Abstract
Mitochondrial dysfunction has been implicated in a multitude of diseases and pathological conditions- the organelles that are essential for life can also be major players in contributing to cell death and disease. Because mitochondria are so well established in our existence, being present in all cell types except for red blood cells and having the responsibility of providing most of our energy needs for survival, then dysfunctional mitochondria can elicit devastating cellular pathologies that can be widespread across the entire organism. As such, the field of “mitochondrial medicine” is emerging in which disease states are being targeted therapeutically at the level of the mitochondrion, including specific antioxidants, bioenergetic substrate additions, and membrane uncoupling agents. New and compelling research investigating novel techniques for mitochondrial transplantation to replace damaged or dysfunctional mitochondria with exogenous healthy mitochondria has shown promising results, including tissue sparing accompanied by increased energy production and decreased oxidative damage. Various experimental techniques have been attempted and each has been challenged to accomplish successful transplantation. The purpose of this review is to present the history of mitochondrial transplantation, the different techniques used for both in vitro and in vivo delivery, along with caveats and pitfalls that have been discovered along the way. Results from such pioneering studies are promising and could be the next big wave of “mitochondrial medicine” once technical hurdles are overcome.
Keywords: oxygen consumption, bioenergetics, oxidative phosphorylation, cellular uptake, replacement strategies
1. Introduction to Mitochondria
Mitochondria are located in the cell cytoplasm and often referred to as the powerhouse of the cell, as they produce most of the cell’s energy in the form of adenosine triphosphate (ATP). However, they are also referred to as the nuclear power plant of the cell as they can efficiently make ATP while simultaneously producing a small but manageable amount of destructive oxidative agents known as reactive oxygen species (ROS). When mitochondria are damaged they can become extremely reactive and damaging to themselves and surrounding mitochondria, to the effect that it can cause cell death. To this end, while mitochondria are essential for life, they are also important factors in unleashing numerous pathways that can lead to both apoptosis and necrosis.
It is theorized that mitochondria were once bacteria that became engulfed by a cell and then utilized for its respiratory capabilities. Evidence of this theory lies in the fact that mitochondria have two lipid membranes and their own circular DNA (Nass & Nass 1963), which allows them to make their own proteins (Mc et al 1958). As evolution progressed, cells became more dependent on mitochondria for their energy supply, even integrating the coding for key mitochondrial proteins within the nuclear DNA. Mitochondrial DNA (mtDNA) only encodes for 13 mitochondrial proteins and needs the cell’s nuclear DNA to provide the rest of the necessary proteins. Mitochondria are inherited maternally (Hutchison et al 1974), meaning that each offspring will have mtDNA identical to their mother’s, with no input from the father’s mtDNA. It is thus possible to track familial lineage using the maternal mitochondria. This also implicates maternal inheritance of mtDNA mutations and disorders. However, the amount of mutated mtDNA in an egg can vary, known as heteroplasmy (Lightowlers et al 1997), resulting in differing phenotypes making inheritance patterns that may not be obvious. Further, in an attempt to bypass inheritance of mtDNA mutations, mitochondrial replacement has been attempted in fertilization techniques to replace defective mtDNA with healthy mtDNA from a donor (Cohen et al 1998, Cohen et al 1997).
1.1 Mitochondrial Function
1.1.1 Oxidative Phosphorylation
Mitochondria provide for most of the cell’s energy needs in the form of ATP (see Figure 1A-1). In the process of oxidative phosphorylation mitochondria use substrates, namely NADH and FADH produced by the Kreb’s cycle in the mitochondrial matrix, to power the electron transport system (ETS) located in the inner mitochondrial membrane (IMM). NADH donates an electron to complex I of the electron transport chain (ETC), an exchange that results in hydrogen atoms being pumped against a concentration gradient from the matrix into the inner membrane space. The electron then passes across the other complexes of the ETC in a series of oxidation/reduction reactions, each time releasing energy and pumping hydrogen from the matrix to the inner membrane space (for comprehensive review see (Nicholls & Ferguson 2013). This creates a membrane potential (between 140 and 180 mV) across the inner mitochondrial membrane, which is used by complex V to form ATP from ADP with simultaneous passage of hydrogen back into the mitochondrial matrix (Reid et al 1966). When the electron reaches complex IV, it is released back into the matrix where it joins with oxygen. The oxygen is held at complex IV until enough electrons are present to allow it to form two water molecules. During oxidative phosphorylation, some ROS are created but are in small amounts that can be counteracted by endogenous antioxidant mechanisms within the mitochondria (see (Cheeseman & Slater 1993, Droge 2002).
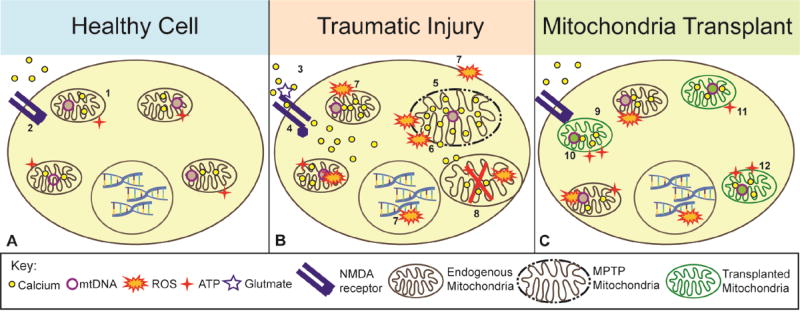
Figure 1. Schematic depiction of how mitochondrial transplantation after injury may promote cell survival.
A. During normal cellular function, mitochondria sequester and store calcium in their matrix and produce ATP (1) while NMDA receptors remain closed (2). B. After traumatic injury, the cell is subjected to glutamate excitotoxicity mediated by activated NMDA receptors (3) which allow massive calcium influx into cells (4) and subsequent uptake into mitochondria. This causes a loss of membrane potential across the inner mitochondrial membrane leading to mitochondrial permeability transition pore (MPTP) formation and subsequent swelling of mitochondria (5). Consequent bursting of the outer mitochondrial membrane then releases calcium, ROS, and apoptotic factors (6). ROS damages membrane lipids, mtDNA, electron transport chain proteins, and nuclear DNA (7), which leads to death of adjacent mitochondria (8). C. After healthy mitochondria, depicted in green, are transplanted into damaged cells they can increase antioxidants to combat ROS release (9) and provide new sources of mtDNA (10). Further, these mitochondria increase ATP production (11) and overall calcium buffering capacity (12) in the compromised cells.
1.1.2 Endogenous Antioxidant Systems
During oxidative phosphorylation, electron flow leads to a small amount of ROS production, namely in the form of superoxide radicals. Electrons released from the ETC can reduce oxygen molecules to superoxides (O2−). Manganese superoxide dismutase (MnSOD) localized within the mitochondria (Weisiger & Fridovich 1973) can oxidize superoxides into less reactive hydrogen peroxide (McCord & Fridovich 1969). Hydrogen peroxide, however, can undergo the Fenton reaction, creating hydroxyl radicals (OH−) that can damage lipids, proteins, and nucleic acids (Winterbourn 1995). Alternatively, glutathione (GSH) peroxidase can convert hydrogen peroxide into water and is present in the intermembrane space of mitochondria (Arai et al 1999). Damage to mitochondria can increase the endogenous antioxidant systems activities. For example, complex I deficiencies increase the expression of MnSOD which turns superoxide into hydrogen peroxide in cultured fibroblasts (Pitkanen & Robinson 1996).
1.1.3 Calcium Buffering
The mitochondrial matrix holds a negative charge of around −240mV, (see (Michelakis 2008) allowing the mitochondria to actively sequester positively charged calcium ions into their matrix (see Figure 1A-1). This is important as calcium is involved in various intracellular signaling pathways. Under normal conditions, concentration of cytoplasmic calcium is 0.1uM, extracellular calcium concentration is 1mM, favoring calcium movement into the cytoplasm, (see (Bianchi et al 2004). The inner mitochondrial membrane has a potential of −180mV, favoring calcium influx into the matrix through the calcium uniporter (Bygrave & Ash 1977), with equilibrium being reached when the calcium concentration within the mitochondrial matrix reaches 106 higher than the cytosol (Bianchi et al 2004). Once inside the matrix, energy is required to export calcium back out of the matrix to overcome the electrochemical force driving calcium influx. It is found that the energy released from ATP hydrolysis (34.1kJ/mol) is sufficient to allow movement of 1 mol of calcium (33kJ) across the inner mitochondrial membrane and into the intermembrane space (Bianchi et al 2004). Importantly, calcium is required for the activation of different metabolic enzymes such as pyruvate dehydrogenase, α-ketoglutaratedehydrogenase, and isocitrate dehydrogenase. This calcium influx into the mitochondrial matrix leads to increased metabolism through interaction with these metabolic enzymes, increasing ATP production for the cell (Jouaville et al 1999).
1.2 Mitochondrial Dysfunction
Mitochondrial dysfunction is implicated in many disease states such as Parkinson’s Disease (Mizuno et al 1989, Winklhofer & Haass 2010), Alzheimer’s Disease (Swerdlow & Khan 2004), muscular dystrophy (Onopiuk et al 2009), macular degeneration (Burdon 1995), and ageing-related neurodegeneration (Lin & Beal 2006), among many others. While the dysfunction may be caused by a genetic defect of the mtDNA in certain disease states, it can also be caused by damage to the ETS of otherwise healthy mitochondria. Such mitochondria dysfunction is also involved in pathologies seen after many traumatic insults including cardiac infarctions (Braunwald & Kloner 1985, Ide et al 2001), stroke (Bolanos et al 2009, Krajewski et al 1999, Sims & Muyderman 2010), traumatic brain injury (TBI) (Aygok et al 2008, Singh et al 2006, Xiong et al 1997), and spinal cord injury (SCI) (Huang et al 2012, Sullivan et al 2007). Accordingly, different therapeutics targeting mitochondrial dysfunction following neurotrauma have included drugs that increase antioxidants or increase mitochondrial substrates and ATP production (Bains & Hall 2012, Jin et al 2014, Patel et al 2010, Patel et al 2014, Petruzzella et al 1992, Smith et al 2008).
1.2.1 Glutamate Excitotoxicity and Calcium Dysregulation
Glutamate is an excitatory neurotransmitter that can also be toxic to neurons in cases of overwhelming exposure. Extracellular glutamate was found to be neurotoxic in the brain as early as 1969 (Olney & Sharpe 1969). During glutamate excitotoxicity, glutamate binds to N-methyl-D-aspartate (NMDA) type receptors located on the cell membrane (see Figure 1A-2) forming channels highly permeable to calcium (MacDermott et al 1986), resulting in an abnormally high influx of calcium into the cell (see Figure 1B-3,4). Increased glutamate levels in the presence of extracellular calcium in vitro causes neuronal degeneration (Choi 1985) that can be inhibited by using NMDA antagonists (Choi 1988). It was found that glutamate exposure to primary cultures of rat cerebellar granule cells results in the translocation of protein kinase C (PKC) to the cell membrane after glutamate is removed (Manev et al 1989). Preventing such translocation decreased the occurrence of sustained post glutamate calcium influx and delayed neuronal death, indicating PKC is an important player in calcium related neuronal death mediated by glutamate excitotoxicity. These findings are important to consider as it is known that mitochondria sequester large amounts of positively charged calcium ions within their negatively charged matrix. In cases of extreme intracellular calcium concentrations, increased calcium sequestration in the mitochondrial matrix results in a loss of membrane potential across the inner mitochondrial membrane as the matrix becomes more positively charged (Rottenberg & Scarpa 1974). This not only results in decreased ATP production but also the formation of the mitochondrial permeability transition pore (MPTP, see Figure 1B-5), in which a mega channel forms allowing water, ions and molecules up to 1500 Daltons to move freely across the inner mitochondrial membrane (Hunter & Haworth 1979, Hunter et al 1976). Upon MPTP formation, water will follow its osmotic gradient and pass into the highly concentrated matrix causing swelling of the mitochondria and bursting of the outer mitochondrial membrane (Figure 1B-6), consequently releasing ROS, reactive nitrogen species (RNS), calcium, and cytochrome c in the cell. Cyclophilin D, a component of the MPTP, interacts with mitochondrial amyloid-beta protein in Alzheimer’s disease as evidenced in a mouse model where cyclophilin D deficiency in cortical neurons increases their mitochondrial Ca2+ buffering capacity whiles improving memory and spatial learning (Du et al 2008). Moreover, small-molecule cyclophilin D inhibitors decrease the detrimental effects of Aβ and calcium-induced MPTP (Valasani et al 2016, Valasani et al 2014), indicating MPTP as a therapeutic target for neuronal disease. In addition to causing potential mitochondrial swelling and MPTP, NMDA receptor activation and calcium influx also leads to activation of nitric oxide synthase, resulting in production of nitric oxide, a powerful oxidant (Dawson et al 1991). Overstimulation of NMDA receptors can result in ROS production including the formation of superoxide radicals (Lafon-Cazal et al 1993). Superoxide radicals then react with nitric oxide to form peroxynitrite (Beckman et al 1990), an oxidant which reacts with lipid membranes, proteins, and DNA and may further cause release of calcium by mitochondria which can be inhibited by cyclosporin A (Packer & Murphy 1994). ROS and RNS can damage nearby proteins and lipids in the mitochondrial membranes, as well as mtDNA (Figure 1B-7). When mitochondria undergo oxidative damage they release higher amounts of ROS, and when the ROS overload becomes too much for the endogenous antioxidant systems to handle, these mitochondria will also undergo MPTP. Damaged mitochondria can then cause a domino effect resulting in widespread mitochondrial impairment until a threshold signals the cell to undergo apoptosis (Figure 1B-8).
1.2.2 Mitochondria as Death Switch for Apoptosis
An early feature of cellular apoptosis is disruption of the ETS. Consequently, there is a drop in ATP production, though this is observed more prevalently in late stages of apoptosis as sufficient ATP levels are necessary for the process of apoptosis, requiring protein translation; in cases of extreme ATP depletion the cell undergoes necrosis (Ankarcrona et al 1995, Eguchi et al 1997). Cytochrome c, a protein in the electron transport chain that shuttles electrons from complex III to complex IV, can act as a signal for apoptosis- when released into the cytosol it can bind to activating factor-1 causing formation of an apoptosome complex that then activates caspases necessary to signal the induction of apoptosis, (see (Riedl & Salvesen 2007). Loss of calcium buffering capacity induced by mitochondrial damage results in calcium dysregulation within the cell such as activation of calpains and phospholipases to induce the release of apoptosis inducing factor (AIF) from mitochondria (Polster et al 2005), downstream release of cytochrome c, and mitochondrial membrane permeability transition (Krajewska et al 2004). Acute insult in vitro using high concentrations of NMDA or free radicals result in necrosis; on the contrary the apoptotic pathway is favored when less concentrated NMDA and free radicals are present. Further, NMDA-induced cell death can be prevented with the use of NMDA receptor antagonists, but not when free radicals are simultaneously present (Bonfoco et al 1995). Mitochondrial function is necessary for cells to undergo apoptosis, as this form of controlled cellular death requires energy, (see (Kroemer et al 1998). Severely damaged mitochondria cause cells to undergo necrosis, which is typically detrimental to nearby cells.
1.2.3 mtDNA Damage
Mitochondria contain their own circular mtDNA located within the matrix. mtDNA replication and transcription occurs in the S and G2 phases of the cell cycle (Pica-Mattoccia & Attardi 1971, Pica-Mattoccia & Attardi 1972), but mitochondrial protein synthesis doubles during interphase and remains relatively constant during the cell cycle (England & Attardi 1974). This DNA is located within close proximity to the ETS, which is prone to release oxidative molecules, putting the mtDNA at a high risk for being damaged (Figure 1B-7). Because of their location, mtDNA undergo mutations at a higher rate than nuclear DNA (Brown et al 1979). Additionally, nuclear DNA has different protection and repair mechanisms that are lacking for mtDNA. mtDNA lacks exons and histones, which protect nuclear DNA by creating a tighter structural formation. Mitochondria have a distinct DNA polymerase separate from that of nuclear DNA (Ch'ih & Kalf 1969, Meyer & Simpson 1970). It has been suggested that there are no recombinant abilities in mtDNA. When creating mouse×rat somatic cell hybrids and rat cytoplasmic hybrids, Hayashi et al (1985) found that there was no mtDNA recombination, even when chloramphenicol (CAP) resistant mtDNA was incorporated into a CAP-sensitive cell. The cell remained resistant to CAP, but the mtDNA was unchanged. The group theorized that since mtDNA is inherited completely maternally, there is no necessity for recombination of mtDNA, and thus it has lost the ability to do so (Hayashi et al 1985).
1.2.4 Immunogenicity of Mitochondria
It is apparent that mitochondria are of bacterial in origin, as evident by common morphological properties including circular DNA, double membrane including cardiolipin, lack of histones, and ability to form N-formyl peptides. Because these properties are also recognized by the body’s immune system as bacterial and thus non-self, or something to be eradicated, the mitochondria can stimulate an immune response similar to that induced by a bacterial infection (Lotze et al 2007). DAMPs (damage-associated molecular patterns) can activate antigen presenting cells such as dendritic cells and macrophages, and thus can be immunostimulatory (Krysko et al 2011, Rubartelli & Lotze 2007). They can be located on the cell surface, secreted to the extracellular matrix, or produced as a byproduct of degradation (Garg et al 2010). Some DAMPs associated with mitochondria include mtDNA (Collins et al 2004, Zhang et al 2010b), N-formylmethionyl proteins (Carp 1982), Cytochrome c, ATP (Ghiringhelli et al 2009, Iyer et al 2009), cardiolipin (Sorice et al 2004), carbamoyl phosphate synthase (Struck et al 2005), and ROS (Kazama et al 2008). In cases of severe tissue trauma, cellular necrosis can release massive quantities of mitochondrial particles, including mtDNA, which have been found to activate neutrophils and create an immune response in a rat model of trauma/hemorrhagic shock (Zhang et al 2010a, Zhang et al 2010b). ATP release from apoptotic cells into the extracellular matrix acts to recruit monocytes and neutrophils in a model of human neutrophil chemotaxis in vitro (Chen et al 2006, Elliott et al 2009) as well as activation of inflammasomes (Schroder & Tschopp 2010).
2. Therapeutic Methods of Targeting Mitochondrial Dysfunction
As proper mitochondrial function is essential for cell survival, mitochondrial dysfunction can have devastating consequences. Therefore, restoring mitochondrial function through various means such as uncoupling, antioxidant addition, and biofuel addition have been tested, with varying degrees of effectiveness.
2.1 Mild Uncoupling
Drugs that uncouple the mitochondrial ETS have been investigated for their potential benefits in retaining mitochondrial function after different models of traumatic nervous tissue damage in vivo (Jin et al 2004, Patel et al 2009, Rodriguez-Jimnez et al 2012, Sullivan et al 2004). Using drugs to mildly uncouple the ETC from complex V allows hydrogen atoms to diffuse freely from the intermembrane space back into the matrix (see (Skulachev 1998). This allows for maximal transport of electrons through the ETC, without conversion of ADP to ATP. When the uncoupling is mild, there can be a buildup and maintenance of membrane potential across the IMM. This maintenance of membrane potential is beneficial in that the mitochondria are less likely to go through MPTP. One group found addition of such an uncoupler, 2,4 dinitrophenol, resulted in neuroprotection in a rat model of quinolinic acid- induced Huntington’s disease (Maragos et al 2003). Others have found that using mild uncoupling proteins (UCP2 and UCP3) reduces ROS production and may be beneficial in treating neurodegenerative diseases (see (Brand & Esteves 2005).
2.2 Antioxidants
The use of antioxidants to supplement the endogenous mitochondrial antioxidant systems have been used to combat free radicals such as ROS and RNS, (see (Bains & Hall 2012, Smith et al 2008). Reactive species can attack and damage mitochondrial electron transport complexes and mtDNA, further increasing mitochondrial dysfunction. Administration of antioxidants or precursors to antioxidants has been shown to have beneficial effects for Parkinson’s disease (Jin et al 2014) and after both traumatic SCI (Patel et al 2014, Patel et al 2009) and TBI (Hall et al 2010). Administration of the antioxidant, resveratrol, has been shown to increase mitochondrial antioxidant enzyme activity (MnSOD) in mouse brains (Robb et al 2008) and reduce oxidative stress in coronary endothelial cell cultures (Ungvari et al 2009). Tempol treatmetn is shown to decrease oxidative stress and mitochondrial dysfunction while increasing cell survival and ATP production in a mouse model of nephrotoxicity (Ahmed et al 2014), and it partially restores mitohcondrial respiration in a model of rat SCI (Xiong & Hall 2009). Further, modified antioxidants are being used in ways to specifically deliver them to mitochondria for optimal effectiveness (see (Sheu et al 2006).
2.3 Substrate Addition
Substrates that feed in to the ETC have also been utilized as mitochondrial therapy. Increasing the amount of substrates such as NADH, acetyl-CoA, and succinate can increase mitochondrial bioenergetics- the subsequent increase in ATP not only provides more energy to the cell, but helps to maintain a healthy membrane potential across the IMM (for review see (Pettegrew et al 2000). Biofuels such as pyruvate and beta-hydroxybutyrate have been found to restore mitochondrial respiration when delivered to cultured neurons after glucose deprivation (Laird et al 2013). Other alternative substrates that can be used by mitochondria for respiration, such as acetyl-L-carnitine, has beneficial effects in clinical trials for Alzheimer’s and Parkinson’s disease (Carta & Calvani 1991, Puca et al 1990), it lowers oxidative damage and restores mitochondrial function in rat models of aging (Liu et al 2002, Petruzzella et al 1992), and has been shown to be neuroprotective following experimental SCI (Patel et al 2010).
3. Mitochondrial Transplantation
A burgeoning approach used in mitochondrial medicine (Armstrong 2007, Luft 1994) is transplanting mitochondria from a healthy, exogenous source into damaged tissues in the effort to rescue cells or tissues from death. Various models of mitochondrial transplantation have been investigated, each with their own caveats and insights, which will be discussed in the ensuing sections. Many of the ‘mitochondrial medicines’ that have been explored, such as antioxidants and alternative substrates (biofuels), have a short therapeutic time window in which to treat damaged mitochondria before the oxidative and energy depletion damage becomes overwhelming. With transplantation methods to replace damaged mitochondria it may be possible to decrease ROS production within the cell (Figure 1C-9), provide a new pool of exogenous mtDNA damaged from oxidative processes (Figure 1C-10), increase energy production (Figure 1C-11), and increase calcium buffering capacity (Figure 1C-12). By providing new mitochondria to the cell it will retain enough energy-producing capacity for survival, thus allowing damaged mitochondria to undergo mitophagy, a form of mitochondria-specific targeted autophagy (see (Ding & Yin 2012), to maintain sufficient energy production sources for self-repair. The prospect of replacing damaged and dysfunctional mitochondria with healthy exogenous mitochondria has already been attempted with promising results.
Mouse models demonstrating the synchronous transfer of cytoplasts from a donor oocyte to a recipient oocyte which led to the development of a healthy zygote (Levron et al 1996) furthered the research efforts of human ooplasmic donation. This process of injecting a small portion of cytoplasm which includes mRNA, protein, and mitochondria increases the chances of compromised eggs to survive. Such cytoplasmic transfer therapy was tested in the clinic to transfer the cytoplasm of a healthy donor oocyte into a compromised oocyte, which was then fertilized and implanted and resulted in successful pregnancy (Cohen et al 1998, Cohen et al 1997). The developing embryo thus contained nuclear DNA from the mother and father, along with mtDNA from the donor egg. While cytoplasmic transfer therapy resulted in successful births, it was banned in the United States in 2001 for ethical reasons; however the practice is still being performed legally in other countries (Ishii 2014).
Since defects in mtDNA can be passed from mother to child, this has also inspired children born with three biological parents using nuclear transfer- a mother and father providing the nuclear DNA, and a separate donor for the mtDNA (Reardon 2016). This therapy involves removal of the nucleus and genetic material from a donor egg with healthy mitochondria. The nucleus is removed from the mother’s egg (containing unhealthy mitochondria) and implanted into the donor egg, resulting in an egg with donor mitochondria (with mtDNA) and cytoplasm, and the mother’s nucleus (with nuclear DNA). The egg is then fertilized with the father’s sperm and an embryo develops with three distinct genetic parents. This therapy has been shown in non-human primates to circumvent the genetic inheritance of mitochondrial disease when it is known that the mother’s mitochondria carry DNA mutations (Tachibana et al 2009). Mitochondrial donation therapy as a modified version of in vitro fertilization was legalized in the United Kingdom in early 2015.
3.1 Methods of Transplantation
While mitochondrial replacement therapy has been proven successful in these types of embryonic genetic therapies, there is a dire need to develop complementary approaches for treating adults who suffer from mtDNA defects and mitochondrial dysfunctions. The transplantation of mitochondria isolated from one source into different recipient cells has been successful in various models and multiple studies have shown incorporation of exogenous mitochondria via direct injection, co-incubation, and cell-mediated transfer, both in vitro and in vivo.
3.1.1 In vitro Transplantation
As early as 1982, the possibility of isolated mitochondrial transplantation into mouse adrenal and embryonal carcinoma cell cultures was shown (Clark & Shay 1982). Briefly, chloramphenicol- and efrapeptin-resistant mitochondria were isolated and purified from mammalian cells and then co-incubated with cells lacking resistance to the mitochondrial-targeting antibiotics. It was found that the isolated mitochondria labeled with rhodamine 123 were not only endocytosed by the cells as visualized by fluorescence microscopy, but they also conferred resistance, indicating successful incorporation of the exogenous mitochondria. This resistance was present in 12 passages of the cells as the mtDNA was retained. Damaging the resistant mitochondria with UV light after isolation before co-incubation rendered them incapable of conferring resistance to co-incubated cells. Moreover, resistance could not be conferred between species (mouse into human), introducing a caveat in mitochondrial transplantations- that various cell types may have more or less propensities to take up mitochondria originating from different sources.
Another group that capitalized on conferred resistance via mitochondria transplantation showed that direct injection of isolated mitochondria into cultured cells is a viable technique for replacing endogenous mtDNA (King & Attardi 1988). Using ethidium bromide, mtDNA was reduced in 143B human osteosarcoma cells to 3% of control levels. Mitochondria were then isolated from chloramphenicol-resistant CAP23 cells and microinjected into mtDNA-depleted 143B cells. Subsequent addition of chloramphenicol to the recipient cells failed to kill them, indicating successful incorporation of CAP23 mitochondria. Further, the cells contained CAP23 mtDNA within five weeks after injection while the nuclear DNA of the cells was only that of 143B cells, indicating the transfer of only mtDNA.
Direct microinjection of isolated mitochondria into cultured cells was thought to be impractical based on the size of mitochondria compared to the gauge of injection needles (Yang & Koob 2012), despite that previous studies used needles with tip diameter of 1 µm or less (King & Attardi 1988). To counteract this problem, mitochondria were first injected into oocytes to allow larger diameter needles to be used and then a mitocytoplast containing the cell membrane, cytoplasm, and the previously injected mitochondria was taken from the oocyte (Yang & Koob 2012). This mitocytoplast could then be fused with recipient lung carcinoma LL2/rho0 cells that are devoid of mtDNA so that mitochondria were then transferred into the recipient cell cytoplasm. An important finding in this study revealed that when a cell is in the presence of two different species of mtDNA, recipient cells will favor the survival of syngeneic mtDNA, similar to the findings of Clark and Shay (1982).
Others have explored direct injection techniques in which mitochondria were isolated from rabbit heart tissue and then injected into regions of ischemic rabbit hearts ex vivo shortly before reperfusion (McCully et al 2009). Autologous mitochondria were directly injected into heart tissue following 29 minutes of ischemia, which resulted in decreased infarct size of the post-ischemic tissue. They then tested injections of frozen, non-functional mitochondria into heart tissue and showed that functional, respiratory competent mitochondria were necessary to decrease ischemic infarct size and maintain function of cardiac tissues.
In addition to direct transfer approaches into host cells, the possibility of mitochondrial transfer by co-incubation has been investigated. Co-incubation of cultured cells with isolated mitochondria results in successful incorporation of the exogenous mitochondria into host cells, often those that had undergone some sort of insult. Xenogenic transfer of mitochondria was evident when isolated mitochondria were co-incubated with cells in vitro, resulting in the successful incorporation of exogenous mitochondria (Katrangi et al 2007). It was shown that healthy murine mitochondria were taken into compromised human mesenchymal stem cells, resulting in their restored respiratory capabilities. The group demonstrated transfer by fluorescently labeling and tracking isolated mitochondria, measuring respiration changes in the cells after transfer, and detecting exogenously-derived mtDNA using polymerase chain reaction (PCR) techniques. These results contrast those found by Clark and Shay (1982) in which mitochondria originating from one species were found not to transfer via co-incubation into host cells of a different species.
Others have studied transfer of isolated mitochondria conjugated to a cell-penetrating peptide, Pep-1 (Chang et al 2013). Pep-1 causes translocation of its cargo in an endosomal-independent manner and has been used to deliver small peptides and DNA sequences, but never entire organelles- for review see (Morris et al 2008). Using mitochondrial specific fluorescent dyes, Chang et al (2013) reported that successful transplantation of mitochondria into recipient cells was found only when mitochondria were conjugated with Pep-1. Conjugated mitochondria-Pep-1 were isolated and co-incubated either with cells derived from myoclonic epilepsy with ragged-red fibers (MERRF) syndrome that had mutated mtDNA hindering mitochondrial protein synthesis or with cells lacking mtDNA. The exogenous mitochondria-Pep-1 co-localized with host mitochondria and resulted in the recovery of mitochondrial membrane potential in cells, increased oxygen consumption, increased ATP production, and decreased lactate production, all indicating a switch from anaerobic to aerobic respiration. This is in contrast to previous reports (Clark & Shay 1982, Katrangi et al 2007) that showed isolated mitochondria could be taken up by host cells in vitro without Pep-1.
In cell-to-cell transfer paradigms, it has been shown that co-culturing human lung epithelial cells containing damaged and/or depleted mitochondria with healthy adult human bone marrow stem cells results in donation of healthy stem cell mitochondria and rescue of epithelial cells that contained functional mitochondria, as indicated by increased ATP production and translation of mitochondrial protein COXII (Spees et al 2006). Microscopy showed that the bone marrow stem cells made cytoplasmic projections towards the epithelial cells, shuttling mitochondria through the extensions between connected cells. In contrast to other studies, this group showed no evidence of isolated mitochondrial transfer into cells using co-incubation methods- when comparing transplantation of isolated mitochondria versus cell-to-cell transplantation, transfer was only attained in a cell-cell fashion, suggesting that movement of exogenous mitochondria into host cells is an active process.
Nanotube formation between cells was reported through which organelles, including mitochondria, were exchanged from one cell to another when co-culturing human bone marrow mesenchymal stem cells with rat embryonic cardiac myocytes (Plotnikov et al 2008). After co-culturing for 24 hours, the cells formed nanotube connections and TMRE-labeled mitochondria were visualized within the nanotubes; Tetramethylrhodamine Ethyl Ester is a membrane potential-dependent mitochondrial dye. Further, the propensity of each cell type to form tunneling nanotubes was higher when co-cultured with the xenogenic cell type compared to a syngeneic cell type. By using calcein-AM, a cell membrane permeant dye, the group was able to demonstrate the movement of cytoplasmic contents in both directions between cultured cells using scanning confocal microscopy, indicating that cytoplasm from both cardiomyocytes and human mesenchymal stem cells was shared. It was also shown that physically disrupting tunneling nanotube formation with gentle shaking inhibited this sharing of cytosolic contents. Using MitoTracker Red and MitoTracker Green FM dyes, each specific for one cell type, they visualized mitochondrial transfer between the co-cultured cells. There was no detectable transfer at 3 hours but was evident after 24 hours of co-culturing. Interestingly, mitochondria movement was apparent from the mesenchymal stem cells to the cardiomyocytes, but there was no reciprocal movement and no co-localization of the MitoTracker markers, indicating that mitochondria originating from the two different sources did not undergo fusion at 24 hours.
Another group found that co-culturing of rat cardiomyoblasts that had undergone oxygen glucose deprivation (OGD) with healthy mouse mesenchymal stem cells resulted in the formation of 200–500 nm diameter nanotubes between cells after 2 hours, and that after 24 hours these nanotubes contained the membrane potential-dependent dye MitoTracker Red (Cselenyak et al 2010). This indicated the presence of functionally competent mitochondria, although the directionality of movement was not obvious using time-lapse confocal microscopy. Importantly, such co-culturing significantly rescued the cardiomyoblasts from OGD.
Others have reported the formation of tunneling nanotubes in cultured cells that underwent ultraviolet radiation stress (Wang & Gerdes 2015). They showed that PC12 cells in the beginning stages of apoptosis formed nanotubes that reached out to healthy, unstressed cells and that when UV-stressed cells were co-cultured with healthy cells there was reduced cell death compared to co-culturing with UV-stressed cells. EdU-labeled mtDNA could be detected in the stressed cells, indicating transfer had occurred into the damaged cells. They further found that mtDNA-deficient PC12 cells lacking electron transport function could not rescue UV-stressed cells whereas PC12 cells with functional mitochondria did.
It is important to note that the reported successful transfer of mitochondria from one cell type to another appears to be most prevalent when transfer occurred from a healthy cell to a cell with dysfunctional and/or depleted mitochondria. While there are many caveats when considering donor vs recipient cell types in the propensity to take up mitochondria, there appear to be mechanisms that allow damaged cells to receive mitochondria more readily than healthy cells. For example, Cho et al. (2012) found that cell-to-cell mitochondrial transfer occurred via partial cell fusion, but only when the receiving cell had dysfunctional mitochondria. The group showed that co-culturing of human mesenchymal stem cells with both human osteosarcoma 143B cells lacking mtDNA and cells with severe R6G toxin-induced mitochondrial dysfunction resulted in the transfer of mitochondria accompanied by their increased ATP and oxygen consumption. However, transfer was not evident when recipient cells had mtDNA mutations, leading the group to conclude that mitochondrial transfer occurs only when mitochondrial function is severely hindered or absent (Cho et al 2012).
3.1.2 In vivo Transplantation
Phylogenetic mapping and mitochondrial transplantation methods have been performed in a number of animal models in vivo. Interestingly, a study investigating the phylogeny of different canine species with canine transmissible venereal tumor (CTVT) by sequencing their mitochondrial genome (Rebbeck et al 2011) revealed an unexpectedly high polymorphism rate in the CTVT samples thought to be caused by transfer of mitochondria from the dogs into the cancerous tumor cells. The group theorized that host (dog) mitochondria may be healthier than that of the CTVT cells, encouraging transfer of mitochondria and subsequent increase in CTVT metabolic health. This theory reflects an endogenous mechanism of mitochondrial transfer in vivo, from one cell to another.
The McCully group who studied direct injection of mitochondria ex vivo (McCully et al 2009) also reported successful mitochondrial transplantation in vivo using a direct injection technique (Masuzawa et al 2013). Autologous transplantation of isolated mitochondria directly into ischemic rabbit hearts immediately before reperfusion resulted in increased mechanical function as soon as 10 minutes after injection, in addition to significantly higher ATP content after 2 days post injection. Further, they showed via confocal microscopy that cardiomyocytes can take in exogenous mitochondria as early as 2 hours after transplantation. The group recently compared direct injection mitochondrial transplantation to a more clinically relevant, less invasive vascular perfusion delivery method (Cowan et al 2016). Rabbit hearts underwent focal ischemia for 30 minutes in situ followed by reperfusion and delivery of mitochondria isolated from adult human cardiac fibroblasts. Mitochondria were delivered either by direct injection into the ischemic heart tissue or they were perfused through the coronary artery. They noted that a large amount of mitochondria were found in the interstitial spaces, but some mitochondria were co-localized within cardiomyocytes. Vascular delivery of mitochondria resulted in a larger dispersal area, while direct injection resulted in a higher concentration in smaller regions of tissue. Importantly, this study showed that both direct injection and vascular delivery of mitochondria afforded functional protection in measures of muscle contractility and decreased infarct size after ischemia-reperfusion injury.
In addition to direct injections in vivo, cell-to-cell transfer of mitochondria has also been reported in vivo. One study instilled mouse bone marrow derived stromal cells (mBMSC) into acutely injured mouse lungs which resulted in connexin pore-dependent attachment of the mBMSC to the injured lung epithelium (Islam et al 2012). This attachment was followed by nanotube formation and delivery of mitochondria-containing vesicles into the injured tissue, leading to increased ATP production and cell survival after injury. The group determined that connexin pore and gap junction channel formation was necessary for such transfer since blocking connexin pore formation inhibited gap junction channel formation, which was then found to inhibit cytosolic mixing between the two cells. When dynamin was inhibited, a GTPase responsible for endocytosis in the eukaryotic cell, then mitochondrial delivery was blocked leading the group to posit endocytosis to be the mechanism responsible for mitochondrial transfer. Again, it is important to note that the mitochondria were transferred to damaged cells or tissues.
Recent compelling studies have shown that endogenous mitochondrial transfer occurs between resident astrocytes and neurons after stroke injury (Hayakawa et al., 2016). The investigators stimulated astrocytes in vitro to release mitochondria by upregulating the CD38 protein, and found that the addition of these astrocyte-derived mitochondria in a model of neuronal cell injury in vitro was successful in increasing neuronal ATP production and cell survival, and that these mitochondria were incorporated into the neuronal cells. They further studied this in vivo by directly injecting astrocyte-derived mitochondria into the injury site 3 days after inducing cerebral ischemia in mice and found these mitochondria were taken into neurons. The endogenous transfer of mitochondria was seen in these transgenic mice with labeled astrocytic mitochondria that were found within neurons 24 hours after ischemic stroke, and cell survival signals such as phosphorylated AKT were increased after this transfer (Hayakawa et al 2016).
3.2 Tracking Exogenous Mitochondria after Transplantation
Various methods have been utilized to verify that exogenous mitochondria are successfully transplanted into host cells. One of the most common procedures is to transplant exogenous mitochondria of one species or strain of animal into a host cell of a different species, and to then perform PCR on the host cell mtDNA showing that the exogenous mtDNA is present (Islam et al 2012, Kitani et al 2014, Yang & Koob 2012). PCR is also performed to show transfer of mtDNA into cells lacking mtDNA (Spees et al 2006) or transfer of mitochondria containing a specific sequence for chloramphenicol resistance (King & Attardi 1988). Alternatively, species-specific mitochondrial antibodies are used to detect exogenous mitochondria (Masuzawa et al 2013). Further, mitochondria can be fluorescently labeled pre-injection to visualize after uptake into the host cells by either transfection methods (Chang et al 2013, Islam et al 2012, Kitani et al 2014, Spees et al 2006, Wang & Gerdes 2015, Yang & Koob 2012), using mitochondrial-specific dyes such as MitoTrackers or rhodamine derivatives (Chang et al 2013, Clark & Shay 1982, Cselenyak et al 2010, Katrangi et al 2007, Masuzawa et al 2013, McCully et al 2009, Plotnikov et al 2008), or transgenic mice in which a subset of mitochondria are fluorescently labeled (Hayakawa et al 2016). Another method labels mitochondria using pHrodo Red, a pH-sensitive label that indicates proton levels. Using this, increased fluorescence of cardiomyocytes in vitro could be measured, indicating mitochondrial internalization that could be quantified as number of mitochondria per nucleus (Pacak et al 2015).
3.3 Mitochondria Transplantation Increases Health of Host Cells and Tissues
Many different outcome measures are being used to show that transplanted mitochondria have beneficial effects on host tissues. Energy production, mitochondrial protein translation, and enzyme activities have all been reported to be increased after transplantation. One group found a three-fold increase in ATP levels and a decrease in lactate levels in cells rescued by cell-to-cell mitochondrial transfer, indicating that the host cells with depleted mtDNA were able to switch from anaerobic respiration to aerobic respiration after receiving mitochondria (Spees et al 2006). Further, the host cells that contained extensive mtDNA damage could again translate mitochondrial proteins, namely COXII, after incorporating exogenous mitochondria. Others showed that mitochondrial transplantation into damaged lung epithelia resulted in increased ATP production approaching control levels, not only in the cells that received exogenous mitochondria, but also in juxtaposition alveoli (Islam et al 2012). The increase in ATP to normal levels rescued functional surfactant excretion by the alveolar cells. Increased cellular ATP content and oxygen consumption rates were also reported after exogenous mitochondria internalization into either cultured cardiomyocytes or HeLa cells lacking mtDNA, and PCR confirmed replacement with exogenous mtDNA in the cells (Pacak et al 2015).
Mitochondrial function and ROS production have also been used to assess the benefits of mitochondrial transplantation. For instance, flow cytometry and the fluorescent dyes rhodamine 123 and JCI have been used to determine changes in mitochondrial membrane potential in culture using MERRF cells (Chang et al 2013). They also measured oxygen consumption rates as well as ATP levels, cell viability, amount of fusion and fission proteins, caspase-3/-7 activity, mtDNA copy number, mitochondrial mass, and mitochondrial calcium uptake. ATP production has also been assessed using fluorometric analyses. TBARS (thiobarbituric acid-reactive substances) has been used to show differences in oxidative damage and TdT-mediated dUTP nick-end labeling (TUNEL) assays have been used to investigate apoptosis (McCully et al 2009). This latter group also employed Mito-Tracker Orange CMTMRos label to show that labeled mitochondria maintained a viable membrane potential and their oxygen consumption rates showed that they were respiration competent.
3.4 Mechanisms for Mitochondrial Incorporation
Cell-to-cell fusion has been disputed as a necessary mechanism of mitochondrial transfer. When co-culturing human mesenchymal stem cells with human alveolar epithelial cells lacking mitochondrial DNA, transfer of mitochondrial but not nuclear DNA was reported (Spees et al 2006). Further, the addition of platelets or isolated mitochondria to cultured cells lacking mtDNA did not result in mtDNA transfer, indicating the incorporation of exogenous mitochondria is not a passive process. In fact, the mesenchymal cells made cytoplasmic extensions towards alveolar cells lacking mtDNA, after which the depleted cells began dividing after being rescued by mitochondrial transfer. It was not shown whether mitochondrial transfer was achieved through nanotube formation or budding of mitochondria-filled vesicles from the donor cells. However, other groups have demonstrated that co-incubation of cells with purified isolated mitochondria results in the uptake of exogenous mitochondria into cells (Clark & Shay 1982, Katrangi et al 2007). This indicates an uncertainty as to whether mitochondria can be passively endocytosed in cell culture. There may be a difference among cell types in the ability to incorporate exogenous materials, as was demonstrated in an earlier study examining incorporation of nuclear DNA among different cell types (Wigler et al 1980). Clark and Shay (1982) similarly showed that different cell lines had a different propensity to uptake co-incubated isolated mitochondria, suggesting that mitochondria are taken up by recipient cells via different endocytic properties according to phenotype.
Islam et al (2012) reported that physical docking of mBMSC to injured lung epithelial cells was necessary for transfer of mitochondria to alveolar cells. mBMSC attachment to injured cells occurred at areas of high CX43 connexin concentration on lung epithelial cells, and specifically blocking CX43 hindered binding and transfer of cytosolic contents from mBMSC to epithelial cells. In vitro, mBMSC formed nanotubes with epithelial cells and released microvesicles containing DsRed-labeled mitochondria. They concluded that gap junctional channels are necessary for attachment, while microvesicles and nanotubes are needed for the movement of mitochondria. Inhibition of dynamin blocked mitochondrial transfer, implicating that endocytosis is responsible for the engulfment of mBMSC microvesicles (Islam et al 2012). Koyanagi et al examined intercellular connections and whether large structures, including organelles such as mitochondria, could be transferred between cells (Koyanagi et al 2005). They found that when human endothelial progenitor cells were co-cultured with cardiomyocytes containing MitoTracker-labeled mitochondria, time lapse imaging showed formation of transient nanotubular structures and the progression of labeled structures moving from the cardiomyocytes to the endothelial progenitor cells. Interestingly, the reverse transport was not evident: when endothelial progenitor cell mitochondria were labeled with MitoTracker there was not transport to the unlabeled cardiomyocytes, indicating there may be cell-type differences that are important to consider for mitochondria transfer. Wang et al showed that tunneling nanotubes can be formed in vitro, providing a pathway for mitochondrial transfer (Wang & Gerdes 2015). PC12 cells stressed by UV created tunneling nanotubes with a larger diameter and containing microtubules that attached to control, unstressed cells. Fluorescence microscopy detected tagged mitochondria located within nanotubes connecting stressed and control cells, but no presence of mitochondria in nanotubes connecting only control, unstressed cells. Live cell imaging techniques allowed tracking of mitochondria from the control cell, through the nanotubes, and into the stressed cells. When tunneling nanotube formation was inhibited using cytochalasin B, an F-actin depolymerizing agent, they found a higher incidence of cell death with the UV stressed/unstressed co-culture suggesting that nanotube formation was necessary for the rescue effect. They also showed positive co-localization of MitoTracker Red-labeled mitochondria with immunostained microtubules located within the nanotubes. They further transgenically labeled mitochondria with DsRed2-mito in the control cells to quantify their transfer into stressed cells. Using cytochalasin B to inhibit the nanotubes decreased the amount of Dsred2-mito in the stressed cells.
An elegant study was performed by the McCully group to determine the mechanism of mitochondrial internalization in vitro (Pacak et al 2015). Chemicals were utilized to inhibit different internalization mechanisms, such as cytochalasin D to block actin polymerization, methyl-β-cyclodextrin to block caveola-dependent clathrin-dependent endocytosis, nocodazole to block tunneling nanotubes, and 5-(N-Ethyl-N-isopropyl) amiloride to block macro-pinocytosis. The only chemical that changed mitochondrial internalization was cytochalasin D, indicating that actin polymerization is involved in mitochondrial uptake into the cardiomyocyte cells, though it was not evident how the mitochondria left the endosomes once they were internalized. The other chemicals had no beneficial effects suggesting that clathrin-dependent endocytosis, tunneling nanotubes, and macro-pinocytosis are not involved. This is in accordance with their previous report that mitochondria were not co-localized with lysosomes, caveolae or autophagosomes upon internalization (Masuzawa et al 2013). In contrast, others utilized fluorescent live imaging to track movement of transgenically-labeled DsRed mitochondria into mesenchymal cells and reported that macropinocytosis is necessary for mitochondrial internalization in vitro (Kitani et al 2014). It should be noted that various dosages of 5-(N-Ethyl-N-isopropyl) amiloride were used in attempts to block macro-pinocytosis in the different cell types used in each study.
4. Discussion
Great strides have been made in the field of mitochondrial replacement and transplantation therapies, and many techniques have proven to be beneficial, although there are still very important questions that must be addressed before such therapies can be used widely and safely. There is disconnection among labs as to the mechanisms of mitochondrial incorporation into host cells. While some argue that it may be endocytosis, others indicate the mechanism is much more complicated. Is the formation of nanotubes between cells necessary or is it the connection supplied by gap junctions or connexin? Are the connections seen in vitro formed in vivo? Some found a carrier peptide to be a necessary factor to incite mitochondrial uptake while others found mitochondrial uptake upon co-incubation in cell culture. Others found connexins or gap junctions necessary for cells to take in extracellular mitochondria.
Further, some laboratories find that co-incubation in vitro results in uptake of exogenous mitochondria and increased bioenergetics in recipient cells while others report no such incorporation of isolated mitochondria. Whether there are major technical differences among the studies or if there are other confounding factors remains unclear. It may be due to different cell types having different metabolic needs or different capacities for endocytosis. Additionally, the type of damage to the host cells may be a crucial factor. Whether the cell undergoes stress, depleted mtDNA, or damage to the ETC may decide whether the cell can endocytose mitochondria as multiple studies have reported that only compromised cells are capable of taking in exogenous mitochondria; still others have noted the incorporation in healthy cells.
The collective findings from the studies reviewed herein support the indication of mitochondrial transplantation as a potentially multifactorial therapy leading to increased ATP production, decreased oxidative stress, mtDNA replacement, and improved enzymatic functions; all to increase overall bioenergetics which ultimately promotes tissue integrity and functionality in models of tissue damage in vivo. Importantly, a seminal finding is that mitochondrial transplantation from healthy cells to injured neurons in vivo occurs naturally, which can be further enhanced by direct injection of exogenous mitochondria into injured tissues that reduces infarct size (Hayakawa et al 2016). Along these lines of evidence, we have recently reported successful transplantation and incorporation of exogenous mitochondria into injured tissues using a rat model of contusion spinal cord injury, which was correlated with increased bioenergetics (VanRooyen et al 2016). This ability of damaged neurons to incorporate extracellular mitochondria after traumatic injuries may open a new avenue for neuroprotective strategies. The studies outlined in this review foster the advancement of ‘mitochondrial medicine’, taking the field in a new and exciting direction of treating mitochondrial dysfunction via replacement strategies.
Acknowledgments
Funding: This work was supported by SCoBIRC Endowment (AGR), NIH F31NS093904-01A1 (JLG)
Abbreviations
- AIF
apoptosis inducing factor
- ATP
adenosine triphosphate
- CAP
chloramphenicol
- CTVT
canine transmissible venereal tumor
- DAMPs
damage-associated molecular patterns
- ETC
electron transport chain
- ETS
electron transport system
- GSH
glutathione
- IMM
inner mitochondrial membrane
- ROS
reactive oxygen species
- MnSOD
manganese superoxide dismutase
- mBMSC
mouse bone marrow derived stromal cells
- mtDNA
Mitochondrial DNA
- MERRF
myoclonic epilepsy with ragged-red fibers
- MPTP
mitochondrial permeability transition pore
- NMDA
N-methyl-D-aspartate
- O2−
superoxides
- OGD
oxygen glucose deprivation
- PCR
polymerase chain reaction
- PKC
protein kinase C
- RNS
reactive nitrogen species
- SCI
spinal cord injury
- TBI
traumatic brain injury
- TMRE
Tetramethylrhodamine Ethyl Ester
- TUNEL
TdT-mediated dUTP nick-end labeling
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: none
References
- Ahmed LA, Shehata NI, Abdelkader NF, Khattab MM. Tempol, a superoxide dismutase mimetic agent, ameliorates cisplatin-induced nephrotoxicity through alleviation of mitochondrial dysfunction in mice. PLoS One. 2014;9:e108889. doi: 10.1371/journal.pone.0108889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, et al. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–73. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- Arai M, Imai H, Koumura T, Yoshida M, Emoto K, et al. Mitochondrial phospholipid hydroperoxide glutathione peroxidase plays a major role in preventing oxidative injury to cells. J Biol Chem. 1999;274:4924–33. doi: 10.1074/jbc.274.8.4924. [DOI] [PubMed] [Google Scholar]
- Armstrong JS. Mitochondrial medicine: pharmacological targeting of mitochondria in disease. Br J Pharmacol. 2007;151:1154–65. doi: 10.1038/sj.bjp.0707288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aygok GA, Marmarou A, Fatouros P, Kettenmann B, Bullock RM. Assessment of mitochondrial impairment and cerebral blood flow in severe brain injured patients. Acta Neurochir Suppl. 2008;102:57–61. doi: 10.1007/978-3-211-85578-2_12. [DOI] [PubMed] [Google Scholar]
- Bains M, Hall ED. Antioxidant therapies in traumatic brain and spinal cord injury. Biochim Biophys Acta. 2012;1822:675–84. doi: 10.1016/j.bbadis.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87:1620–4. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi K, Rimessi A, Prandini A, Szabadkai G, Rizzuto R. Calcium and mitochondria: mechanisms and functions of a troubled relationship. Biochim Biophys Acta. 2004;1742:119–31. doi: 10.1016/j.bbamcr.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Bolanos JP, Moro MA, Lizasoain I, Almeida A. Mitochondria and reactive oxygen and nitrogen species in neurological disorders and stroke: Therapeutic implications. Adv Drug Deliv Rev. 2009;61:1299–315. doi: 10.1016/j.addr.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Bonfoco E, Krainc D, Ankarcrona M, Nicotera P, Lipton SA. Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-D-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc Natl Acad Sci U S A. 1995;92:7162–6. doi: 10.1073/pnas.92.16.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005;2:85–93. doi: 10.1016/j.cmet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Braunwald E, Kloner RA. Myocardial reperfusion: a double-edged sword? The Journal of clinical investigation. 1985;76:1713–9. doi: 10.1172/JCI112160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WM, George M, Jr, Wilson AC. Rapid evolution of animal mitochondrial DNA. Proceedings of the National Academy of Sciences of the United States of America. 1979;76:1967–71. doi: 10.1073/pnas.76.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdon RH. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free radical biology & medicine. 1995;18:775–94. doi: 10.1016/0891-5849(94)00198-s. [DOI] [PubMed] [Google Scholar]
- Bygrave FL, Ash GR. Development of mitochondrial calcium transport activity in rat liver. FEBS Lett. 1977;80:271–4. doi: 10.1016/0014-5793(77)80455-9. [DOI] [PubMed] [Google Scholar]
- Carp H. Mitochondrial N-formylmethionyl proteins as chemoattractants for neutrophils. J Exp Med. 1982;155:264–75. doi: 10.1084/jem.155.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta A, Calvani M. Acetyl-L-carnitine: a drug able to slow the progress of Alzheimer's disease? Ann N Y Acad Sci. 1991;640:228–32. doi: 10.1111/j.1749-6632.1991.tb00223.x. [DOI] [PubMed] [Google Scholar]
- Ch'ih JJ, Kalf GF. Studies on the biosynthesis of the DNA polymerase of rat liver mitochondria. Arch Biochem Biophys. 1969;133:38–45. doi: 10.1016/0003-9861(69)90485-8. [DOI] [PubMed] [Google Scholar]
- Chang JC, Liu KH, Li YC, Kou SJ, Wei YH, et al. Functional recovery of human cells harbouring the mitochondrial DNA mutation MERRF A8344G via peptide-mediated mitochondrial delivery. Neurosignals. 2013;21:160–73. doi: 10.1159/000341981. [DOI] [PubMed] [Google Scholar]
- Cheeseman KH, Slater TF. An introduction to free radical biochemistry. Br Med Bull. 1993;49:481–93. doi: 10.1093/oxfordjournals.bmb.a072625. [DOI] [PubMed] [Google Scholar]
- Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–5. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- Cho YM, Kim JH, Kim M, Park SJ, Koh SH, et al. Mesenchymal stem cells transfer mitochondria to the cells with virtually no mitochondrial function but not with pathogenic mtDNA mutations. PLoS One. 2012;7:e32778. doi: 10.1371/journal.pone.0032778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW. Glutamate neurotoxicity in cortical cell culture is calcium dependent. Neurosci Lett. 1985;58:293–7. doi: 10.1016/0304-3940(85)90069-2. [DOI] [PubMed] [Google Scholar]
- Choi DW. Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci. 1988;11:465–9. doi: 10.1016/0166-2236(88)90200-7. [DOI] [PubMed] [Google Scholar]
- Clark MA, Shay JW. Mitochondrial transformation of mammalian cells. Nature. 1982;295:605–7. doi: 10.1038/295605a0. [DOI] [PubMed] [Google Scholar]
- Cohen J, Scott R, Alikani M, Schimmel T, Munne S, et al. Ooplasmic transfer in mature human oocytes. Mol Hum Reprod. 1998;4:269–80. doi: 10.1093/molehr/4.3.269. [DOI] [PubMed] [Google Scholar]
- Cohen J, Scott R, Schimmel T, Levron J, Willadsen S. Birth of infant after transfer of anucleate donor oocyte cytoplasm into recipient eggs. Lancet. 1997;350:186–7. doi: 10.1016/S0140-6736(05)62353-7. [DOI] [PubMed] [Google Scholar]
- Collins LV, Hajizadeh S, Holme E, Jonsson IM, Tarkowski A. Endogenously oxidized mitochondrial DNA induces in vivo and in vitro inflammatory responses. J Leukoc Biol. 2004;75:995–1000. doi: 10.1189/jlb.0703328. [DOI] [PubMed] [Google Scholar]
- Cowan DB, Yao R, Akurathi V, Snay ER, Thedsanamoorthy JK, et al. Intracoronary Delivery of Mitochondria to the Ischemic Heart for Cardioprotection. PLoS One. 2016;11:e0160889. doi: 10.1371/journal.pone.0160889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cselenyak A, Pankotai E, Horvath EM, Kiss L, Lacza Z. Mesenchymal stem cells rescue cardiomyoblasts from cell death in an in vitro ischemia model via direct cell-to-cell connections. BMC Cell Biol. 2010;11:29. doi: 10.1186/1471-2121-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson VL, Dawson TM, London ED, Bredt DS, Snyder SH. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci U S A. 1991;88:6368–71. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding WX, Yin XM. Mitophagy: mechanisms, pathophysiological roles, and analysis. Biol Chem. 2012;393:547–64. doi: 10.1515/hsz-2012-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Du H, Guo L, Fang F, Chen D, Sosunov AA, et al. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease. Nat Med. 2008;14:1097–105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi Y, Shimizu S, Tsujimoto Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 1997;57:1835–40. [PubMed] [Google Scholar]
- Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–6. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England JM, Attardi G. Expression of the mitochondrial genome in HeLa cells. XXI. Mitochondrial protein synthesis during the cell cycle. J Mol Biol. 1974;85:433–44. doi: 10.1016/0022-2836(74)90442-2. [DOI] [PubMed] [Google Scholar]
- Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV, Agostinis P. Immunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation. Biochim Biophys Acta. 2010;1805:53–71. doi: 10.1016/j.bbcan.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–8. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- Hall ED, Vaishnav RA, Mustafa AG. Antioxidant therapies for traumatic brain injury. Neurotherapeutics. 2010;7:51–61. doi: 10.1016/j.nurt.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, et al. Transfer of mitochondria from astrocytes to neurons after stroke. Nature. 2016;535:551–5. doi: 10.1038/nature18928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi J, Tagashira Y, Yoshida MC. Absence of extensive recombination between inter- and intraspecies mitochondrial DNA in mammalian cells. Exp Cell Res. 1985;160:387–95. doi: 10.1016/0014-4827(85)90185-5. [DOI] [PubMed] [Google Scholar]
- Huang SF, Tsai YA, Wu SB, Wei YH, Tsai PY, Chuang TY. Effects of intravascular laser irradiation of blood in mitochondria dysfunction and oxidative stress in adults with chronic spinal cord injury. Photomed Laser Surg. 2012;30:579–86. doi: 10.1089/pho.2012.3228. [DOI] [PubMed] [Google Scholar]
- Hunter DR, Haworth RA. The Ca2+-induced membrane transition in mitochondria. I. The protective mechanisms. Archives of biochemistry and biophysics. 1979;195:453–9. doi: 10.1016/0003-9861(79)90371-0. [DOI] [PubMed] [Google Scholar]
- Hunter DR, Haworth RA, Southard JH. Relationship between configuration, function, and permeability in calcium-treated mitochondria. J Biol Chem. 1976;251:5069–77. [PubMed] [Google Scholar]
- Hutchison CA, 3rd, Newbold JE, Potter SS, Edgell MH. Maternal inheritance of mammalian mitochondrial DNA. Nature. 1974;251:536–8. doi: 10.1038/251536a0. [DOI] [PubMed] [Google Scholar]
- Ide T, Tsutsui H, Hayashidani S, Kang D, Suematsu N, et al. Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ Res. 2001;88:529–35. doi: 10.1161/01.res.88.5.529. [DOI] [PubMed] [Google Scholar]
- Ishii T. Potential impact of human mitochondrial replacement on global policy regarding germline gene modification. Reprod Biomed Online. 2014;29:150–5. doi: 10.1016/j.rbmo.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Islam MN, Das SR, Emin MT, Wei M, Sun L, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18:759–65. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer SS, Pulskens WP, Sadler JJ, Butter LM, Teske GJ, et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci U S A. 2009;106:20388–93. doi: 10.1073/pnas.0908698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Kanthasamy A, Ghosh A, Anantharam V, Kalyanaraman B, Kanthasamy AG. Mitochondria-targeted antioxidants for treatment of Parkinson's disease: preclinical and clinical outcomes. Biochim Biophys Acta. 2014;1842:1282–94. doi: 10.1016/j.bbadis.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, McEwen ML, Nottingham SA, Maragos WF, Dragicevic NB, et al. The mitochondrial uncoupling agent 2,4-dinitrophenol improves mitochondrial function, attenuates oxidative damage, and increases white matter sparing in the contused spinal cord. J Neurotrauma. 2004;21:1396–404. doi: 10.1089/neu.2004.21.1396. [DOI] [PubMed] [Google Scholar]
- Jouaville LS, Pinton P, Bastianutto C, Rutter GA, Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc Natl Acad Sci U S A. 1999;96:13807–12. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katrangi E, D'Souza G, Boddapati SV, Kulawiec M, Singh KK, et al. Xenogenic transfer of isolated murine mitochondria into human rho0 cells can improve respiratory function. Rejuvenation Res. 2007;10:561–70. doi: 10.1089/rej.2007.0575. [DOI] [PubMed] [Google Scholar]
- Kazama H, Ricci JE, Herndon JM, Hoppe G, Green DR, Ferguson TA. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity. 2008;29:21–32. doi: 10.1016/j.immuni.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MP, Attardi G. Injection of mitochondria into human cells leads to a rapid replacement of the endogenous mitochondrial DNA. Cell. 1988;52:811–9. doi: 10.1016/0092-8674(88)90423-0. [DOI] [PubMed] [Google Scholar]
- Kitani T, Kami D, Matoba S, Gojo S. Internalization of isolated functional mitochondria: involvement of macropinocytosis. J Cell Mol Med. 2014;18:1694–703. doi: 10.1111/jcmm.12316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi M, Brandes RP, Haendeler J, Zeiher AM, Dimmeler S. Cell-to-cell connection of endothelial progenitor cells with cardiac myocytes by nanotubes: a novel mechanism for cell fate changes? Circ Res. 2005;96:1039–41. doi: 10.1161/01.RES.0000168650.23479.0c. [DOI] [PubMed] [Google Scholar]
- Krajewska M, Rosenthal RE, Mikolajczyk J, Stennicke HR, Wiesenthal T, et al. Early processing of Bid and caspase-6, -8, -10, -14 in the canine brain during cardiac arrest and resuscitation. Exp Neurol. 2004;189:261–79. doi: 10.1016/j.expneurol.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Krajewski S, Krajewska M, Ellerby LM, Welsh K, Xie Z, et al. Release of caspase-9 from mitochondria during neuronal apoptosis and cerebral ischemia. Proc Natl Acad Sci U S A. 1999;96:5752–7. doi: 10.1073/pnas.96.10.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annual review of physiology. 1998;60:619–42. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- Krysko DV, Agostinis P, Krysko O, Garg AD, Bachert C, et al. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. 2011;32:157–64. doi: 10.1016/j.it.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Lafon-Cazal M, Pietri S, Culcasi M, Bockaert J. NMDA-dependent superoxide production and neurotoxicity. Nature. 1993;364:535–7. doi: 10.1038/364535a0. [DOI] [PubMed] [Google Scholar]
- Laird MD, Clerc P, Polster BM, Fiskum G. Augmentation of normal and glutamate-impaired neuronal respiratory capacity by exogenous alternative biofuels. Transl Stroke Res. 2013;4:643–51. doi: 10.1007/s12975-013-0275-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levron J, Willadsen S, Bertoli M, Cohen J. The development of mouse zygotes after fusion with synchronous and asynchronous cytoplasm. Hum Reprod. 1996;11:1287–92. doi: 10.1093/oxfordjournals.humrep.a019373. [DOI] [PubMed] [Google Scholar]
- Lightowlers RN, Chinnery PF, Turnbull DM, Howell N. Mammalian mitochondrial genetics: heredity, heteroplasmy and disease. Trends Genet. 1997;13:450–5. doi: 10.1016/s0168-9525(97)01266-3. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–95. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Liu J, Head E, Gharib AM, Yuan W, Ingersoll RT, et al. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-L-carnitine and/or R-alpha -lipoic acid. Proc Natl Acad Sci U S A. 2002;99:2356–61. doi: 10.1073/pnas.261709299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze MT, Zeh HJ, Rubartelli A, Sparvero LJ, Amoscato AA, et al. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunological reviews. 2007;220:60–81. doi: 10.1111/j.1600-065X.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- Luft R. The development of mitochondrial medicine. Proc Natl Acad Sci U S A. 1994;91:8731–8. doi: 10.1073/pnas.91.19.8731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermott AB, Mayer ML, Westbrook GL, Smith SJ, Barker JL. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. Nature. 1986;321:519–22. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- Manev H, Favaron M, Guidotti A, Costa E. Delayed increase of Ca2+ influx elicited by glutamate: role in neuronal death. Mol Pharmacol. 1989;36:106–12. [PubMed] [Google Scholar]
- Maragos WF, Rockich KT, Dean JJ, Young KL. Pre- or post-treatment with the mitochondrial uncoupler 2,4-dinitrophenol attenuates striatal quinolinate lesions. Brain Res. 2003;966:312–6. doi: 10.1016/s0006-8993(02)04225-7. [DOI] [PubMed] [Google Scholar]
- Masuzawa A, Black KM, Pacak CA, Ericsson M, Barnett RJ, et al. Transplantation of autologously derived mitochondria protects the heart from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2013;304:H966–82. doi: 10.1152/ajpheart.00883.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mc LJ, Cohn GL, Brandt IK, Simpson MV. Incorporation of labeled amino acids into the protein of muscle and liver mitochondria. The Journal of biological chemistry. 1958;233:657–63. [PubMed] [Google Scholar]
- McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–55. [PubMed] [Google Scholar]
- McCully JD, Cowan DB, Pacak CA, Toumpoulis IK, Dayalan H, Levitsky S. Injection of isolated mitochondria during early reperfusion for cardioprotection. Am J Physiol Heart Circ Physiol. 2009;296:H94–H105. doi: 10.1152/ajpheart.00567.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer RR, Simpson MV. Deoxyribonucleic acid biosynthesis in mitochondria. Purification and general properties of rat liver mitochondrial deoxyribonucleic acid polymerase. J Biol Chem. 1970;245:3426–35. [PubMed] [Google Scholar]
- Michelakis ED. Mitochondrial medicine: a new era in medicine opens new windows and brings new challenges. Circulation. 2008;117:2431–4. doi: 10.1161/CIRCULATIONAHA.108.775163. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Ohta S, Tanaka M, Takamiya S, Suzuki K, et al. Deficiencies in complex I subunits of the respiratory chain in Parkinson's disease. Biochem Biophys Res Commun. 1989;163:1450–5. doi: 10.1016/0006-291x(89)91141-8. [DOI] [PubMed] [Google Scholar]
- Morris MC, Deshayes S, Heitz F, Divita G. Cell-penetrating peptides: from molecular mechanisms to therapeutics. Biol Cell. 2008;100:201–17. doi: 10.1042/BC20070116. [DOI] [PubMed] [Google Scholar]
- Nass S, Nass MM. Intramitochondrial Fibers with DNA Characteristics. Ii. Enzymatic and Other Hydrolytic Treatments. The Journal of cell biology. 1963;19:613–29. doi: 10.1083/jcb.19.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls DG, Ferguson SJ. Bioenergetics. Amsterdam: Academic Press, Elsevier; 2013. pp. xiv–419. [Google Scholar]
- Olney JW, Sharpe LG. Brain lesions in an infant rhesus monkey treated with monsodium glutamate. Science. 1969;166:386–8. doi: 10.1126/science.166.3903.386. [DOI] [PubMed] [Google Scholar]
- Onopiuk M, Brutkowski W, Wierzbicka K, Wojciechowska S, Szczepanowska J, et al. Mutation in dystrophin-encoding gene affects energy metabolism in mouse myoblasts. Biochem Biophys Res Commun. 2009;386:463–6. doi: 10.1016/j.bbrc.2009.06.053. [DOI] [PubMed] [Google Scholar]
- Pacak CA, Preble JM, Kondo H, Seibel P, Levitsky S, et al. Actin-dependent mitochondrial internalization in cardiomyocytes: evidence for rescue of mitochondrial function. Biology open. 2015;4:622–6. doi: 10.1242/bio.201511478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer MA, Murphy MP. Peroxynitrite causes calcium efflux from mitochondria which is prevented by Cyclosporin A. FEBS Lett. 1994;345:237–40. doi: 10.1016/0014-5793(94)00461-7. [DOI] [PubMed] [Google Scholar]
- Patel SP, Sullivan PG, Lyttle TS, Rabchevsky AG. Acetyl-L-carnitine ameliorates mitochondrial dysfunction following contusion spinal cord injury. J Neurochem. 2010;114:291–301. doi: 10.1111/j.1471-4159.2010.06764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SP, Sullivan PG, Pandya JD, Goldstein GA, VanRooyen JL, et al. N-acetylcysteine amide preserves mitochondrial bioenergetics and improves functional recovery following spinal trauma. Exp Neurol. 2014;257:95–105. doi: 10.1016/j.expneurol.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SP, Sullivan PG, Pandya JD, Rabchevsky AG. Differential effects of the mitochondrial uncoupling agent, 2,4-dinitrophenol, or the nitroxide antioxidant, Tempol, on synaptic or nonsynaptic mitochondria after spinal cord injury. J Neurosci Res. 2009;87:130–40. doi: 10.1002/jnr.21814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruzzella V, Baggetto LG, Penin F, Cafagna F, Ruggiero FM, et al. In vivo effect of acetyl-L-carnitine on succinate oxidation, adenine nucleotide pool and lipid composition of synaptic and non-synaptic mitochondria from cerebral hemispheres of senescent rats. Arch Gerontol Geriatr. 1992;14:131–44. doi: 10.1016/0167-4943(92)90048-9. [DOI] [PubMed] [Google Scholar]
- Pettegrew JW, Levine J, McClure RJ. Acetyl-L-carnitine physical-chemical, metabolic, and therapeutic properties: relevance for its mode of action in Alzheimer's disease and geriatric depression. Mol Psychiatry. 2000;5:616–32. doi: 10.1038/sj.mp.4000805. [DOI] [PubMed] [Google Scholar]
- Pica-Mattoccia L, Attardi G. Expression of the mitochondrial genome in HeLa cells. V. Transcription of mitochondrial DNA in relationship to the cell cycle. J Mol Biol. 1971;57:615–21. doi: 10.1016/0022-2836(71)90113-6. [DOI] [PubMed] [Google Scholar]
- Pica-Mattoccia L, Attardi G. Expression of the mitochondrial genome in HeLa cells. IX. Replication of mitochondrial DNA in relationship to cell cycle in HeLa cells. J Mol Biol. 1972;64:465–84. doi: 10.1016/0022-2836(72)90511-6. [DOI] [PubMed] [Google Scholar]
- Pitkanen S, Robinson BH. Mitochondrial complex I deficiency leads to increased production of superoxide radicals and induction of superoxide dismutase. J Clin Invest. 1996;98:345–51. doi: 10.1172/JCI118798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikov EY, Khryapenkova TG, Vasileva AK, Marey MV, Galkina SI, et al. Cell-to-cell cross-talk between mesenchymal stem cells and cardiomyocytes in co-culture. Journal of cellular and molecular medicine. 2008;12:1622–31. doi: 10.1111/j.1582-4934.2007.00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polster BM, Basanez G, Etxebarria A, Hardwick JM, Nicholls DG. Calpain I induces cleavage and release of apoptosis-inducing factor from isolated mitochondria. J Biol Chem. 2005;280:6447–54. doi: 10.1074/jbc.M413269200. [DOI] [PubMed] [Google Scholar]
- Puca FM, Genco S, Specchio LM, Brancasi B, D'Ursi R, et al. Clinical pharmacodynamics of acetyl-L-carnitine in patients with Parkinson's disease. International journal of clinical pharmacology research. 1990;10:139–43. [PubMed] [Google Scholar]
- Reardon S. 'Three-parent baby' claim raises hopes- and ethical concerns. Nature News 2016 [Google Scholar]
- Rebbeck CA, Leroi AM, Burt A. Mitochondrial capture by a transmissible cancer. Science. 2011;331:303. doi: 10.1126/science.1197696. [DOI] [PubMed] [Google Scholar]
- Reid RA, Moyle J, Mitchell P. Synthesis of adenosine triphosphate by a protonmotive force in rat liver mitochondria. Nature. 1966;212:257–8. doi: 10.1038/212257a0. [DOI] [PubMed] [Google Scholar]
- Riedl SJ, Salvesen GS. The apoptosome: signalling platform of cell death. Nat Rev Mol Cell Biol. 2007;8:405–13. doi: 10.1038/nrm2153. [DOI] [PubMed] [Google Scholar]
- Robb EL, Winkelmolen L, Visanji N, Brotchie J, Stuart JA. Dietary resveratrol administration increases MnSOD expression and activity in mouse brain. Biochem Biophys Res Commun. 2008;372:254–9. doi: 10.1016/j.bbrc.2008.05.028. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Jimnez FJ, Alastrue-Agudo A, Erceg S, Stojkovic M, Moreno-Manzano V. FM19G11 favors spinal cord injury regeneration and stem cell self-renewal by mitochondrial uncoupling and glucose metabolism induction. Stem Cells. 2012;30:2221–33. doi: 10.1002/stem.1189. [DOI] [PubMed] [Google Scholar]
- Rottenberg H, Scarpa A. Calcium uptake and membrane potential in mitochondria. Biochemistry. 1974;13:4811–7. doi: 10.1021/bi00720a020. [DOI] [PubMed] [Google Scholar]
- Rubartelli A, Lotze MT. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 2007;28:429–36. doi: 10.1016/j.it.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–32. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Sheu SS, Nauduri D, Anders MW. Targeting antioxidants to mitochondria: a new therapeutic direction. Biochim Biophys Acta. 2006;1762:256–65. doi: 10.1016/j.bbadis.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Sims NR, Muyderman H. Mitochondria, oxidative metabolism and cell death in stroke. Biochim Biophys Acta. 2010;1802:80–91. doi: 10.1016/j.bbadis.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Singh IN, Sullivan PG, Deng Y, Mbye LH, Hall ED. Time course of post-traumatic mitochondrial oxidative damage and dysfunction in a mouse model of focal traumatic brain injury: implications for neuroprotective therapy. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2006;26:1407–18. doi: 10.1038/sj.jcbfm.9600297. [DOI] [PubMed] [Google Scholar]
- Skulachev VP. Uncoupling: new approaches to an old problem of bioenergetics. Biochimica et biophysica acta. 1998;1363:100–24. doi: 10.1016/s0005-2728(97)00091-1. [DOI] [PubMed] [Google Scholar]
- Smith RA, Adlam VJ, Blaikie FH, Manas AR, Porteous CM, et al. Mitochondria-targeted antioxidants in the treatment of disease. Ann N Y Acad Sci. 2008;1147:105–11. doi: 10.1196/annals.1427.003. [DOI] [PubMed] [Google Scholar]
- Sorice M, Circella A, Cristea IM, Garofalo T, Di Renzo L, et al. Cardiolipin and its metabolites move from mitochondria to other cellular membranes during death receptor-mediated apoptosis. Cell Death Differ. 2004;11:1133–45. doi: 10.1038/sj.cdd.4401457. [DOI] [PubMed] [Google Scholar]
- Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci U S A. 2006;103:1283–8. doi: 10.1073/pnas.0510511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struck J, Uhlein M, Morgenthaler NG, Furst W, Hoflich C, et al. Release of the mitochondrial enzyme carbamoyl phosphate synthase under septic conditions. Shock. 2005;23:533–8. [PubMed] [Google Scholar]
- Sullivan PG, Krishnamurthy S, Patel SP, Pandya JD, Rabchevsky AG. Temporal characterization of mitochondrial bioenergetics after spinal cord injury. J Neurotrauma. 2007;24:991–9. doi: 10.1089/neu.2006.0242. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Springer JE, Hall ED, Scheff SW. Mitochondrial uncoupling as a therapeutic target following neuronal injury. J Bioenerg Biomembr. 2004;36:353–6. doi: 10.1023/B:JOBB.0000041767.30992.19. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Khan SM. A "mitochondrial cascade hypothesis" for sporadic Alzheimer's disease. Med Hypotheses. 2004;63:8–20. doi: 10.1016/j.mehy.2003.12.045. [DOI] [PubMed] [Google Scholar]
- Tachibana M, Sparman M, Sritanaudomchai H, Ma H, Clepper L, et al. Mitochondrial gene replacement in primate offspring and embryonic stem cells. Nature. 2009;461:367–72. doi: 10.1038/nature08368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Labinskyy N, Mukhopadhyay P, Pinto JT, Bagi Z, et al. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H1876–81. doi: 10.1152/ajpheart.00375.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valasani KR, Sun Q, Fang D, Zhang Z, Yu Q, et al. Identification of a Small Molecule Cyclophilin D Inhibitor for Rescuing Abeta-Mediated Mitochondrial Dysfunction. ACS Med Chem Lett. 2016;7:294–9. doi: 10.1021/acsmedchemlett.5b00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valasani KR, Vangavaragu JR, Day VW, Yan SS. Structure based design, synthesis, pharmacophore modeling, virtual screening, and molecular docking studies for identification of novel cyclophilin D inhibitors. J Chem Inf Model. 2014;54:902–12. doi: 10.1021/ci5000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRooyen JL, Patel SP, Mashburn C, Eldahan KC, Cox D, et al. Transplanted mitochondria significantly maintain cellular respiration after acute contusion spinal cord injury - Abstracts from the 34th Annual National Neurotrauma Symposium. Journal of Neurotrauma. 2016;33:A-1–A-139. [Google Scholar]
- Wang X, Gerdes HH. Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells. Cell Death Differ. 2015;22:1181–91. doi: 10.1038/cdd.2014.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisiger RA, Fridovich I. Superoxide dismutase. Organelle specificity. The Journal of biological chemistry. 1973;248:3582–92. [PubMed] [Google Scholar]
- Wigler M, Perucho M, Kurtz D, Dana S, Pellicer A, et al. Transformation of mammalian cells with an amplifiable dominant-acting gene. Proc Natl Acad Sci U S A. 1980;77:3567–70. doi: 10.1073/pnas.77.6.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winklhofer KF, Haass C. Mitochondrial dysfunction in Parkinson's disease. Biochim Biophys Acta. 2010;1802:29–44. doi: 10.1016/j.bbadis.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Winterbourn CC. Toxicity of iron and hydrogen peroxide: the Fenton reaction. Toxicol Lett. 1995;82–83:969–74. doi: 10.1016/0378-4274(95)03532-x. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Gu Q, Peterson PL, Muizelaar JP, Lee CP. Mitochondrial dysfunction and calcium perturbation induced by traumatic brain injury. J Neurotrauma. 1997;14:23–34. doi: 10.1089/neu.1997.14.23. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Hall ED. Pharmacological evidence for a role of peroxynitrite in the pathophysiology of spinal cord injury. Exp Neurol. 2009;216:105–14. doi: 10.1016/j.expneurol.2008.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YW, Koob MD. Transferring isolated mitochondria into tissue culture cells. Nucleic Acids Res. 2012;40:e148. doi: 10.1093/nar/gks639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Itagaki K, Hauser CJ. Mitochondrial DNA is released by shock and activates neutrophils via p38 map kinase. Shock. 2010a;34:55–9. doi: 10.1097/SHK.0b013e3181cd8c08. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010b;464:104–7. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]