma, non–small cell lung cancer, and recently also in bladder cancer (13, 22). While no correlation with disease stage was observed (Figure 2, A and B), the presence of liver metastasis was associated with reduced percentages of tumor-infiltrating peCTLs (Figure 2, C and D). Elevated LDH levels have been reported to correlate with reduced objective response rates (ORRs) to anti–PD-1 monotherapy (4, 11, 24, 25). Thus, we examined whether there was an association between LDH levels and both peCTL and Treg percentages. Neither of these T cell subsets was associated with elevated LDH (Figure 2, E and F).
Figure 2. Percentage of partially exhausted cytotoxic T lymphocytes (peCTLs, CTLA-4hiPD-1hi) and regulatory T lymphocytes (Tregs, CD25+FoxP3+CD4+) in relation to disease stage, liver metastasis, and lactate dehydrogenase (LDH) levels.
Flow cytometric data from metastatic tumors taken and pregated on live CD45+CD3+CD8+ cells. (A) peCTLs as a percentage of total CD8+ T cells, with disease stage on the x axis (n = 102). (B) peCTLs as a percentage of total CD8+ T cells, with disease stage on the x axis (n = 102). (C) Tregs as a percentage of total CD8+ T cells, with presence of liver metastasis on the x axis (n = 93). (D) Percentage of Tregs in the total CD4+ population, with the presence or absence of liver metastasis on the x axis (n = 93). Statistical significance was determined by the Mann-Whitney test; 2-sided P value is shown. NS, not significant. (E) peCTLs as a percentage of total CD8+ T cells, with LDH levels on the x axis (n = 102). (F) Percentage of Tregs in the total CD4+ population, with the presence or absence of LDH elevation on the x axis (n = 93). Statistical significance was determined by the Mann-Whitney test; 2-sided P value is shown.
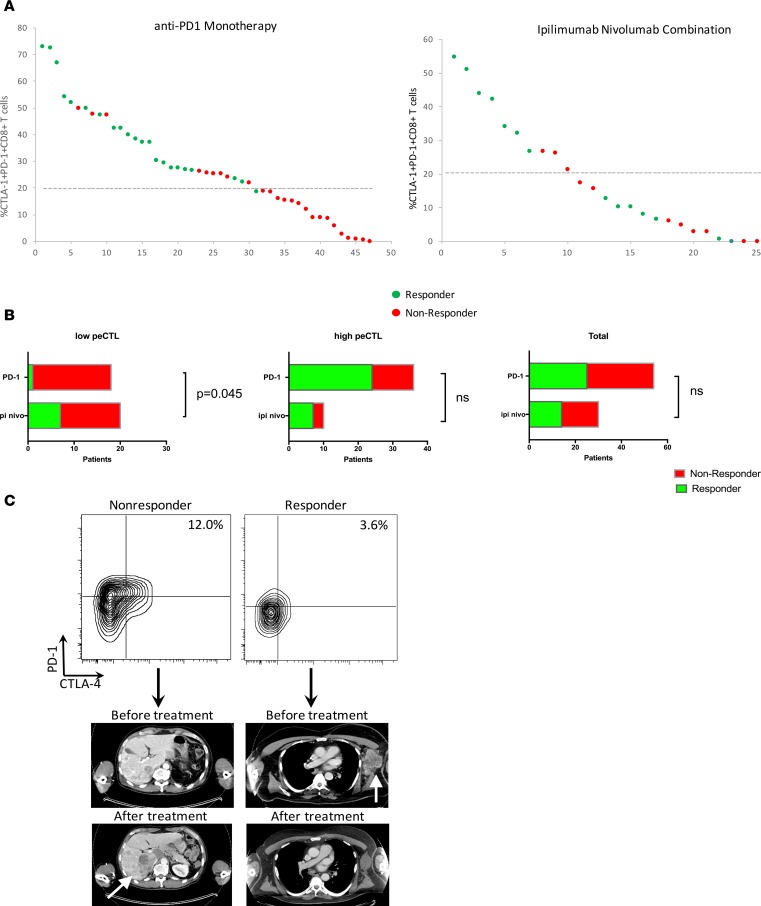
While the relative abundance of tumor-infiltrating peCTLs correlates with response to anti–PD-1 monotherapy (18), it is currently unknown whether this metric predicts response to ipilimumab/nivolumab combination therapy. Thus, we quantified peCTLs and ORRs by Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1) criteria (26) to ipilimumab/nivolumab combination therapy (n = 29) and compared these with anti–PD-1 monotherapy (n = 64) (Figure 3). Across all patients treated with either single-agent or combination checkpoint therapy, there was a significant correlation between percentage of peCTLs and ORR (CR+PR). Responders had a peCTL fraction of 30.5% versus 15.4% in nonresponders (P < 0.0001, Mann-Whitney test). Responses to PD-1 monotherapy were significantly more likely when the peCTL fraction was greater than 20% (65.8% vs. 5%, P = 0.0001), while with combination immunotherapy, responses were not significantly different with high or low peCTL fraction (72.7% vs. 38.9%, P = 0.1281). We have previously reported that a peCTL fraction of 20% or lower is associated with lack of response to anti–PD-1 monotherapy (18). Using the same metric, only 1 of 18 patients (5.6%) with peCTL 20% or lower responded to anti–PD-1 monotherapy, whereas 7 of 20 patients (35%) with peCTL 20% or lower responded to combination ipilimumab and nivolumab. The relative risk of response in the low-peCTL population was 6.3 (95% CI 1.2–37.7), and this difference was statistically significant (P = 0.045, Fisher’s exact test). In contrast, there was no difference in patients with high (>20%) peCTL. In these patients, 70% of patients treated with ipilimumab/nivolumab responded, while 66.7% of patients treated with anti–PD-1 monotherapy responded (P > 0.9999, Fisher’s exact test). In the illustrative example shown in Figure 3C, a patient with a peCTL fraction of 12% failed to respond to anti–PD-1 monotherapy (Figure 3C), while a patient with a peCTL fraction of 3.6% responded to ipilimumab/nivolumab combination therapy (Figure 3C), illustrating the lower threshold needed for combination immunotherapy response. There was no significant correlation between ORR and Treg percentage (Figure 4, Treg panel) (P = 0.0925, Mann-Whitney test), while peCTL level was highly correlated with response status across all patients (Figure 4, peCTL panel) (P = 0.0001, Mann-Whitney test).
Figure 3. Treatment response to anti–programmed death 1 (PD-1) monotherapy or ipilimumab/nivolumab combination therapy correlated with pretreatment partially exhausted cytotoxic T lymphocytes (peCTLs, CTLA-4hiPD-1hi).
(A) Flow cytometric data from metastatic tumors taken and pregated on live CD45+CD3+CD8+ cells. peCTLs as a percentage of total CD8+ T cells is shown on the y axis, and response to either anti–PD-1 monotherapy (n = 64) or ipilimumab/nivolumab combination (n = 29) is designated by color. Patients with partial or complete responses (PR+CR) by Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1) are shown in green, and patients with stable or progressive disease (SD+PD) are shown in red. (B) Responders and nonresponders to anti–PD-1 monotherapy and combination therapy relative to pretreatment peCTL count. Low peCTL count is 20% or lower, high peCTL count is greater than 20%. For patients with low peCTLs (left), the difference in objective response rate was 35% for ipilimumab plus nivolumab, while it was 5.6% for anti–PD-1 monotherapy (P = 0.045 by Fisher’s exact test). The difference for patients with high peCTLs (middle) was not significantly different: 70% for ipilimumab plus nivolumab and 66.7% for anti–PD-1 monotherapy. For the overall cohort (right), the difference was also not statistically significant: objective response rate was 46.7% for ipilimumab plus nivolumab versus 46.3% for anti–PD-1 monotherapy. NS, not significant. (C) Illustrative examples for 2 patients. Left: A nonresponder to anti–PD-1 monotherapy with 12% peCTLs with progression in the liver (white arrow). Right: A responder to ipilimumab plus nivolumab combination therapy with 3.6% peCTLs shows a response in a large left axillary mass (white arrow).
Figure 4. Percentage of partially exhausted cytotoxic T lymphocytes (peCTLs, CTLA-4hiPD-1hi) and regulatory T lymphocytes (Tregs, CD25+FoxP3+CD4+) in relation to objective response.
Flow cytometric data from metastatic tumors pregated on live CD45+CD3+CD8+ cells. peCTL (left) shows activated, exhausted peCTLs as a percentage of total CD8+ T cells, with response status on the x axis. Patients with a complete or partial response (CR+PR) were considered to be responders, and those with stable disease or progressive disease (SD+PD) as best response were considered to be nonresponders. Treg (right) shows Tregs as a percentage of total CD4+ T cell population, with response status on the x axis. Statistical significance was determined by the Mann-Whitney test; P value is shown. NS, not significant.
Discussion
While PD-1 inhibition represents a major advance in the treatment of melanoma and other cancers, it is well recognized that in most cancers only a minority of patients benefit from this approach (3, 4, 25), and that host and tumor factors play a role in response to these agents (11, 15, 17). Previously, we studied the abundance of tumor-infiltrating peCTLs in freshly isolated melanoma tumors using a novel assay that uses multiparameter flow cytometry and found that the fraction of peCTL/total CD8+ T cells within tumors directly correlated with response to anti–PD-1 monotherapy in a 40-patient cohort (18). Here, we utilized this approach in an expanded cohort of patients to understand the relationship between host factors and the abundance of peCTLs within the tumor microenvironment. Additionally, studying Tregs in the tumor microenvironment using a fractional Treg score (Tregs/total CD4+ T cells) found no correlation between the frequency of these cells with host demographics and disease characteristics. Importantly, female sex and the presence of liver metastasis significantly correlated with reduced peCTLs and reduced ORRs, while AJCC disease stage (stage III vs. IV) and elevated LDH did not. Although the association of elevated LDH with reduced response rate to anti–PD-1 therapy is well documented (4, 14), the lack of association with reduced peCTLs suggests that other immune cells such as NK cells (27) or tumor macrophages (28) may be involved in mediating the effects of LDH on immunotherapy. The effects of liver metastasis have been recently shown to reduce ORR and progression-free survival (PFS) to anti–PD-1 antibodies in melanoma and non–small cell lung cancer (22), and also in urothelial cancer (13) in immunotherapy-treated patients, and remains an area of active investigation. Preclinical data suggest that activated CD8+ T cells can be selectively deleted in the liver (29) and that liver tolerance (30) is operational in allotransplantation. Further studies in other tumor types and animal models may reveal additional information about the mechanism of liver metastasis–induced tolerance (31). The effect of female sex on reduced response rate to PD-1 has been recently reported (22, 32). Additional data are needed to determine the validity and generalizability of this observation.
Combined blockade of the PD-1/CTLA-4 checkpoints represents a major advance in immunotherapy, resulting in increased response rates and PFS in melanoma (33). However, this approach also exposes patients to high rates of severe autoimmune toxicities (34), and careful patient selection is key to using this potent combination versus single-agent anti–PD-1 (35). Recent studies have utilized PD-L1 immunohistochemical staining as a biomarker for response, with recent data showing that patients with low tumor PD-L1 expression (<1% or <5%) have better PFS and overall survival (OS) with anti–PD-1 combination therapy than with anti–PD-1 monotherapy (33, 36). In this current study, we sought to improve resolution by stratifying patients by low (≤20%) versus high (>20%) peCTL frequency. Using this cutoff, derived from our previous analysis in patients treated with anti–PD-1 monotherapy (18), we found that low-peCTL patients had increased response rates with combination checkpoint blockade, while high-peCTL patients had equivalent response rates with either combination or monotherapy. Taken together, these results suggest that fewer tumor-infiltrating peCTLs are required to achieve a response to ipilimumab/nivolumab combination therapy. Since peCTLs express CTLA-4 (18, 21), our data imply that dual blockade is additive or synergistic when peCTLs are infrequent in the tumor microenvironment. However, when peCTLs are abundant the additional stimulus of CTLA-4 blockade may not be necessary for maximal antitumor effect. These results complement the PD-L1 subset analysis data from the PD-1/CTLA-4 randomized combination trial discussed above, though studies of peCTLs in other tumor types and in animal models are needed to validate these observations.
There is a complex interplay between the environment, the microbiome, the host immune system, and the tumor (6, 15, 17). The results presented here provide a framework with which we can begin to understand why specific patient subsets have a paucity of tumor-infiltrating peCTLs and thus a lower likelihood of responding to immune checkpoint blockade. Future studies will require the discovery of specific therapies that reinvigorate this population and are tailored toward specific constellations of host factors and peCTL frequency.
Methods
Study design and tumor sample procurement.
Between January 2014 and August 2016, 102 patients with advanced melanoma at UCSF underwent biopsy for tumor-infiltrating lymphocyte profiling. Biopsies of accessible melanoma tumors were obtained after patients provided informed consent under the UCSF Committee on Human Research Protocol 138510. Biopsy samples were obtained with a 16- or 18-gauge needle, or a 4-mm punch tool. Fresh tumor samples were immediately placed on ice and transported to the laboratory for dissociation and analysis. Of these, 64 patients underwent treatment with monotherapy PD-1, 29 patients underwent treatment with ipilimumab plus nivolumab, and 9 patients with other regimens (anti–PD-1/indoleamine deoxygenase combination).
Study approval.
Melanoma tumor biopsies were performed after written informed consent was obtained from patients under the UCSF Committee on Human Research Protocol 138510.
Treatment outcome groups and efficacy analysis.
Two treatment outcome groups, responders and nonresponders, were defined using radiologic imaging following anti–PD-1 monotherapy or ipilimumab/nivolumab combination treatment. Responders included patients with tumor target lesions that met RECIST v1.1 criteria for complete response (>99% reduction in the target lesions) or partial response (≥30% reduction in target lesions). Nonresponders included patients with tumor target lesions that met RECIST v1.1 criteria for progression (≥20% increase in the target lesions) or stable disease (<30% reduction or <20% increase in tumor target lesions). Efficacy and immunological data available as of August 2016 were included in all the analyses. The efficacy analysis was limited to best overall response (BOR), defined as the best tumor response according to RECIST v1.1 criteria from the start of treatment to the time of disease progression or death. The laboratory team was blinded to all demographic and clinical data (including treatment response) throughout all studies.
Flow cytometric analyses.
Multiparameter flow cytometry was performed on pretreatment samples obtained from metastatic tumors as previously described. Freshly isolated samples were minced and digested overnight with buffer consisting of collagenase type 4 (Worthington, 4188), DNAse (Sigma-Aldrich, DN25-1G), 10% FBS, 1% HEPES, and 1% penicillin/streptavidin in RPMI medium. Single-cell suspensions were double filtered, centrifuged, and counted. For intracellular cytokine analysis, digested tumor cell suspensions were stimulated with PMA/ionomycin for 4 hours as previously described (37). Approximately 2 × 106 cells were stained with multiple fluorochrome-conjugated monoclonal antibodies. The following antibodies were used (eBioscience, unless otherwise stated): anti-hCD3 (UCHT1), anti-hCD8 (RPA-T8), anti-hCD45 (HI30), anti-CD4 (SK3), anti-Foxp3 (PCH101), anti–hCTLA-4 (14D3), anti–PD-1 (EH12.2H7; Biolegend), anti–HLA-DR (LN3), anti–PD-L1 (MIH1), and LIVE/DEAD Fixable Aqua Dead Cell Stain (Life Technologies). Data were acquired by an LSRFortessa (BD Biosciences) and analyzed using FlowJo software (Tree Star, Inc.).
Flow cytometry standardization and gating strategy.
All the samples were fresh and acquired by the Fortessa at different time points. To standardize voltages over time, Sphero Ultra Rainbow beads (Spherotech) were used to calibrate and normalize to baseline intensity. Gates were determined using both isotype control antibody staining and an internal negative control cell population (i.e., PD-1 and CTLA-4 expression on CD3– cells).
Statistics.
Patients with tumors exhibiting a threshold of less than 20% CTLA-4hiPD-1hi CD8+ T cells were noted to have infrequent responses in initial testing. This threshold was used in subsequent correlative analyses. The Mann-Whitney test was used to compare peCTL and Treg levels across paired demographic and disease variables assuming nonparametric distributions. Fisher’s exact test was used to analyze contingency tables. A P value of less than or equal to 0.05 was considered significant. In all figures quantifying flow cytometric data, the mean value ± SEM is depicted. Statistical analysis was done using Prism software (GraphPad).
Author contributions
AID, KL, KKT, and MDR designed the research studies. AID, KL, KKT, KM, MLP, and PS conducted experiments. AID, KL, KKT, KM, J Liu, LSL, MP, and LC acquired data. AID, MDR, KL, KKT, KM, J Lee, MP, PS, AN, LC, and APA analyzed data. AID, JH, KL, KKT, MDR, MA, and MFK wrote the manuscript.
Acknowledgments
This work was supported by NIH grants DP2-AR068130, K08-AR062064, and R21-AR066821, and Burroughs Wellcome Fund CAMS-1010934 (to M.D.R.); and grants from the Helen Diller Comprehensive Cancer Center and the Kelly Family, Amoroso and Cook Fund (to A.I.D.).
Footnotes
Conflict of interest: A.P. Algazi discloses institutional research funding from Novartis, Merck, Bristol-Myers Squibb (BMS), OncoSec, Acerta, AstraZeneca, MedImmune, Tessa, Celldex, Celgene, and Plexxikon. M.F. Krummel discloses ownership of shares in Pionyr Immunotherapeutics, and lab funding from BMS, Amgen, and Abbvie. A.I. Daud discloses ownership of shares in OncoSec Inc.
Reference information: JCI Insight. 2017;2(14):e93433. https://doi.org/10.1172/jci.insight.93433.
Contributor Information
Kimberly Loo, Email: Kimberly.Loo@ucsf.edu.
Kelly Mahuron, Email: kelly.mahuron@ucsf.edu.
Jacqueline Liu, Email: jacquieliu@ucla.edu.
Mariela L. Pauli, Email: Mariela.Pauli@ucsfmedctr.org.
Adi Nosrati, Email: adinosrati@gmail.com.
James Lee, Email: James.Lee4@ucsf.edu.
Lawrence Chen, Email: lawrence.chen@ucsf.edu.
Jimmy Hwang, Email: Jimmy.hwang@ucsf.edu.
Michael D. Rosenblum, Email: rosenblummd@derm.ucsf.edu.
References
- 1.Pitt JM, Marabelle A, Eggermont A, Soria JC, Kroemer G, Zitvogel L. Targeting the tumor microenvironment: removing obstruction to anticancer immune responses and immunotherapy. Ann Oncol. 2016;27(8):1482–1492. doi: 10.1093/annonc/mdw168. [DOI] [PubMed] [Google Scholar]
- 2.Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36(4):265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brahmer JR, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamid O, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rizvi NA, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257–265. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rizvi NA, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGranahan N, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351(6280):1463–1469. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snyder A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vétizou M, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sivan A, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264):1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribas A, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315(15):1600–1609. doi: 10.1001/jama.2016.4059. [DOI] [PubMed] [Google Scholar]
- 12. Goldinger SM et al. Correlation between metastatic site and response to anti-Programmed Death-1 (PD-1) agents in melanoma. Abstract presented at ASCO 2016 Annual Meeting; June 3–7, 2016; Chicago, IL. Abstract 9579. [Google Scholar]
- 13.Bellmunt J, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herbst RS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blank CU, Haanen JB, Ribas A, Schumacher TN. CANCER IMMUNOLOGY. The “cancer immunogram”. Science. 2016;352(6286):658–660. doi: 10.1126/science.aaf2834. [DOI] [PubMed] [Google Scholar]
- 16.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541(7637):321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 17.Pitt JM, et al. Resistance mechanisms to immune-checkpoint blockade in cancer: tumor-intrinsic and -extrinsic factors. Immunity. 2016;44(6):1255–1269. doi: 10.1016/j.immuni.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Daud AI, et al. Tumor immune profiling predicts response to anti-PD-1 therapy in human melanoma. J Clin Invest. 2016;126(9):3447–3452. doi: 10.1172/JCI87324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmadzadeh M, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114(8):1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gros A, et al. PD-1 identifies the patient-specific CD8+ tumor-reactive repertoire infiltrating human tumors. J Clin Invest. 2014;124(5):2246–2259. doi: 10.1172/JCI73639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang AC, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545(7652):60–65. doi: 10.1038/nature22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tumeh PC, et al. Liver metastasis and treatment outcome with anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res. 2017;5(5):417–424. doi: 10.1158/2326-6066.CIR-16-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balch CM, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med. 2012;366(26):2517–2519. doi: 10.1056/NEJMe1205943. [DOI] [PubMed] [Google Scholar]
- 25.Robert C, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384(9948):1109–1117. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 26.Eisenhauer EA, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 27.Crane CA, et al. Immune evasion mediated by tumor-derived lactate dehydrogenase induction of NKG2D ligands on myeloid cells in glioblastoma patients. Proc Natl Acad Sci U S A. 2014;111(35):12823–12828. doi: 10.1073/pnas.1413933111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seth P, et al. Deletion of lactate dehydrogenase-A in myeloid cells triggers antitumor immunity. Cancer Res. doi: 10.1158/0008-5472. [published online ahead of print April 26, 2017]. https://doi.org/10.1158/0008-5472.CAN-16-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowen DG, Zen M, Holz L, Davis T, McCaughan GW, Bertolino P. The site of primary T cell activation is a determinant of the balance between intrahepatic tolerance and immunity. J Clin Invest. 2004;114(5):701–712. doi: 10.1172/JCI21593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crispe IN, Giannandrea M, Klein I, John B, Sampson B, Wuensch S. Cellular and molecular mechanisms of liver tolerance. Immunol Rev. 2006;213:101–118. doi: 10.1111/j.1600-065X.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 31.Brodt P. Role of the microenvironment in liver metastasis: from pre- to prometastatic niches. Clin Cancer Res. 2016;22(24):5971–5982. doi: 10.1158/1078-0432.CCR-16-0460. [DOI] [PubMed] [Google Scholar]
- 32. Tsai KK, et al. Outcome disparities by sex in melanoma patients treated with anti-PD-1 therapy. Journal for ImmunoTherapy of Cancer 2016, 4(Suppl 1):P115.2016. [Google Scholar]
- 33.Larkin J, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolchok JD, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luke JJ, Flaherty KT, Ribas A, Long GV. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol. doi: 10.1038/nrclinonc.2017. [published online ahead of print: April 4, 2017]. https://doi.org/10.1038/nrclinonc.2017.43. [DOI] [PubMed] [Google Scholar]
- 36. Larkin J, et al. Overall survival results from a phase III trial of nivolumab combined with ipilimumab in treatment-naïve patients with advanced melanoma (CheckMate-067): press conference. In: Proceedings from the 2017 American Association for Cancer Research Annual Meeting; April 2–5, 2017; Washington DC. Abstract CT075. [Google Scholar]
- 37.Sanchez Rodriguez R, et al. Memory regulatory T cells reside in human skin. J Clin Invest. 2014;124(3):1027–1036. doi: 10.1172/JCI72932. [DOI] [PMC free article] [PubMed] [Google Scholar]