Abstract
The current review is guided by the theoretical perspective that emphasizes the regulating role of executive functioning (Carver, Johnson, & Joormann, 2009) and presents studies that elucidate the ways that executive functioning (inhibition and working memory) explain individual differences in adolescent substance use independently or by regulating the reactive system (reward and punishment sensitivity). Behavioral studies indicate that main effects of executive functioning on adolescent substance use are often nonsignificant or weak in effect sizes. In contrast, emerging evidence suggests consistent and stronger regulating effects of executive functioning over reward and punishment sensitivity. Functional neuroimaging studies reveal significant associations between executive functioning task-related hemodynamic responses and substance use with strong effect sizes. There is also direct evidence from studies testing statistical interactions of the regulating effects of EF-related brain activation, and indirect evidence in studies examining functional connectivity, temporal discounting, and reinforced control. We note key future directions and ways to address limitations in existing work.
Keywords: Executive functioning, substance use, reward sensitivity, punishment sensitivity, cognitive contro
1. Introduction
Recent research efforts have acknowledged the need for a specific focus on health risk behaviors as they apply to developmental trajectories of risky decision making in adolescence. Identifying factors contributing to the propensity to use substances (tobacco, alcohol, and other drug use) during adolescence is especially important because experimentation and the process of addiction most often starts in adolescence during which the brain undergoes dramatic developmental changes (Chambers, Taylors, & Potenza, 2003; Koob & Volkow, 2016). Early onset of substance use (i.e., 14 years or younger) is also associated with increased risk for lifetime substance use disorders and addiction (Grant & Dawson, 1998). Substance use behaviors not only place adolescents at increased risk for negative outcomes such as injury, drug addiction, and sexually transmitted diseases including human immunodeficiency virus infection, but also serve as the leading cause of mortality and morbidity among adolescents (CDC, 2012).
Within research on substance use, it has been theorized that deficits in executive functioning (EF) contribute to substance use problems as a result of poor cognitive regulation of behavior (Giancola & Mezzich, 2003). In the neuroscience literature, adolescent risk taking such as substance use has been explained by imbalances in neural development, including rapid development of subcortical functioning in conjunction with slow development of prefrontal functioning (Casey, Getz, & Galvan, 2008; Ernst, Pine, & Hardin, 2006; Steinberg, 2008). Although considerable conceptual work in the neuroscience of adolescent risk taking and empirical work on EF impairment among substance abusers has been published, we do not have a clear answer to the following foundational questions: Can deficits in EF be regarded as risk factors for adolescent substance use? If so, how? Therefore, our primary goal is to provide a comprehensive integration of established and emerging evidence from both behavioral and human neuroscience literature regarding the role of EF in the development of substance use throughout adolescence. We propose a conceptual model that highlights the regulating role of EF and examine how individual differences in EF contribute to adolescent substance use, independently or by interacting with the motivational reactivity (represented by reward and punishment sensitivity). In doing so, we first review key behavioral studies that examined the main effects of two primary dimensions of EF—inhibition and working memory—in relation to substance use. We then draw on evidence of statistical interaction effects to support the proposed regulating effects of EF. Subsequently, we review EF task-based functional neuroimaging studies examining main effects of EF on substance use outcomes. Then, we present evidence from neuroimaging research suggesting the regulating effects of EF—illustrated directly by statistical interactions between regulatory and reactive brain systems, and indirectly by task-based functional connectivity, temporal discounting, and reinforced control. Finally, we conclude by synthesizing the contributions of both behavioral and neuroimaging studies of EF to aid in understanding the development of substance use behaviors and point towards promising future directions and ways to address limitations in existing work.
The literature and databases regarding the prevalence of substance use across age groups consistently identify the overlapping periods of adolescence (ages 10–19; World Health Organization, 2016) and emerging adulthood (ages 18–25; Arnett, 2005) as characterized by a peak in risk taking and substance use behaviors. Literature further indicates that brain function underlying cognitive control continues to develop into the 20s (Ordaz, Foran, Velanova, & Luna, 2013). Accordingly, we define adolescence in the current review as encompassing ages 10–25, which includes emerging adults such as samples of university students. We examine tobacco, alcohol, and marijuana use because they are the most commonly used drugs in the U.S. and polysubstance use of these three substances is prevalent among U.S. adolescents (Johnston, O’Malley, Bachman, & Schulenberg, 2016). We limited our review to substance use studies of adolescents’ initiation (e.g., age of onset), progression (e.g., increases in use with age) and severity (e.g., frequency and quantity of use). We focus on the basic scientific understanding of the association between EF and substance use and do not intend to characterize specific substance use disorders.
To date, no clear consensus exists as to whether neurodevelopmental abnormalities among adolescent substance users may reflect predisposing risk factors for substance use disorders, the neurotoxic effects of the substances, or both. Behavioral research reviewed here includes many prospective longitudinal studies documenting changes in substance use behaviors among initially substance use-naive adolescents, permitting inferences regarding the contributions of prior and concurrent EF deficits to growth in substance use over time. By comparison, there is a lack of prospective longitudinal neuroimaging studies, which limits our capacity to draw directional inferences regarding the EF-substance use association. Nevertheless, data from human brain imaging studies suggest that abnormalities in brain systems related to EF may be a predisposing risk factor for developing substance use disorder in the future (e.g., Ersche et al., 2012; Squeglia, Jacobus, Nguyen-Louie, & Tapert, 2014). Also, prefrontal cortex impairment may further contribute to disinhibited behavior and inability to discontinue substance use (e.g., Goldstein & Volkow, 2002; Meier et al., 2012; see Silveri, Dager, Cohen-Gilbert, & Sneider, 2016 for review).
1.1. Conceptualization of EF
Executive function is a multi-faceted construct involving various cognitive processes that guide goal-directed behavior necessary for adaptive functioning in life. These cognitive processes are distinct from one another but at the same time may overlap and be interdependent on one another. Thus, there exists a hierarchy in EF with the development of more basic functions necessary for enhancement of more complex functions (Miyake et al., 2000). One operationalization of EF as a higher-order cognitive construct involved in planning, initiation, and regulation of goal-directed behavior comprises three core components: (1) inhibition of prepotent responses, which involves inhibiting dominant or automatic responses, in the service of goal-directed behavior, (2) monitoring and updating of working memory representations, which involve the ability to manipulate current information in working memory, and (3) shifting of mental sets, or the ability to shift back and forth between different tasks or cognitive states (Miyake et al., 2000). Inhibition and working memory are hypothesized to play critical roles in risky decision-making such that successful achievement of goals requires suppression of behavioral impulses and goal-irrelevant responses as well as maintenance and representation of internal goals (Luna, Padmanabhan, & O’Hearn, 2010). Similar to behavioral studies of EF, functional neuroimaging studies to date have primarily focused on studying brain responses associated with inhibition of prepotent responses and impairments in working memory processes that interfere with goal-directed behavior (Luna, Marek, Larsen, Tervo-Clemmens, & Chahal, 2015). Indeed, existing literature examining the effects of EF on adolescent substance use almost exclusively focuses on inhibition and working memory; thus, we examine primarily these two dimensions.
1.2. Moderation by EF is key for understanding individual differences in substance use
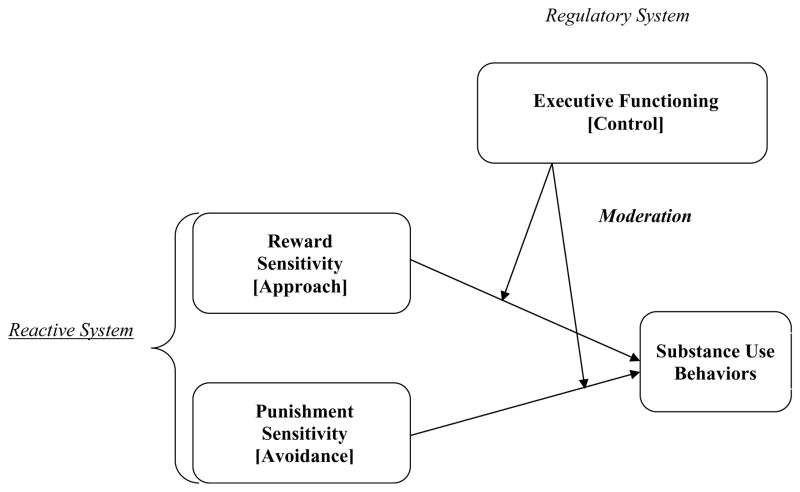
Our conceptual model is graphically represented in Figure 1 and highlights the interaction between two main systems involved in the regulation of motivated behaviors: reward and punishment sensitivity that represents the reactive system and EF that represents the regulatory system. We propose that evaluating the interaction between these systems is key to understanding the etiology of individual differences in adolescent substance use behaviors. The regulatory system refers to EF processes that modulate the operation of the reactive system in the service of goal directed behavior, which for some may result in substance use. This approach is guided by the work of Carver and colleagues (2009) whose two-mode model of self-regulation dictates that the nervous system is structured to allow humans to simultaneously process information from experiences in two simultaneous but distinct methods of processing (Carver, Johnson, & Joormann, 2009). Thus, processing occurs from both a reflexive lower order system that is reactive in nature (e.g., reward and punishment sensitivity) and a reflective or regulatory higher order system that is more strategic or deliberate (e.g., EF). Therefore, risk-taking behaviors such as substance use can be viewed as resulting not only from an inability to inhibit behaviors that pursue reward seeking but also from an inability to inhibit behaviors that will likely result in punishment. We view EF as regulating the operation of the reactive system toward achieving the goal, rather than simply suppressing it. That is, adolescents with higher EF will be better at regulating reward and punishment sensitivity to achieve the desired goal—whether the goal is to engage in or avoid substance use, in accordance with their beliefs and values—compared to their counterparts with lower EF.
Figure 1.
The interaction between the regulatory system and the reactive system predicting substance use behaviors in adolescence
One common way the reactive system is conceptualized originates from Gray’s (1982) neuropsychological theory of personality termed Reinforcement Sensitivity Theory (RST). Specifically, the RST delineates two statistically independent motivational systems involved in the inhibition or activation of behavior: the Behavioral Inhibition System (BIS) and the Behavioral Activation System (BAS). Although a conceptual link exists between the reactive system involving reward and punishment sensitivity presented in Figure 1 and the BIS/BAS in RST, a clear distinction between them is noted. That is, Gray’s RST focuses on how BIS/BAS motivational systems interface with each other to produce behaviors without accounting for superordinate regulatory system that can override and countermand BIS/BAS systems’ functioning (Carver, 2005).
Within the neuroscience literature, our conceptual model is in line with the triadic model that emphasizes the interplay between the functional modules of approach, avoidance, and control (Ernst & Fudge, 2009; Ernst et al., 2006). As we indicated in Figure 1, the triadic model outlines three neural systems that are responsible for approach, avoidance, and control, and emphasizes the potential imbalance in maturation across these brain regions as neural bases of adolescent risk-taking behaviors (Ernst, Daniele, & Frantz, 2011; Ernst & Fudge, 2009; Ernst et al., 2006; Richards, Plate, & Ernst, 2013). Notably, these three functional domains also characterize the primary processes involved in addiction (Koob & Volkow, 2016). The approach neural system is related to reward sensitivity and comprises subcortical and cortical structures that are stimulated by a dramatic and rapid increase in dopaminergic activity during adolescence, including the striatum and orbitofrontal cortex. The avoidance neural system is related to punishment sensitivity and comprises the amygdala, hippocampus, and insula. Finally, the control neural system refers to the regulatory system, involving EF, that modulates subcortical function—the approach and the avoidance system—through top-down cognitive control. This system comprises prefrontal cortical structures including the dorsolateral prefrontal cortex, ventral prefrontal cortex, and anterior cingulate cortex. Prefrontal cortex regions have been shown to undergo maturation, including increased myelination and experience-dependent synaptogenesis and pruning, throughout adolescence and into early adulthood (Paus, 2005).
Similar to the triadic model, the imbalance model (Casey et al., 2008) describes adolescent self-control development as an imbalance in neural maturational patterns between the cortical-control system (involving the prefrontal cortex) and the limbic system (involving brain regions such as the striatum and amygdala). Both the triadic model and the imbalance model are “neuromaturational models” of ontogeny (i.e., species-typical age-based changes) that seek to explain the normative heightened risk-taking seen in adolescence as reflecting the developmental gap between a faster-maturing subcortical reactive system and a slower-maturing cortical regulation system. As discussed later, these models emphasize age-typical associations between subcortical and cortical regions, such as connectivity, as the primary way to capture the interplay between the two neural systems. Although similar in some respects to the triadic and imbalance models, the dual systems model of risk taking posits the orthogonal development of the control network and the reward-seeking network (Shulman et al., 2016; Steinberg, 2008), and emphasizes independent contributions of these two networks to age-typical, ontogenic development of adolescent risk taking. In contrast to these three established models, our model focuses on the processes through which the reactive and the regulatory systems interact with each other, to explain individual differences (rather than age-typical changes, i.e., ontogeny) in adolescent risk taking—processes that may generalize to most or all time points of the adolescent phase of development. Our position is that the effects on risk-taking behaviors of the reactive neural system can be meaningfully evaluated only while simultaneously considering the level of self-control available arising from the regulatory neural system (i.e., tested as the statistical interaction between the reactive and the regulatory systems) to identify which adolescents are most likely to be vulnerable to risk-taking behaviors.
The role of the regulatory system may become critical during adolescence due to the heightened sensitivity to reward during this developmental period. Past research has shown that adolescents report greater reward sensitivity than do adults and reward sensitivity increases markedly from early to late adolescence (Urošević, Collins, Muetzel, Lim & Luciana, 2012). Human neuroimaging work also provides evidence that adolescents are hyper-responsive to rewards: A meta-analysis of functional neuroimaging studies of reward processing reveals that regions of the striatum that receive projections from midbrain dopamine neurons are more active in adolescents relative to adults in response to and in anticipation of rewards (Silverman, Jedd, & Luciana, 2015). Indeed, the reward circuits involved in the ventral striatum mature at an early age, and mesoaccumbens dopamine that modulates reward motivation increases in adolescence before declining into adulthood, resulting in increased risk taking during adolescence (Luciana, Wahlstrom, Porter, & Collins, 2012; Spear, 2007).
Following, we review studies evaluating the roles of EF in relation to adolescent substance use directly (i.e., main effects) or by regulating the effects of reactivity (i.e., interactive effects). As shown in the Appendix, we present the summarized information of the reviewed behavioral studies examining the association between EF and substance use, including the measure of EF, and sample description (see Table 1). We also present similar information for the reviewed functional neuroimaging studies with additional information regarding contrasts and neural correlates (see Table 2). We report effect sizes when available or when we were able to calculate. We focus on studies examining EF based on behavioral performance tasks in the neuropsychology tradition, as opposed to behavioral ratings via self- or other report (Nigg, in press).
2. Behavioral Studies of EF Related to Substance Use
2.1. Main effects of EF
Substance use is linked with impaired cognitive functioning, with some previous research using high-risk samples reporting significant main effects of EF on adolescent substance use; yet the effect sizes are often notably small. In a seminal study examining the association between EF and substance use, Aytaclar and colleagues (1999) contrasted high-risk male adolescents (having fathers with a psychoactive substance use disorder) and low-risk male adolescents (having fathers with no history of psychoactive substance use). They found that an EF composite score (including inhibition, shifting, and planning) was related to the total number of drugs and severity of substance involvement at a two-year follow-up, after controlling for the baseline level of conduct disorder and parental substance use disorder history (Aytaclar, Tarter, Kirisci, & Lu, 1999). In this study, effect sizes for the EF effects were not reported and not enough statistical information was provided to calculate them. However, in a separate study involving a large sample of high-risk adolescents from families with alcoholism, poor inhibition predicted later alcohol and drug related problems and illicit drug use, but explained only about 1% of the variance (Nigg et al., 2006).
Other research contrasting high-risk versus low-risk adolescents has not found clear associations between EF and substance use especially when examined prospectively. For example, in a cross-sectional study, a composite measure of EF was related to drug use frequency among adolescent males with a family history of substance use disorder but not their control counterparts (Shoal & Giancola, 2001). However, a prospective longitudinal study by this same research team revealed that a composite measure of EF did not predict later drug use among adolescent boys with and without a family history of substance use disorder (Giancola & Parker, 2001). Similarly, a longitudinal study comparing adolescents from high-risk families (those with alcoholic fathers) and matched controls reported that earlier inhibition did not predict later binge drinking or alcohol problems (Wong, Brower, Nigg, & Zucker, 2010). Longitudinal analyses examining adolescents with and without attention-deficit/hyperactivity disorder (ADHD) also failed to find significant associations between EF deficits and substance use onset and disorders, regardless of the presence of ADHD (Groenman et al., 2015; Wilens et al., 2011).
Findings for the main effects of EF on substance use in nonclinical, normative populations are mixed and generally produce nonsignificant or significant yet weak effects of EF. In a cross-sectional study of a large sample of adolescents from the National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA), no significant difference was found between the no/low drinking group and the hazardous drinking group with respect to working memory performance (Sullivan et al., 2016). One longitudinal study found that working memory at baseline was related to the baseline level and change slope in adolescent alcohol use over four years, although the effect sizes were small (Khurana et al., 2013). Another longitudinal study based on a large sample of Dutch adolescents found no significant effects of working memory on the likelihood of initiating smoking or becoming daily smokers a few years later. Although inhibition was not predictive of future daily smoking either, adolescents with low inhibition at ages 10–11 years were more likely to endorse that they have ever smoked at ages 15–17 years, compared to those with high inhibition (Harakeh et al., 2012). However, predicting complete abstinence throughout adolescence may not be as meaningful as evidence of early onset or patterns of substance use in predicting future abuse and addiction (e.g., Stone, Becker, Huber, & Catalano, 2012). Two other longitudinal studies indicated that adolescents who transitioned into moderate- to heavy drinkers did not differ on working memory performance compared to matched controls (Squeglia, Spadoni, Infante, Myers, & Tapert, 2009), and adolescents who later transitioned into alcohol and marijuana users did not differ on working memory and inhibition performance compared to those who remained substance-naive (Squeglia et al., 2014).
Studies involving individuals with externalizing problems demonstrate similar results with respect to the connection between poorer EF and more substance use. A prospective study of high risk adolescents with externalizing problems found that working memory predicted alcohol use six months later, with a small effect size (Peeters, Monshouwer, Janssen, Wiers, & Vollebergh, 2014). Similarly, a survival analysis study using a combined sample of adolescents recruited from both mainstream and special education schools (mainly due to high externalizing problems) found that working memory and inhibition predicted the likelihood of initiating alcohol use between the ages of 12 and 17 years, with a small effect size and a medium effect size, respectively (Peeters et al., 2015). As we noted earlier, however, there is no clear evidence that failure to remain abstinent throughout adolescence is a precursor for future problematic use.
Interestingly, the role of externalizing problems in the EF-substance use connection may be nonlinear in the sense that such effects are no longer present among adolescents with clinical diagnoses of substance use disorders. For example, in a study comparing a clinical sample of adolescent females with a substance use disorder with controls, Giancola and colleagues (2001) found that antisocial behavior moderated the association between EF and drug use: Lower EF was related to higher drug use involvement only among adolescents with low antisocial behavior (Giancola, Shoal, & Mezzich, 2001). These findings were interpreted to mean that adolescents with high antisocial behavior had so little EF capacity that these cognitive skills were ineffective in inhibiting drug use. The results of Giancola et al.’s (2001) study highlight the need for further research to clarify the role of externalizing problems in the association between EF and substance use in both male and female adolescents.
2.2. Regulating effects of EF
Although modest and mixed evidence supports the main effects of EF on substance use among adolescents, its regulating effects have not been extensively studied. However, a few cross-sectional studies have directly tested the statistical interaction between EF and reward and punishment sensitivity. The results of these studies provide support for the regulating role of EF in the association between the reactive system and adolescent substance use. Patrick and colleagues (2008) conducted the first study examining such an interaction in a sample of female college students and reported non-significant main effects of EF (working memory and inhibition) on their risk-taking behaviors, including alcohol and drug use. A significant interaction (with a medium effect size) indicated that the combination of higher reward sensitivity (measured by BAS) and better working memory was related to higher levels of alcohol and drug use among female college students. The results were interpreted that college women with stronger reward sensitivity and better working memory both enjoy the stimulation of taking risks and may be better at cognitively regulating their behavior to avoid negative consequences of their actions. In this study, there was also a marginally significant interaction (with a small-to-medium effect size) between reward sensitivity and inhibition suggesting that higher reward sensitivity was related to higher levels of alcohol use among women with lower inhibition but not among women with higher inhibition (Patrick, Blair, & Maggs, 2008).
These results provide a nuanced understanding of the modulating effects of EF by illustrating individual differences in the role of EF. As suggested by Reyna and Rivers (2008), risk taking in real life is often intentional, involving an analytical process of weighing magnitudes of risks and benefits. For some whose risk behavior is intentional, their strong working memory capacity is likely to help them to achieve their goal of using substances by maintaining relevant information and shielding it from interference, especially when they possess strong approach motive. For others whose risky behavior is not intentional or analytically planned, failure to regulate acting on their strong approach motive is likely to result in substance use.
Another study used a sample of college students consisting of both light and heavy alcohol users found a significant interaction between inhibition and punishment sensitivity suggesting that low punishment sensitivity was significantly related to high alcohol use among those with weak inhibition, but not among those with strong inhibition (Jonker, Ostafin, Glashouwer, van Hemel-Ruiter, & de Jong, 2014). Although effect sizes for the simple effect slopes were not reported in this study, the interaction between inhibition and punishment sensitivity explained 6% of the variance in alcohol use indicating a medium effect size. More recently, in a study examining whether inhibition interacts with reward sensitivity (measured by BAS) and punishment sensitivity (measured by BIS) to predict substance use among early adolescents, significant moderating effects of inhibition on the link between reward sensitivity and the age of substance use initiation indicated that higher levels of reward sensitivity were predictive of earlier onset of substance use (including cigarette, alcohol, and marijuana) among adolescents with low inhibition (with a medium effect size) but not among adolescents with high inhibition (Kim-Spoon et al., 2016).
Similarly, another study reported a significant interaction between temperamental frustration and cognitive control (i.e., a combination of self-reported effortful control and behavioral performance on inhibitory control tasks) such that the link between frustration (i.e., negative affect related to interruption of ongoing tasks or goal blocking) and adolescent risk-taking behavior (including substance use) was significantly attenuated among those with high cognitive control (Youssef et al., 2016). Supporting evidence of EF’s moderating role extends beyond cross-sectional studies. A longitudinal study demonstrated that attentional control regulated reactivity to predict changes in adolescent risk-taking behaviors in a large sample of participants from the Study of Early Child Care and Youth Development (SECCYD). Specifically, increased anger reactivity (i.e., approach motivation) between 9 and 11 years was related to increased risk-taking behaviors (including substance use) between 11 and 15 years only for adolescents with low attentional control (Kim-Spoon, Holmes, & Deater-Deckard, 2015).
2.3. Summary
As a whole, available evidence from extant behavioral research provides rather modest support (i.e., statistically nonsignificant or significant but mostly small effect sizes) for the association between substance use and EF—both inhibition and working memory. In comparison, emerging evidence from behavioral research points towards more consistent and stronger regulating effects of EF over reactivity related to adolescent substance use, potentially accounting for the weak main effects of EF found in previous behavioral studies. Thus, adolescent substance use can result from heightened reward sensitivity and diminished punishment sensitivity, but for those with strong EF regulating capacity the biased processing of reward and risk can be modulated resulting in reduction of substance use behaviors. Next, we turn to key functional neuroimaging studies pertaining to EF effects on adolescent substance use.
3. Relation between EF Neural Responses and Substance Use
3.1. Main effects of EF neural responses
The use of functional neuroimaging has enhanced our ability to identify potential neurobiological mechanisms that contribute to EF. Previous studies have identified brain regions involved in inhibition, including the basal ganglia (such as caudate, putamen, globus pallidus), that are thought to be involved in inhibition of inappropriate responses, and prefrontal regions (such as inferior, medial, and dorsolateral prefrontal cortices) that receive inputs from the limbic basal ganglia thalamocortical circuit and represent and maintain relevant information for goal directed behaviors (Aron, Robbins, & Poldrack, 2014; Booth et al., 2003; Casey, Durston & Fossella, 2001). Another core component of EF that has strong connections to inhibition is working memory processes involving the ability to temporarily store goal-related information in mind. The tripartite conceptual framework of working memory (Baddeley, 1986, 1992; Baddeley & Hitch, 1994) consisting of a central executive system that directs subordinate systems (visuospatial and phonological) has played an influential role in guiding neuroimaging research efforts to better understand the development of working memory. Lesion studies in humans and animals using spatial delay response tasks have implicated the dorsolateral prefrontal cortex in working memory (Curtis & D’Esposito, 2003; D’Esposito & Postle, 1999; Goldman-Rakic, 1987; Smith & Jonides, 1995). Further, a meta-analysis of human neuroimaging studies provides converging evidence showing that activation of prefrontal and parietal cortices is related to the maintenance of information (Wager & Smith, 2003). Indeed, recruitment of lateral prefrontal cortex differs depending on whether tasks require manipulation of information, which recruits dorsolateral activity, or maintenance of information, which recruits ventrolateral activity (D’Esposito & Postle, 1999; Smith & Jonides, 1995).
A small but growing number of neuroimaging studies provide preliminary evidence suggesting that neural inhibitory responses are related to concurrent substance use and may be predictive of future substance use among adolescents, often even in the absence of behavioral differences. For instance, a study of adolescents comparing heavy smokers and nonsmokers revealed higher levels of smoking to be associated with lower inhibition-related blood oxygenation level—dependent (BOLD) responses in the inferior frontal gyrus, middle frontal gyrus, superior frontal gyrus, cingulate cortex, and the supplementary motor area (Galvan, Poldrack, Baker, McGlennen & London, 2011). Similarly, lower inhibitory-related BOLD responses in the inferior frontal gyrus and right insula were related to high levels of alcohol and marijuana use in adolescents (Feldstein Ewing, Houck, & Bryan, 2015). Interestingly, one study found that adolescent marijuana users and non-users showed no differences in terms of brain activation during an inhibition task, but for marijuana users there were significantly higher positive correlations between cerebellar and bilateral posterior parietal regions, implying aberrant connectivity within inhibition networks (Behan et al., 2014).
Furthermore, longitudinal studies of adolescents have found that lower inhibitory-related BOLD responses in the dorsolateral, inferior and medial prefrontal cortices, supplementary motor area, cingulate, and putamen are predictive of later alcohol use (Heitzeg et al., 2014; Norman et al., 2011; Wetherill, Squeglia, Yang & Tapert, 2013), and lower inhibitory-related BOLD responses in the medial prefrontal cortex predicted later alcohol dependence among those with a history of high frequency substance use (Mahmood et al., 2013). During typical neural maturation, adolescents are expected to exhibit less activation compared to children while completing inhibition tasks, reflecting refined and more efficient neural functioning with development (Luna et al., 2010). Therefore, the findings regarding neural responses related to inhibition suggest that more developmentally “mature” prefrontal functioning is related to greater risks for adolescent substance use initiation and problems (Squeglia et al., 2017).
With respect to working memory, several functional neuroimaging studies have demonstrated differentiated neural responses on working memory tasks in adolescents who use alcohol compared to adolescents who do not use alcohol. A cross-sectional study demonstrated that adolescents with alcohol use disorders, relative to those who do not, displayed less BOLD responses in left precentral gyrus and bilateral cerebellar areas, while exhibiting greater BOLD responses in bilateral parietal cortices (Tapert et al., 2004). Similarly, a cross-sectional study comparing adolescent binge drinkers and non-drinkers indicated that binge drinkers, compared with non-drinkers, showed significantly higher BOLD responses in working memory systems (e.g., right superior frontal and bilateral posterior parietal cortices; Schweinsburg, McQueeny, Nagel, Eyler, & Tapert, 2010). In contrast, a recent longitudinal study reported that those who showed significantly lower BOLD responses in the right posterior cingulate and right superior temporal regions during a working memory task at ages 12–14 years, were more likely to become moderate to heavy alcohol drinkers by age 18 years (Squeglia et al., 2017).
The seeming contradiction between studies showing either higher or lower BOLD responses being related to more substance use, may be clarified by a longitudinal study that examined changes in brain activation before and after the transition to heavy drinking. In a prospective study of adolescents, Squeglia and colleagues (2012) reported that those who transitioned into heavy drinking showed lower BOLD response during working memory performance at baseline, compared to those who remained as non-drinkers. However, those adolescents transitioned into heavy drinking showed increasing activation in frontal and parietal regions during working memory tasks over three years, whereas continuous nondrinkers showed lessening activation over time (Squeglia et al., 2012). Such a divergent activation pattern over time may suggest that adolescent heavy drinkers had to work harder to achieve similar levels of behavioral performance as nondrinkers because of their less efficient processing. Thus, the results imply that heavy drinkers’ brains became less efficient in processing information, whereas non-drinkers’ brains became more efficient over time.
Taken together, the altered developmental patterns of BOLD responses in substance-using adolescents during inhibition and working memory tasks may suggest that, prior to substance use initiation, early maturation of neural functioning related to EF may be a risk factor that increases the likelihood of engaging in substance use behaviors (Squeglia et al., 2017). Once adolescents have started using substances regularly, compensatory neural functioning occurs; adolescents exhibit difficulty in recruiting appropriate brain areas and thus need to recruit additional brain areas not relevant to task demands to achieve the same behavioral performance as those adolescents who do not use substances (e.g., Feldstein Ewing, Sakhardande, & Blakemore, 2014).
The pattern of neural results for working memory found in adolescents who use alcohol is consistent with other studies examining adolescents who use other substances such as marijuana. In particular, marijuana users exhibited hypoactivation in the right temporal gyrus, thalamus, pulvinar, and left parahippocampal gyrus, as well as hyperactivation of the left temporal gyrus and anterior cingulate cortex during a spatial working memory task, which was the opposite pattern found in controls (Padula, Schweinsburg, & Tapert, 2007; Schweinsburg, Schweinsburg, Nagel, Eyler, & Tapert, 2011). Evidence also suggested that marijuana users, compared to controls, showed exaggerated BOLD responses in prefrontal and posterior parietal regions during verbal working memory performance (Jager, Block, Luijten, & Ramsey, 2010). The findings support the idea of compensatory neural functioning by showing that marijuana users show overactive frontal lobe functioning and recruit additional brain areas in order to achieve the same behavioral performance as non-users.
3.2. Regulating effects of EF neural responses
3.2.1. Statistical interaction
Following the conceptual model illustrated in Figure 1, individual differences in the modulation of EF may be considered behavioral manifestations of underlying brain mechanisms involved in regulation of reactivity (e.g., reward and punishment sensitivity). Our most recent neuroimaging study presented the first evidence for a statistical interaction between reactive brain activation and regulatory brain activation predicting adolescent health risk behaviors (Kim-Spoon et al., in press). Specifically, we tested the interaction effects between brain activation during a lottery task measuring tendency to avoid risky decision options and brain activation during a multi-source interference task measuring inhibition predicts late adolescents’ health risk behaviors (a composite based on severity and onset of cigarette, alcohol, and marijuana use, severity of risky sexual behaviors, and age at sexual debut). The results indicated that risk-related hemodynamic activity in the anterior insula during anticipation of uncertain outcomes predicted health risk behaviors among adolescents requiring greater dorsal anterior cingulate cortex activity during a cognitive interference task (i.e., poor inhibition), explaining 44% of the variance. However, dorsal anterior cingulate cortex activity was not associated with health risk behaviors among adolescents requiring less dorsal anterior cingulate cortex activity to achieve the same level of performance (i.e., high inhibition), explaining 5% of the variance. The findings suggest a significant moderating effect of the neural substrates involved in EF (particularly inhibition) on the link between punishment sensitivity and health risk behaviors in adolescents.
Similarly, our previous neuroimaging study investigating whether neural processes related to inhibition interact with self-reported BAS to predict substance use among early adolescents demonstrated significant moderating effects (with a medium effect size) of neural inhibition on the link between BAS and the age of substance use initiation (including cigarette, alcohol, and marijuana). Specifically, lower levels of BAS were significantly predictive of earlier onset of substance use, only among adolescents with poor inhibition who showed high activation in the regulatory neural networks systems (e.g., medial and dorsolateral frontal cortices) during a cognitive interference task (Kim-Spoon et al., 2016). Taken together, these findings suggest significant moderating effects of the neural substrates involved in EF (particularly inhibition) on the link between reactive systems and substance use behaviors among adolescents, supporting the theoretically hypothesized regulating role of EF and its associated frontal lobe activity.
Next, we turn to alternative ways that neuroimaging researchers have examined the joint operations of reactive and regulatory brain networks in predicting adolescent substance use. These studies do not involve direct statistical evaluation of main versus interaction effects of EF on adolescent substance use, so the statistical details of their findings are not included in the tables in the Appendix. Nevertheless, their findings offer indirect evidence of the regulating role of the EF neural system on the reactive neural system.
3.2.2. Task-based functional connectivity
In recent years, neuroimaging researchers have begun to study how the regulatory system interfaces with the reactive system by examining functional connectivity (see Ernst, Torrisi, Balderston, Grillon, & Hale, 2015). Functional connectivity refers to statistical dependence or mutual information between two neuronal systems (Friston, Moran, & Seth, 2013). Such an approach seems reasonable given that although motivational reactivity and cognitive regulation may be operationalized as distinct psychological entities in behavioral studies, it is far less clear that they have separable representations in the brain (Braver et al., 2014). Both the triadic model (Ernst & Fudge, 2009; Ernst et al., 2006) and the imbalance model (Casey, 2015; Casey et al., 2008) suggest that the prefrontal cortex can modulate both the amygdala and ventral striatum via functional connectivity between these regions. Casey and colleagues (2016) further propose that a plausible temporal mechanism for developmental shifts in cognitive capacity from childhood to adulthood is a fine-tuning of circuits from subcortico-subcortical to cortico-subcortical to cortico-cortical.
Evidence shows stronger subcortico-subcortical coupling (i.e., correlated temporal dynamics) but weaker cortico-subcortical and cortico-cortical coupling among adolescents, compared to adults. Somerville and colleagues (2011) conducted a psychophysiological interaction (PPI) analysis on a ventral frontostriatal circuit with a seed region in the right inferior frontal gyrus. Their results indicated that, when suppressing approach responses toward appetitive cues, only adolescents showed significant ventral-dorsal striatal coupling although both adolescents and adults showed significant coupling of dorsal striatal-prefrontal responses (Somerville, Hare, & Casey, 2011). In contrast, during a cognitive control task accompanying varying monetary rewards, adolescents showed less functional coupling between the ventral striatum and prefrontal regions compared to adults, as indicated by the PPI analysis with the ventral striatum as a seed region (Teslovich et al., 2014). Similarly, during cognitive control tasks accompanying varying emotional arousal states, adolescents showed less functional coupling between the dorsal anterior cingulate/posterior parietal cortices and ventromedial prefrontal cortex compared to adults, as indicated by the PPI analysis with dorsolateral prefrontal cortex and ventromedial prefrontal cortex as seed regions (Cohen et al., 2016).
Although the link between EF task-based functional connectivity and adolescent substance use has not been studied, functional connectivity studies using reward or risk-taking tasks present evidence for beneficial effects of the decoupling of reward sensitivity and cognitive control networks (i.e., cortico-subcortical connectivity) and strong coupling within the cognitive control network (i.e., cortico-cortical connectivity). These findings are consistent with Casey and colleagues’ (2016) illustration of hierarchical developmental shifts in connectivity changing from cortico-subcortical to cortico-cortical circuits from adolescence to adulthood. Studies linking functional connectivity during a reward related task to adolescent substance use indicate positive associations between stronger reward-control connectivity (e.g., nucleus accumbens-superior parietal lobule; nucleus accumbens-supplementary sensorimotor area) and higher levels of substance use craving and behavior (Do & Galvan, 2016; Weiland et al., 2013). Furthermore, longitudinal declines in functional coupling in frontostriatal connectivity (i.e., ventral striatum and medial prefrontal cortex) measured during a risk-taking task were associated with decreases in self-reported risk-taking behaviors including substance use among adolescents (Qu, Galvan, Fuligni, & Telzer, 2015). This finding is consistent with the regulating role of the medial prefrontal cortex, suggesting that the medial prefrontal cortex may be involved in top-down regulation of ventral striatum inputs resulting in decreases in adolescent risk-taking behaviors.
3.2.3. Temporal discounting
In brain research on decision making, one way researchers have simultaneously studied the regulatory system and reactive system is by using tasks assessing temporal discounting of rewards (i.e., the preference for either a smaller amount immediately or a larger amount later). In particular, Competing Neurobehavioral Decision Systems theory (Bickel et al., 2007) posits that decision making between immediate and delayed reinforcers is related to the regulatory balance of activation in two distinct neural systems: the impulsive system which consists of portions of the limbic and paralimbic areas (i.e., the amygdala, nucleus accumbens, ventral pallidum, and related structures) and the executive system which consists of the prefrontal cortices. Functional neuroimaging research has shown greater BOLD responses in the reward network when preference is for an immediate option, and greater BOLD responses in the cognitive control network when preference is for a later reward (McClure, Laibson, Loewenstein, & Cohen, 2004). The “competition” between the two neural systems has been indicated by significant negative correlations between reward valuation and cognitive control neural systems during temporal discounting decision making (e.g., Hare, Camerer, & Rangel, 2009; Stanger et al., 2013). Further, studies examining task-based functional connectivity has revealed the beneficial contributions to less impulsive decision making among adolescents, of negative functional coupling between the control and the reward systems (i.e., functional connectivity between the dorsolateral prefrontal cortex and the medial striatum during larger, later choices versus smaller, sooner choices; van den Bos, Rodriguez, Schweitzer, & McClure, 2015) as well as the increased activation coherence within the control system (i.e., functional connectivity between the ventromedial prefrontal cortex and dorsolateral prefrontal and parietal cortices; Christakou, Brammer, & Rubia, 2011).
In the addiction literature, temporal discounting has been consistently associated with the development and maintenance of addiction (Bickel, Koffarnus, Moody, & Wilson, 2014). Past behavioral studies have shown more powerful prediction of adolescent substance use by temporal discounting compared to EF, implying that the interaction between the reward and control systems may be a stronger predictor than the control system alone. For example, in a study comparing a group of high-risk boys with alcohol abusing parents to a community control group, data failed to support the hypothesis that the highest risk adolescent boys (who came from antisocial alcoholic families) were characterized by weak EF; instead, those high-risk boys exhibited relative weakness in the ability to delay gratification (Nigg et al., 2004). Similarly, a longitudinal study demonstrated stronger effects of temporal discounting than working memory in predicting changes in adolescent alcohol use (Khurana et al., 2013). Though neuroimaging research on temporal discounting among substance abusing adolescents is rare, available findings reveal a substantial negative correlation between the neural systems involved in reward valuation and cognitive control, suggesting that these two systems function in opposition during intertemporal choice (Stanger et al., 2013).
3.2.4. Reinforced control studies
Another way researchers have simultaneously studied both the regulatory and the reactive systems is by manipulating levels of reward and punishment sensitivity stimuli in cognitive control tasks (see Richards et al., 2013 for a review). This line of research has primarily focused on evaluating bottom-up regulation through which reward or punishment sensitivity alters performance of cognitive control. Therefore, this “bottom up” approach is differentiated from our “top down” approach that focuses on the regulating effects of EF on the reactive system when trying to explain individual differences in substance use outcomes.
One early example of the “bottom up” approach research can be seen in a cross-sectional study of emerging adults with externalizing problems and early-onset alcoholism. The researchers found that these individuals had poorer inhibition compared to healthy controls, and that this effect was even stronger when the inhibition task condition involved punishment (Finn, Mazas, Justus, & Steinmetz, 2002). Subsequently, a study using a rewarded antisaccade task (i.e., earning money by performing better) demonstrated that, compared to adults, adolescents show greater activation not only in striatal regions but also in inhibitory regions during preparation for reward-related inhibition (Geier, Terwilliger, Teslovich, Velanova, & Luna, 2010). That study also showed that adolescents performed at adult levels during incentive trials but not neutral trials. Another study using a rewarded antisaccade task demonstrated that unlike healthy non-using adolescents, substance using adolescents (i.e., marijuana use disorder) showed enhanced inhibition behavior by monetary reward, along with increased activation in brain regions associated with inhibition (e.g., dorsolateral prefrontal cortex) when preparing to inhibit (Chung et al., 2011). Such findings illustrate that the greater the reward-seeking tendency, the more that cognitive control resources are recruited—perhaps more so for adolescents than for adults (Luna, Paulsen, Padmanabhan, & Geier, 2013 for review).
However, more longitudinal research is needed on developmental changes in reinforced control. Several past studies have failed to find significant differences in the influence of incentives on inhibition between adolescents and adults in neural activation (Strang & Pollak, 2014) or behavioral performance (Hardin, Schroth, Pine, & Ernst, 2007; Jazbec et al., 2006; Strang & Pollak, 2014). A recent longitudinal study of 10 to 22 year olds provides possible explanations for the lack of age-dependent differences in the effects of incentives shown in the prior research (Paulsen, Hallquist, Geier, & Luna, 2015). Behaviorally, the effects of incentives were diverse; they enhanced performance for some but they hampered performance for others regardless of age. Neural results indicated no age difference in striatal BOLD responses during the reward trials. Instead, during neutral trials (i.e., no incentives), increased striatal BOLD responses were associated with better performance for younger participants, but worse performance for older participants. The findings highlight the need for further studies examining individual differences in reinforced control using within-person longitudinal designs. Relatedly, functional neuroimaging research on task-based effective connectivity (referring to the influence that one neural system exerts over another) indicates that the reward neurocircuitry (i.e., nucleus accumbens, thalamus, and insula connectivity) functions similarly between adolescents and adults (Cho et al., 2012). Taken together, these findings provide indirect evidence supporting the regulating role of EF and associated prefrontal functioning by suggesting that heightened vulnerability to problematic substance use among adolescents is likely to stem from impaired top-down regulation of EF (e.g., “choking” by overwhelming focus on reward) rather than inadequate bottom-up processing of the reward system.
3.3. Summary
Overall, neuroimaging studies utilizing inhibition and working memory tasks suggest a significant association between substance use and impaired neural circuitry, especially in the prefrontal cortex. Emerging evidence also implies interactions of neural systems involved in risky decision making and behaviors that give rise to adolescent substance use. Specifically, neuroimaging studies using separate tasks that assess distinguishable neural networks involved in reactive and regulatory systems demonstrate interactions between these two systems in the prediction of adolescent substance use (e.g., statistical moderation, in which the statistical effect of one system varies depending on the level of the other system). In comparison, temporal discounting studies demonstrate associations between these two systems (e.g., bivariate correlations) to indicate that these systems work in opposition to each other as shown by a negative correlation in optimal decision making. Similarly, studies using functional connectivity indicate whether the two brain systems activate in synchrony or not. Knowing the strength of the association between the regulatory and the reactive systems, however, does not indicate whether the regulatory system moderates the link between the reactive system and substance use outcomes. In fact, statistically, whether two variables (in this case, individual differences in reactivity and regulation) are associated with each other or not is independent of whether those two variables statistically interact in their effects (Baron & Kenny, 1986).
Furthermore, our methodological approach to examining the statistical interactions between the reactivity and regulation systems has clear advantages over studies using reinforced control methods. First, considering ranges of reward or punishment sensitivity on a continuum better reflects individual differences in reward and punishment sensitivity than comparing reactions to discrete levels of reinforcement factors (e.g., such as money gain, money loss, and no money incentive conditions). Second, measuring individual differences in both reactive and regulatory systems and performing direct evaluations of statistical interactions between these two systems enhance our ability to understand why subgroups of adolescents are vulnerable to developing maladaptive substance use behaviors. For example, some adolescents may be vulnerable due to their extremely strong reward sensitivity, whereas others may be vulnerable due to their extremely weak EF.
Most importantly, it is unknown whether statistical interactions between the reactive and the regulatory neural responses may predict individual differences in adolescent substance use; this awaits future investigation. Additionally, measuring functional connectivity will offer complementary information about the processes that explain how the reactive and the regulatory neural networks jointly contribute to typical versus atypical functioning (e.g., how the brain orchestrates information processing among identified ROIs).
4. Conclusions and Future Directions
4.1. Summary of supporting evidence for the modulating role of EF
Despite growing interest in the association between EF and adolescent risky decision making and behaviors, to date, we do not have a clear picture of how deficits in EF may contribute to adolescent substance use. In the present review, we aimed to clarify the role of EF in the development of substance use by reviewing behavioral and functional neuroimaging studies to evaluate empirical evidence of main and interactive effects of EF on substance use throughout adolescence. Although current theories of adolescent risk taking emphasize joint contributions of the reactive and the regulatory systems, empirical research thus far has fallen short in evaluating how these systems interact to determine adolescent substance use. Based on the theoretical perspective that posits the modulating role of the regulatory systems over the reactive systems (Carver et al., 2009), we have proposed a conceptual model emphasizing that EF interacts with reward and punishment sensitivity to produce differential vulnerability to substance use among adolescents. Our model outlines that adolescent substance use can result from heightened approach toward potential positive consequences or diminished avoidance of potential negative consequences, but cognitive regulation capacity may further modulate the biased processing of outcomes. Emerging evidence of the moderating effects of EF at both behavioral and neural levels suggest interactions between the reactive and regulative systems that give rise to adolescent substance use behaviors. If true, this provides an explanation for the weak association between EF and substance use previously found in behavioral studies focusing on main effects.
4.2. Future studies examining statistical interactions
More studies are needed to examine the role of EF as a regulatory system that modulates the contributions of reward and punishment sensitivity to adolescent substance use. Existing behavioral research indicates that the main effects of EF on substance use in adolescents are often non-significant or show relatively weak effect sizes even when significant, whereas statistical interaction effects reveal consistently stronger effect sizes compared to main effects. Based on the empirical findings as well as theory regarding the regulating role of EF, we recommend that researchers consider testing statistical interactions between EF and reward and punishment sensitivity in future studies. Statistical interactions between brain networks associated with the reactive versus regulatory systems can be tested using task based fMRI data (e.g., BOLD responses to a reward sensitivity task and BOLD responses to a cognitive control task). To do so, statistical approaches that accommodate multiple regions of interest, such as latent factor modeling, will be essential (e.g., Kim-Spoon et al., 2016; Nees et al., 2012).
In comparison to behavioral research, functional neuroimaging research on EF and substance use shows more consistent and powerful main effects of EF—for both inhibition and working memory—on substance use. Indeed, in most functional neuroimaging studies reviewed, differences in neural activation (during both inhibition and working memory tasks) were detected even in the absence of observable differences in behavioral performance. Such a discrepancy may be in part due to the fact that behavior tested using a laboratory task may be limited in representing real-life behaviors, whereas task-related neural responses capture individual differences in neurobiological vulnerability more sensitively (Richards et al., 2013). This discrepancy may also reflect differences in methodological approaches between behavioral and neuroimaging research. That is, neuroimaging research examines differences in activation across multiple brain regions (i.e., within-person repeated measures) and this approach provides greater power to detect individual differences in EF rather than using a single parameter of behavioral performance. At the same time, there are some methodological pitfalls in neuroimaging research that misrepresent the true effect due to inflated false positive rates (i.e., Type II error), including incorrect use or failure of multiple comparison corrections as well as use of nonindependent analyses—i.e., computing separate correlations for individual voxels and reporting means of only those voxels exceeding chosen thresholds (Eklund, Nichols, & Knutsson, 2016; Vul, Harris, Winkielman, & Pashler, 2009). With these cautions in mind, we suggest that examining statistical interactions between neural substrates of EF and the reactive system is helpful for inferences regarding underlying mechanisms for behavior. Imaging data, in conjunction with behavioral data, can provide a powerful tool for identifying adolescents who may be susceptible to substance use.
4.3. Future studies examining brain connectivity
In delineating neurobiological determinants of substance use, fruitful directions for future neuroimaging research include examining functional connectivity within frontolimbic circuitry underlying EF regulation of the reactive system in predicting substance use behaviors. Past studies of task-based functional connectivity we reviewed have limitations that need to be addressed in future work. First and foremost, research examining the associations between EF task-based functional connectivity and adolescent substance use is needed. As reviewed earlier, to our knowledge, there has been no direct examination of the association between task-based functional connectivity during EF tasks and adolescent substance use behaviors per se. Additionally, future research should attempt to clarify the direction of functional coupling (i.e., bottom-up versus town-down processing) when examining the association between functional connectivity and adolescent substance use. The two prominent neurodevelopmental models of adolescents’ motivated behavior propose that reduction in impulsive behaviors such as substance use is primarily explained by top-down regulation: The triadic model that proposes the importance of relative prefrontal cortical control over subcortical functioning (Ernst & Fudge, 2009) and the imbalance model that specifies age-graded (from childhood to adulthood) developmental shifts in connectivity from subcortico-subcortical to cortico-subcortical to cortico-cortical circuits (Casey et al., 2016). Yet, any inference regarding causal relationships among ROIs or brain circuits awaits further investigation. We note, but do not discuss here, that advanced analytic approaches for effective connectivity offer promising directions for modeling associations among neural networks, including dynamic causal modeling (Friston, Harrison, & Penny, 2003) and the extended unified structural equation modeling (Gates, Molenaar, Hillary, & Slobounov, 2011).
4.4. Consolidation of EF operationalization and measurement
From a methodological viewpoint, clarity and consistency in how EF is operationalized and measured will help to alleviate the problem of ‘task impurity’ that often occurs in EF research because of the use of tasks that tap into multiple dimensions of EF (Miyake et al., 2000). Variation in results across studies is due in part to differences in task characteristics and demands. Therefore, employing multiple tasks to assess different subcomponents of EF and using a latent variable approach that captures what is shared among the multiple tasks for each EF dimension will alleviate the task impurity problem. For example, if the goal is to distinguish inhibition from working memory, one may use latent variable modeling of behavioral tasks (based on multiple tasks assessing inhibition and multiple tasks assessing working memory) as well as latent variable modeling of neural ROIs (based on multiple ROIs assessed during an inhibition task and multiple ROIs assessed during a working memory task). This, in turn, will aid in our understanding of differential developmental trajectories as well as relative contributions of different EF dimensions to substance use.
Our reviews of neuroimaging research point to the overlap in neural circuitry (e.g., dorsolateral prefrontal, medial frontal, anterior cingulate) between inhibition and working memory, suggesting that these two EF components may work together in the regulation of behavior for successful goal achievement. These findings are in line with conceptual models that integrate working memory and inhibition (Baddeley & Hitch, 1994; Diamond, 2013; Kane & Engle, 2002). Importantly, EF components such as working memory and inhibition may interact with each other in the service of goal-directed behavior (e.g., Badre, 2011). Therefore, understanding how specific dimensions of EF may operate not only independently but also interactively is an important task for future research to better understand the role of EF in the development of substance use behaviors.
4.5. Future studies examining transactional processes using longitudinal designs
In the extant literature, the lack of longitudinal examinations of ontogenic changes in reactive and regulatory systems dynamically interacting to contribute to substance use presents challenges to advancing our understanding of the role of EF in substance use development. Although many neuroimaging studies have demonstrated age-dependent differences in BOLD responses related to reward seeking and EF, these findings are primarily based on cross-sectional comparisons among different age groups instead of within-person developmental changes over time. We emphasize that the heavy reliance on cross-sectional data in neuroimaging research complicates inferences about the directionality of the relation between substance use and abnormal neural responses in task-related inhibition and working memory networks. Notably, longitudinal studies of behavioral and neural indicators of EF and substance use are becoming more common (e.g., the Adolescent Brain Cognitive Development or ABCD study).
As reviewed earlier, prospective neuroimaging studies that statistically predicted later substance use from earlier EF show that more “mature” neural functioning (i.e., less BOLD response during EF tasks indicating more efficient processing) is related to greater rates of transitioning to heavy substance use among adolescents (e.g., Mahmood et al., 2013; Norman et al., 2011; Squeglia et al., 2017; Wetherill et al., 2013). This paradox may be explained by longitudinal examination of changes in brain activation before and after the transition to heavy substance use. For example, adolescents who transitioned into heavy drinking showed less BOLD response at baseline but they showed increasing activation in frontal and parietal regions over time, whereas continuous nondrinkers showed lessening activation over time (Squeglia et al., 2012). As such, brain activation patterns that are associated with healthy vs. pathological functioning can be meaningfully evaluated only by examining longitudinal trajectories across multiple time points throughout adolescence.
To be most useful for guiding prevention and intervention strategies, future research should consider reciprocal effects among reactive and regulative systems and substance use over time, within complete longitudinal designs in which all relevant constructs are measured at each time point. This will permit examination of developmental trajectories of EF in conjunction with those of reward and punishment sensitivity; doing so will provide critical information for clarifying who are vulnerable and when should be targeted. Such information can enhance our ability to identify adolescents at heightened risk for substance use initiation and progression due to aberrant developmental trajectories of reward and punishment sensitivity and EF. Based on our conceptual model highlighting the regulating role of EF, we may expect that adolescents showing developmental patterns of increased reward sensitivity and decreased punishment sensitivity will be most vulnerable to the escalation of substance use when they also show impaired development of EF. In turn, substance use may influence adolescent brain development related to EF such that, for adolescents who are involved in substance use behaviors repeatedly, regulatory abilities may be impaired and reward sensitivity and punishment sensitivity may be augmented and reduced, respectively, placing them at increased risk for substance-related and addictive disorders.
Also importantly, future research should consider contextual factors that may determine the directions of the regulating effects of EF on reward and punishment sensitivity to predict adolescent substance use. In their Fuzzy Trace theory, Reyna and Farley (2006) proposed two distinct routes to risk taking: a reactive route resulting in impulsive risk taking driven by a failure to inhibit behavior versus a reasoned route resulting in intentional risk taking. The regulating role of EF may be shown by reining in extreme levels of reward or punishment sensitivity to lead to lower substance use. At the same time, better EF may promote well-planned risky choices towards substance use. Thus, future research would benefit from examining whether EF modulation demotes impulsive risk taking or promotes intentional risk taking depending on internal (e.g., goals) and external (e.g., peers) contexts.
4.6. Closing remarks
In order to enhance our understanding of the ways through which EF contributes to substance use development, we reviewed the current state of behavioral and neuroimaging research related to adolescent substance use guided by a theoretically based model emphasizing the regulating role of EF over the reactive system (reward and punishment sensitivity). Simultaneous consideration of the regulating effects of EF at both neural and behavioral levels is particularly promising to identify adolescents who are most vulnerable to developing problematic substance use behaviors. Regarding implications for policy and practice, improvements in the regulatory system should be targeted in preventive intervention strategies for substance use and will be most impactful among adolescents with reactive susceptibility to risky and health-compromising behaviors.
Research Highlights.
Extant literature suggests a regulatory system represented by executive functioning (EF).
Main effects of EF on adolescent substance use are weak in behavioral research.
The reward/punishment sensitivity and EF interaction predicts substance use.
Research should consider regulating effects of EF at neural and behavioral levels.
Acknowledgments
We would like to thank Alexis Brieant, Jacob Elder, Toria Herd, Dominique Maciejewski, and Kristin Peviani for their help in editing and proofreading the manuscript.
Funding
This work was supported by the grant from the National Institutes of Health (DA036017).
Appendix
Table 1.
Summary of the reviewed behavioral studies on EF and substance use, including authors, measures, sample size, age range, sex, and effect size for the effect size for the association between EF and substance use (i.e., cigarette, alcohol, and/or marijuana use).
| Study | Measures | N | Age | Sex | Effect size |
|---|---|---|---|---|---|
|
Aytaclar et al. 1999 |
Composite: Stroop; Porteus maze task; Vigilance task; Motor restraint task; Forbidden toy task; Block design task |
275 (106 high- risk youth; 169 low-risk youth) |
10–12 (baseline) |
100% male | β = .28* to −2.25* a |
|
Giancola & Parker 2001 |
Composite: Porteus maze task; Motor restraint task; Vigilance task; Forbidden toy task; Block design task |
187 (69 with family history of substance use disorder, SUD; 21 with family history other than SUD; 97 controls) |
10–12 (baseline) |
100% male | r = −.02 |
|
Giancola et al. 2001 |
Composite: Porteus maze task; Motor restraint task; Stroop task; Vigilance task; Block design task; Picture arrangement task; Object assembly task |
282 (188 with substance use disorder; 94 controls) |
14–18 | 100% female |
b* = −.16*/.08 for low/high antisocial behavior group |
|
Groenman et al. 2015 |
Stop task; Shifting attentional set task; Visuospacial sequencing; Digit span backward task |
669 (294 ADHD probands; 161 unaffected siblings; 214 controls) |
11–12 (baseline) |
78%, 41%, 39% male, respectively |
All p values > .05 |
|
Harakeh et al. 2012 |
Memory search task and Shifting set task |
1797 community youth |
11 (baseline) |
49% male | OR = 1.21 and 2.24* |
|
Jonker et al. 2014 |
Attentional network task |
78 undergraduate students |
18–32 | 34% male |
ΔR2 = .06* for the interaction between EF and punishment sensitivity |
|
Khurana et al. 2013 |
Latent factor: Digit span backward task; Spatial working memory task; Corsi block tapping task; Letter two-back task |
358 community youth |
10–12 (baseline) |
48% male | b* = −.02* |
|
Kim- Spoon et al. 2015 |
Attentional control questionnaire |
822 community youth |
9 (baseline) |
51% male |
b* = .20*/−.04 for low/high control group |
|
Kim- Spoon et al. 2016 |
Multi-source interference task |
156 community youth |
13–14 | 48% male |
b* = .25*/.08 reward sensitivity effects for low/high control group |
|
Nigg et al. 2006 |
Stopping task | 498 (high risk youth and controls) |
12–14 (baseline) |
73% male | b* = .03 to .11* |
|
Patrick et al. 2008 |
N-back task and Go- no-go task |
72 undergraduate students |
19–24 | 0% male |
b* = −.04 to .16 for main effects; b* = − .22 to .28* for interaction effects between EF and reward sensitivity |
|
Peeters et al. 2014 |
Self-ordered pointing task |
374 special education youth |
12–14 (baseline) |
88% male | b* = .05** |
|
Peeters et al. 2015 |
Self-ordered pointing task; Stroop task |
534 (from 250 mainstream school and 374 special education youth) |
12–14 (baseline) |
69% male | OR = 0.15* to 3.12* |
|
Shoal & Giancola 2001 |
Composite: Porteus maze task; Motor restraint task; Stroop task; Vigilance task |
250 (90 with family history of substance use disorder, SUD; 160 controls) |
15–17 | 100% male |
b* = .36*/ .01 for SUD/control group |
| Sullivan et al. 2014 |
N-back task (accuracy and speed) |
831 community youth (130 hazardous drinkers; 692 no/low drinkers) |
12–21 | 49% male | d = 0.002 and 0.17 b |
|
Squeglia et al. 2014 |
Color-word interference task and Digit span task |
175 community youth (105 substance use transitioners; 70 continuous nonusers) |
12–14 (baseline) |
59% male | d = 0.16 and 0.24 b |
|
Wilens et al. 2011 |
Composite: Stroop task; Rey-Osterrieth complex figure; Auditory continuous performance test; Wisconsin card sort test; Freedom from distractibility index; Wide range achievement of memory and learning test for younger children or California verbal learning test for older children |
435 (232 with attention deficit hyperactivity disorder and 203 controls; among them 90 with executive function deficit, EFC) |
15–16 (baseline) |
59% male | All p values > .05 |
|
Wong et al. 2010 |
Stopping task | 386 (290 high risk youth; 96 controls) |
15–17 (baseline) |
76% male | All p values > .05 |
|
Youssef et al. 2016 |
Latent factor score: Stroop task; Multi- source interference task; Effortful control questionnaire |
177 community youth |
15–16 | 47% male | Main b* = −.30* Interaction b* = − .38* |
Notes. EF = executive functioning.
Aytaclar et al. did not clarify whether they reported unstandardized or standardized regression estimates.
Effect size estimates were calculated by authors.
p < .05.
Table 2.
Summary of the reviewed functional neuroimaging studies on EF and substance use, including authors, measures, sample size, age range, sex, contrast, brain regions, and the effect size for the association between EF and substance use (i.e., cigarette, alcohol, and/or marijuana use).
| Study | Measures | N | Age | Sex | Contrast | Brain Regions | Effect Size |
|---|---|---|---|---|---|---|---|
| Behan et al. 2014 | Go-no-go task | 35 (17 marijuana users; 18 controls) | 14–19 | 94 % male | successful stop > baseline ; group difference (marijuana users vs. controls) | B frontal gyrus, R medial frontal gyrus, B middle gyrus, L superior frontal gyrus, B cingulate gyrus, B insula, B middle temporal gyrus, B inferior parietal lobule, lentiform nucleus, tuber, culmen | All p values > .05 |
| Feldstein Ewing et al. 2015 | Go-no-go task | 95 high-risk youth | 14–18 | 81 % male | no-go > go; correlate d with substance use frequency | L inferior frontal gyrus, R insula | r = −.34* and −.37*a |
| Galvan et al. 2011 | Stop-signal task | 50 (25 smokers ; 25 non-smokers) | 15–21 | 58 % male | successful stop > baseline ; correlate d with heaviness of smoking successful stop > go; correlate d with heaviness of smoking | B middle frontal gyrus, cingulate gyrus, supplementary motor area, orbitofrontal cortex, R superior frontal gyrus, L inferior frontal gyrus | d = 1.54* to > 14*a |
| d = 3.98*a | |||||||
| Jager et al. 2010 | Verbal working memory task | 47 (23 marijuana users; 24 controls) | 13–19 | 100 % mal e | group (marijuana user vs. control) X task (before vs. after practice) interaction | L inferior frontal gyrus, L precentral/dorsol ateral prefrontal cortex, anterior cingulate cortex | d = 0.69* to 1.41*a |
| Heitzeg et al. 2014 | Go-no-go task | 45 (13 high risk youth with problem alcohol use; 13 high risk youth with no alcohol use; 19 controls) | 9–12 (baseline) | 77 % mal e | failed inhibition > correct inhibition; group difference (problem user vs. non-user) | L middle frontal gyrus | d = 1.36* |
| Kim-Spoon et al. in press | Multi-source interference task | 24 community youth | 17–20 | 75 % mal e | interference > neutral conditions; cognitive control brain activation moderates anterior insula effects | dorsal anterior cingulate cortex | b* = −.66*/−.23 for low/high control group |
| Kim-Spoon et al. 2016 | Multi-source interference task | 156 community youth | 13–14 | 48 % mal e | interference > neutral conditions; cognitive control brain activation moderates reward sensitivity effects | factor score based on L posterior-medial prefrontal cortex, B inferior parietal cortex, R inferior frontal gyrus, R insula, L middle frontal gyrus, and R superior frontal gyrus | b* = .25*/ .04 for low/high control group |
| Mahmood et al. 2013 | Go-no-go task | 80 (39 high frequency drug users, HF; 41 low frequency drug users, LF) | 16–19 (baseline) | 69 % mal e | no-go > go; predicting alcohol dependence | L angular gyrus and medial prefrontal cortex | b* = .07 and −.31 for HF and b* = .17 and −.08 for LF |
| Norman et al. 2011 | Go-no-go task | 38 (21 transitioning to heavy alcohol use, TU; 17 controls, CON | 12–14 (baseline) | 50 % mal e | TU < CON for no-go trials | L dorsolateral prefrontal cortex, L superior and middle frontal gyrus, R inferior frontal gyrus, B middle frontal gyrus, B paracentral lobule, cingulate gyrus, L cingulate, B middle temporal gyrus, B inferior parietal lobule, L putamen, and pons | d = 1.17* to 2.66* |
| Padula et al. 2007 | Spatial working memory task | 34 (17 marijuana users, MH; 17 controls, CON) | 16–18 | 65 % male | MJ > CON | B superior parietal lobules, B precuneus, R putamen, caudate, thalamus, globus pallidus, insula | d = 0.77* to 1.47*a |
| Schweinsburg et al. 2010 | Verbal working memory task | 24 (12 binge drinkers, BD; 12 controls, CON) | 16–18 | 75 % mal e | BD > CON | R superior frontal gyrus, B inferior parietal lobule, B cingulate gyrus | d = 1.45* to 1.75*a |
| BD < CON | B cuneus, lingual gyrus, parahippocampal gyrus, R precuneus | d = 2.34*a | |||||
| Schweinsburg et al. 2011 | Verbal working memory task | 74 (16 binge drinkers, BD; 8 marijuana users, MJ; 28 binge drinking marijuana users, BD+MJ ; 22 controls, CON) | 16–18 | 76 % mal e | BD & BD+MJ > CON & MJ | R post-central gyrus/inferior parietal lobule, R superior frontal gyrus | η2 = 0.16* to 0.20* |
| CON & MJ > BD & BD + MJ | L fusiform/parahip pocampal gyrus/cuneus, L precuneus/inferior parietal lobule, L inferior frontal/precentral gyrus, R middle/inferior frontal gyrus, L medial frontal/cingulate gyrus | η2 = 0.13* to 0.20* | |||||
| Squeglia et al. 2012 | Spatial working memory task | 40 (20 heavy drinking transitioners; 20 continuous nondrinkers) | 12–16 | 70 % mal e | spatial working memory > vigilance conditions; group (transitioners vs. nondrinkers) X time (baseline vs. age 17–18) interaction | L medial frontal gyrus, R interior parietal lobule | η2 = 0.19* to 0.23* |
| Squeglia et al. 2017 | Spatial working memory task | 137 (70 moderate to heavy alcohol users; 67 continuous nonuser s) | 12–18 | 56 % mal e | spatial working memory > vigilance conditions; Random forest classification to predict alcohol initiator status | R superior temporal, R posterior cingulate | d = .22* to .42* |
| Tapert et al. 2004 | Spatial working memory task | 34 (15 alcohol use disorder ed, AUD; 19 controls, CON) | 15–17 | 62 % mal e | spatial working memory > vigilance conditions; AUD < CON | R precuneus, B middle occipital gyrus, L superior occipital gyrus, L inferior temporal gyrus, L precentral gyrus, B cerebellar semilunar lobule, R cerebellar culmen and declive | d = 3.46* to 7.81* |
| spatial working memory > vigilance conditions; AUD > CON | B precuneus and B superior parietal lobule | d = 7.22* to 9.29* | |||||
| Wetherill et al. 2013 | Go-no-go task | 40 (20 heavy drinkers ; 20 controls) | 11–16 (baseline) | 55 % mal e | no-go > go; group (heavy drinker vs. control) X time (baseline vs. follow-up) interaction | B middle frontal gyrus, R inferior parietal lobule, L putamen, L cerebellar tonsil | η2 = 0.18* to 0.30* |
Notes. EF = executive functioning; L = left; R = right; B = bilateral.
Effect size coefficients were calculated by authors.
p < .05.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting galley proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnett JJ. The developmental context of substance use in emerging adulthood. Journal of Drug Issues. 2005;35:235–254. doi: 10.1177/002204260503500202. [DOI] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex: One decade on. Trends in Cognitive Sciences. 2014;18:177–185. doi: 10.1016/j.tics.2013.12.003. http://dx.doi.org/10.1016/j.tics.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Aytaclar S, Tarter RE, Kirisci L, Lu S. Association between hyperactivity and executive cognitive functioning in childhood and substance use in early adolescence. Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38:172–178. doi: 10.1097/00004583-199902000-00016. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory. New York, NY: Oxford University Press; 1986. [Google Scholar]
- Baddeley A. Working memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Hitch GJ. Developments in the concept of working memory. Neuropsychology. 1994;8:485–493. doi: 10.1037/0894-4105.8.4.485. [DOI] [Google Scholar]
- Badre D. Defining an ontology of cognitive control requires attention to component interactions. Topics in Cognitive Science. 2011;3:217–221. doi: 10.1111/j.1756-8765.2011.01141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037/0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Behan B, Connolly CG, Datwani S, Doucet M, Ivanovic J, Morioka R, … Garavan H. Response inhibition and elevated parietal-cerebellar correlations in chronic adolescent cannabis users. Neuropharmacology. 2014;84:131–137. doi: 10.1016/j.neuropharm.2013.05.027. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Miller ML, Yi R, Kowal BP, Lindquist DM, Pitcock JA. Behavioral and neuroeconomics of drug addiction: Competing neural systems and temporal discounting processes. Drug and Alcohol Dependence. 2007;90:S85–S91. doi: 10.1016/j.drugalcdep.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Koffarnus MN, Moody L, Wilson AG. The behavioral-and neuro-economic process of temporal discounting: A candidate behavioral marker of addiction. Neuropharmacology. 2014;76:518–527. doi: 10.1016/j.neuropharm.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Lei Z, Trommer BL, Davenport ND, … Mesulam MM. Neural development of selective attention and response inhibition. Neuroimage. 2003;20:737–751. doi: 10.1016/S1053-8119(03)00404-X. [DOI] [PubMed] [Google Scholar]
- Braver TS, Krug MK, Chiew KS, Kool W, Westbrook JA, Clement NJ, … Somerville LH. Mechanisms of motivation–cognition interaction: Challenges and opportunities. Cognitive, Affective & Behavioral Neuroscience. 2014;14(2):443–472. doi: 10.3758/s13415-014-0300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS. Impulse and constraint: Perspectives from personality psychology, convergence with theory in other areas, and potential for integration. Personality and Social Psychology Review. 2005;9:312–333. doi: 10.1207/s15327957pspr0904_2. [DOI] [PubMed] [Google Scholar]
- Carver CS, Johnson SL, Joormann J. Two-mode models of self-regulation as a tool for conceptualizing effects of the serotonin system in normal behavior and diverse disorders. Current Directions in Psychological Science. 2009;18:195–199. doi: 10.1111/j.1467-8721.2009.01635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ. Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annual Review of Psychology. 2015;66:295–319. doi: 10.1146/annurev-psych-010814-015156. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Durston S, Fossella JA. Evidence for a mechanistic model of cognitive control. Clinical Neuroscience Research. 2001;1:267–282. doi: 10.1016/S1566-2772(01)00013-5. [DOI] [Google Scholar]
- Casey BJ, Galván A, Somerville LH. Beyond simple models of adolescence to an integrated circuit-based account: A commentary. Developmental Cognitive Neuroscience. 2016;17:128–130. doi: 10.1016/j.dcn.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolescent brain. Developmental Review. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Youth Risk Behavior Surveillance – United States, 2012. MMWR. 2012;2012(4):61. [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: A critical period of addiction vulnerability. American Journal of Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YT, Fromm S, Guyer AE, Detloff A, Pine DS, Fudge JL, Ernst M. Nucleus accumbens, thalamus and insula connectivity during incentive anticipation in typical adults and adolescents. Neuroimage. 2012;66:508–521. doi: 10.1016/j.neuroimage.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakou A, Brammer M, Rubia K. Maturation of limbic corticostriatal activation and connectivity associated with developmental changes in temporal discounting. NeuroImage. 2011;54:1344–1354. doi: 10.1016/j.neuroimage.2010.08.067. [DOI] [PubMed] [Google Scholar]
- Chung T, Geier C, Luna B, Pajtek S, Terwilliger R, Thatcher D, Clark DB. Enhancing response inhibition by incentive: Comparison of adolescents with and without substance use disorder. Drug and Alcohol Dependence. 2011;115(1–2):43–50. doi: 10.1016/j.drugalcdep.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AO, Breiner K, Steinberg L, Bonnie RJ, Scott ES, Taylor-Thompson KA, … Casey BJ. When is an adolescent an adult? Assessing cognitive control in emotional and nonemotional contexts. Psychological Science. 2016;27:549–562. doi: 10.1177/0956797615627625. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends in Cognitive Sciences. 2003;7:415–423. doi: 10.1016/S1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR. The dependence of span and delayed-response performance on prefrontal cortex. Neuropsychologia. 1999;37:1303–1315. doi: 10.1016/S0028-3932(99)00021-4. [DOI] [PubMed] [Google Scholar]
- Diamond A. Executive functions. Annual Review of Psychology. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do KT, Galván A. Neural sensitivity to smoking stimuli is associated with cigarette craving in adolescent smokers. Journal of Adolescent Health. 2016;58:186–194. doi: 10.1016/j.jadohealth.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of National Academy of Sciences. 2016;113:7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Daniele T, Frantz K. New perspectives on adolescent motivated behavior: Attention and conditioning. Developmental Cognitive Neuroscience. 2011;1:377–389. doi: 10.1016/j.dcn.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: Anatomy, connectivity, and ontogeny of the triadic nodes. Neuroscience and Biobehavioral Reviews. 2009;33:367–382. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Torrisi S, Balderston N, Grillon C, Hale EA. fMRI functional connectivity applied to adolescent neurodevelopment. Annual Review of Clinical Psychology. 2015;11:361–377. doi: 10.1146/annurev-clinpsy-032814-112753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal brain structure implicated in stimulant drug addiction. Science. 2012;335(6068):601–604. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Sakhardande A, Blakemore SJ. The effect of alcohol consumption on the adolescent brain: A systematic review of MRI and fMRI studies of alcohol-using youth. NeuroImage: Clinical. 2014;5:420–437. doi: 10.1016/j.nicl.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Houck JM, Bryan AD. Neural activation during response inhibition is associated with adolescents’ frequency of risky sex and substance use. Addictive Behaviors. 2015;44:80–87. doi: 10.1016/j.addbeh.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn PR, Mazas CA, Justus AN, Steinmetz J. Early-onset alcoholism with conduct disorder: Go/no go learning deficits, working memory capacity, and personality. Alcoholism: Clinical and Experimental Research. 2002;26:186–206. [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. NeuroImage. 2003;19:1273–1302. doi: 10.1016/S1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Moran R, Seth A. Analyzing connectivity with Granger causality and dynamic causal modelling. Current Opinion in Neurobiology. 2013;23:172–178. doi: 10.1016/j.conb.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Poldrack RA, Baker CM, McGlennen KM, London ED. Neural correlates of response inhibition and cigarette smoking in late adolescence. Neuropsychopharmacology. 2011;36:970–978. doi: 10.1038/npp.2010.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates KM, Molenaar PCM, Hillary FG, Slobounov S. Extended unified SEM approach for modeling event-related fMRI data. NeuroImage. 2011;54:1151–1158. doi: 10.1016/j.neuroimage.2010.08.051. [DOI] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cerebral Cortex. 2010;20:1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancola PR, Mezzich AC. Executive functioning, temperament, and drug use involvement in adolescent females with a substance use disorder. Journal of Child Psychology and Psychiatry. 2003;44:857–866. doi: 10.1111/1469-7610.00170. [DOI] [PubMed] [Google Scholar]
- Giancola PR, Parker AM. A six-year prospective study of pathways toward drug use in adolescent boys with and without a family history of a substance use disorder. Journal of Studies on Alcohol and Drugs. 2001;62:166–178. doi: 10.15288/jsa.2001.62.166. [DOI] [PubMed] [Google Scholar]
- Giancola PR, Shoal GD, Mezzich AC. Constructive thinking, executive functioning, antisocial behavior, and drug use involvement in adolescent females with a substance use disorder. Experimental and Clinical Psychopharmacology. 2001;9:215–227. doi: 10.1037/1064-1297.9.2.215. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Circuitry of primate prefrontal cortex and regulation of behavior by representational memory. In: Plum F, editor. Handbook of physiology: Vol. V. Higher functions of the brain. Section I. The nervous system. Bethesda, MD: American Psychological Society; 1987. pp. 373–417. [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV drug abuse and dependence: Results from the National longitudinal alcohol epidemiologic survey. Journal of Substance Abuse. 1998;10:163–173. doi: 10.1016/S0899-3289(99)80131-X. [DOI] [PubMed] [Google Scholar]
- Gray JA. The neuropsychology of anxiety: an enquiry into the functions of the septo-hippocampal system. 1. Oxford: Oxford University Press; 1982. [Google Scholar]
- Groenman AP, Oosterlaan J, Greven CU, Vuijk PJ, Rommelse N, Franke B, … Buitelaar J. Neurocognitive predictors of substance use disorders and nicotine dependence in ADHD probands, their unaffected siblings, and controls: A 4-year prospective follow-up. Journal of Child Psychology and Psychiatry. 2015;56:521–529. doi: 10.1111/jcpp.12315. [DOI] [PubMed] [Google Scholar]
- Harakeh Z, de Sonneville L, van den Eijnden RJJM, Huizink AC, Reijneveld SA, Ormel J, … Vollebergh WAM. The association between neurocognitive functioning and smoking in adolescence: The TAILS study. Neuropsychology. 2012;26:541–550. doi: 10.1037/a0029217. [DOI] [PubMed] [Google Scholar]
- Hardin MG, Schroth E, Pine DS, Ernst M. Incentive-related modulation of cognitive control in healthy, anxious, and depressed adolescents: Development and psychopathology related differences. Journal of Child Psychology and Psychiatry. 2007;48:446–454. doi: 10.1111/j.1469-7610.2006.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPMC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Heitzeg MM, Nigg JT, Hardee JE, Soules M, Steinberg D, Zubieta JK, Zucker RA. Left middle frontal gyrus response to inhibitory errors in children prospectively predicts early problem substance use. Drug and Alcohol Dependence. 2014;141:51–57. doi: 10.1016/j.drugalcdep.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager G, Block RI, Luijten M, Ramsey NF. Cannabis use and memory brain function in adolescent boys: a cross-sectional multicenter functional magnetic resonance imaging study. Journal of American Academy of Child Adolescent Psychiatry. 2010;49:561–572. doi: 10.1016/j.jaac.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazbec S, Hardin M, Schroth E, McClure E, Pine D, Ernst M. Age-related influence of contingencies on a saccade task. Experimental Brain Research. 2006;174:754–762. doi: 10.1007/s00221-006-0520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975–2015: Overview, key findings on adolescent drug use. Ann Arbor, MI: Institute for Social Research, The University of Michigan; 2016. [Google Scholar]
- Jonker NC, Ostafin BD, Glashouwer KA, van Hemel-Ruiter ME, de Jong PJ. Reward and punishment sensitivity and alcohol use: The moderating role of executive control. Addictive Behaviors. 2014;39:945–948. doi: 10.1016/j.addbeh.2013.12.011. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: An individual-differences perspective. Psychonomic Bulletin & Review. 2002;9:637–671. doi: 10.3758/BF03196323. [DOI] [PubMed] [Google Scholar]
- Khurana A, Romer D, Betancourt LM, Brodsky NL, Giannetta JM, Hurt H. Working memory ability predicts trajectories of early alcohol use in adolescents: The mediational role of impulsivity. Addiction. 2013;108:506–515. doi: 10.1111/add.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Spoon J, Deater-Deckard K, Lauharatanahirun N, Farley JP, Chiu PH, Bickel WK, King-Casas B. Neural interaction between risk sensitivity and cognitive control predicting health risk behaviors among late adolescents. Journal of Research on Adolescence. doi: 10.1111/jora.12295. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Spoon J, Deater-Deckard K, Holmes CJ, Lee JI, Chiu PH, King-Casas B. Behavioral and neural inhibitory control moderates the effects of reward sensitivity on adolescent substance use. Neuropsychologia. 2016;91:318–326. doi: 10.1016/j.neuropsychologia.2016.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Spoon J, Holmes C, Deater-Deckard K. Attention regulates anger and fear to predict changes in adolescent risk-taking behaviors. Journal of Child Psychology and Psychiatry. 2015;56:756–765. doi: 10.1111/jcpp.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M, Wahlstrom D, Porter JN, Collins PF. Dopaminergic modulation of incentive motivation in adolescence: Age-related changes in signaling, individual differences, and implications for the development of self-regulation. Developmental Psychology. 2012;48:844–861. doi: 10.1037/a0027432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Marek S, Larsen B, Tervo-Clemmens B, Chahal R. An integrative model of the maturation of cognitive Control. Annual Review of Neuroscience. 2015;38:151–170. doi: 10.1146/annurev-neuro-071714-034054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Padmanabhan A, O’Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain and Cognition. 2010;72:101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Paulsen DJ, Padmanabhan A, Geier C. Cognitive control and motivation. Current Directions in Psychological Science. 2013;22:94–100. doi: 10.1177/0963721413478416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood OM, Goldenberg D, Thayer R, Migliorini R, Simmons AN, Tapert SF. Adolescents’ fMRI activation to a response inhibition task predicts future substance use. Addictive Behaviors. 2013;38:1435–1441. doi: 10.1016/j.addbeh.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, … Moffitt TE. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proceedings of the National Academy of Sciences. 2012;109:E2657–E2664. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Nees F, Tzschoppe J, Patrick CJ, Vollstadt-Klein S, Steiner S, Poustka L … tI Consortium. Determinants of early alcohol use in healthy adolescents: The differential contribution of neuroimaging and psychological factors. Neuropsychopharmacology. 2012;37:986–995. doi: 10.1038/npp.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT. On the relations among self-regulation, self-control, executive functioning, effortful control, cognitive control, impulsivity, risk-taking, and inhibition for developmental psychopathology. Journal of Child Psychology and Psychiatry. doi: 10.1111/jcpp.12675. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Glass JM, Wong MM, Poon E, Jester JM, Fitzgerald HE, … Zucker RA. Neuropsychological executive functioning in children at elevated risk for alcoholism: Findings in early adolescence. Journal of Abnormal Psychology. 2004;113:302–314. doi: 10.1037/0021-843X.113.2.302. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM, … Zucker RA. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:468–475. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- Norman AL, Pulido C, Squeglia LM, Spadoni AD, Paulus MP, Tapert SF. Neural activation during inhibition predicts initiation of substance use in adolescence. Drug and Alcohol Dependence. 2011;119:216–223. doi: 10.1016/j.drugalcdep.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordaz SJ, Foran W, Velanova K, Luna B. Longitudinal growth curves of brain function underlying inhibitory control through adolescence. Journal of Neuroscience. 2013;33(46):18109–18124. doi: 10.1523/jneurosci.1741-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padula CB, Schweinsburg AD, Tapert SF. Spatial working memory performance and fMRI activation interaction in abstinent adolescent marijuana users. Psychology of Addictive Behaviors. 2007;21:478–487. doi: 10.1037/0893-164X.21.4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick ME, Blair C, Maggs JL. Executive function, approach sensitivity, and emotional decision making as influences on risk behaviors in young adults. Journal of Clinical and Experimental Neuropsychology. 2008;30:449–462. doi: 10.1080/13803390701523109. [DOI] [PubMed] [Google Scholar]
- Paulsen DJ, Hallquist MN, Geier CF, Luna B. Effects of incentives, age, and behavior on brain activation during inhibitory control: A longitudinal fMRI study. Developmental Cognitive Neuroscience. 2015;11:105–115. doi: 10.1016/j.dcn.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends in Cognitive Sciences. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Peeters M, Janssen T, Monshouwer K, Boendermaker W, Pronk T, Wiers R, Vollenbergh W. Weaknesses in executive functioning predict the initiating of adolescents’ alcohol use. Developmental Cognitive Neuroscience. 2015;16:139–146. doi: 10.1016/j.dcn.2015.04.003. http://dx.doi.org/10.1016/j.dcn.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters M, Monshouwer K, Janssen T, Wiers RW, Vollebergh WA. Working memory and alcohol use in at-risk adolescents: A 2-year follow-up. Alcoholism: Clinical and Experimental Research. 2014;38:1176–1183. doi: 10.1111/acer.12339. [DOI] [PubMed] [Google Scholar]
- Qu Y, Galvan A, Fuligni AJ, Lieberman MD, Telzer EH. Longitudinal changes in prefrontal cortex activation underlie declines in adolescent risk taking. Journal of Neuroscience. 2015;35:11308–11314. doi: 10.1523/JNEUROSCI.1553-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyna VF, Farley F. Risk and rationality in adolescent decision making: Implications for theory, practice, and public policy. Psychological Science in the Public Interest. 2006;7:1–44. doi: 10.1111/j.1529-1006.2006.00026.x. [DOI] [PubMed] [Google Scholar]
- Reyna VF, Rivers SE. Current theories of risk and rational decision making. Developmental Review. 2008;28:1–11. doi: 10.1016/j.dr.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JM, Plate RC, Ernst M. A systematic review of fMRI reward paradigms used in studies of adolescents vs. adults: The impact of task design and implications for understanding neurodevelopment. Neuroscience & Biobehavioral Reviews. 2013;37:976–991. doi: 10.1016/j.neubiorev.2013.03.004. http://dx.doi.org/10.1016/j.neubiorev.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, McQueeny T, Nagel BJ, Eyler LT, Tapert SF. A preliminary study of fMRI response during verbal encoding among adolescent binge drinkers. Alcohol. 2010:111–117. doi: 10.1016/j.alcohol.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Nagel BJ, Eyler LT, Tapert SF. Neural correlates of verbal learning in adolescent alcohol and marijuana users. Addiction. 2011;106:564–573. doi: 10.1111/j.1360-0443.2010.03197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoal GD, Giancola PR. Executive cognitive functioning, negative affectivity, and drug use in adolescent boys with and without a family history of a substance use disorder. Journal of Child & Adolescent Substance Abuse. 2001;10:111–121. doi: 10.1300/J029v10n04_11. [DOI] [Google Scholar]
- Shulman EP, Smith AR, Silva K, Icenogle G, Duell N, Chein J, Steinberg L. The dual systems model: Review, reappraisal, and reaffirmation. Developmental Cognitive Neuroscience. 2016;17:103–117. doi: 10.1016/j.dcn.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Dager AD, Cohen-Gilbert JE, Sneider JT. Neurobiological signatures associated with alcohol and drug use in the human adolescent brain. Neuroscience & Biobehavioral Reviews. 2016;70:244–259. doi: 10.1016/j.neubiorev.2016.06.0420149-7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman MH, Jedd K, Luciana M. Neural networks involved in adolescent reward processing: An activation likelihood estimation meta-analysis of functional neuroimaging studies. NeuroImage. 2015;122:427–439. doi: 10.1016/j.neuroimage.2015.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Working memory in humans: Neuropsychological evidence. The MIT Press; 1995. [Google Scholar]
- Somerville LH, Hare T, Casey BJ. Frontostriatal Maturation Predicts Cognitive Control Failure to Appetitive Cues in Adolescents. Journal of Cognitive Neuroscience. 2011;23:2123– 2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Assessment of adolescent neurotoxicity: rationale and methodological considerations. Neurotoxicology and Teratology. 2007;29:1–9. doi: 10.1016/j.ntt.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Ball TM, Jacobus J, Brumback T, McKenna BS, Nguyen-Louie TT, … Tapert SF. Neural predictors of initiating alcohol use during adolescence. American Journal of Psychiatry. 2017;174:172–179. doi: 10.1176/appi.ajp.2016.15121587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J, Nguyen-Louie TT, Tapert SF. Inhibition during early adolescence predicts alcohol and marijuana use by late adolescence. Neuropsychology. 2014;28:782–790. doi: 10.1037/neu0000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Pulido C, Wetherill RR, Jacobus J, Brown GG, Tapert SF. Brain response to working memory over three years of adolescence: influence of initiating heavy drinking. Journal of Studies on Alcohol and Drugs. 2012;73:749–760. doi: 10.15288/jsad.2012.73.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychology of Addictive Behaviors. 2009;23:715–722. doi: 10.1037/a0016516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger C, Elton A, Ryan SR, James GA, Budney AJ, Kilts CD. Neuroeconomics and adolescent substance abuse: Individual differences in neural networks and delay discounting. Journal of the American Academy of Child & Adolescent Psychiatry. 2013;52:747–755. doi: 10.1016/j.jaac.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone AL, Becker LG, Huber AM, Catalano RF. Review of risk and protective factors of substance use and problem use in emerging adulthood. Addictive Behaviors. 2012;37:747–775. doi: 10.1016/j.addbeh.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Strang NM, Pollak SD. Developmental continuity in reward-related enhancement of cognitive control. Developmental Cognitive Neuroscience. 2014;10:34–43. doi: 10.1016/j.dcn.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Brumback T, Tapert SF, Fama R, Prouty D, Brown SA, … Pfefferbaum A. Cognitive, emotion control, and motor performance of adolescents in the NCANDA study: Contributions from alcohol consumption, age, sex, ethnicity, and family history of addiction. Neuropsychology. 2016;30:449–473. doi: 10.1037/neu0000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, Brown SA, Frank LR, Brown GG, Meloy MJ. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcoholism: Clinical and Experimental Research. 2004;28:1577–1586. doi: 10.1097/01.ALC.0000141812.81234.A6. [DOI] [PubMed] [Google Scholar]
- Teslovich T, Mulder M, Franklin NT, Ruberry EJ, Millner A, Somerville LH, … Casey BJ. Adolescents let sufficient evidence accumulate before making a decision when large incentives are at stake. Developmental Science. 2014;17:59–70. doi: 10.1111/desc.12092. [DOI] [PubMed] [Google Scholar]
- Urošević S, Collins P, Muetzel R, Lim K, Luciana M. Longitudinal changes in behavioral approach system sensitivity and brain structures involved in reward processing during adolescence. Developmental Psychology. 2012;48:1488–1500. doi: 10.1037/a0027502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos W, Rodriguez CA, Schweitzer JB, McClure SM. Adolescent impatience decreases with increased frontostriatal connectivity. Proceedings of the National Academy of Sciences. 2015;112(29):E3765–E3774. doi: 10.1073/pnas.1423095112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijvenvoorde ACK, Achterberg M, Braams BR, Peters S, Crone EA. Testing a dual-systems model of adolescent brain development using resting-state connectivity analyses. NeuroImage. 2016;124:409–420. doi: 10.1016/j.neuroimage.2015.04.069. [DOI] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspectives on Psychological Science. 2009;4:274–290. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory. Cognitive, Affective, & Behavioral Neuroscience. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Weiland BJ, Welsh RC, Yau WYW, Zucker RA, Zubieta JK, Heitzeg MM. Accumbens functional connectivity during reward mediates sensation-seeking and alcohol use in high-risk youth. Drug and Alcohol Dependence. 2013;128(1–2):130–139. doi: 10.1016/j.drugalcdep.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Squeglia LM, Yang TT, Tapert SF. A longitudinal examination of adolescent response inhibition: Neural differences before and after the initiation of heavy drinking. Psychopharmacology. 2013;230:663–671. doi: 10.1007/s00213-013-3198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Martelon M, Joshi G, Bateman C, Fried R, Petty C, Biederman J. Does ADHD predict substance-use disorders? A 10-year follow-up study of young adults with ADHD. Journal of the American Academy of Child & Adolescent Psychiatry. 2011;50:543–553. doi: 10.1016/j.jaac.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MM, Brower KJ, Nigg JT, Zucker RA. Childhood sleep problems, response inhibition, and alcohol and drug outcomes in adolescence and young adulthood. Alcoholism: Clinical and Experimental Research. 2010;34(6):1033–1044. doi: 10.1111/j.1530-0277.2010.01178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. 2016 http://www.who.int/maternal_child_adolescent/topics/adolescence/dev/en/ retrieved on April 27, 2016.
- Youssef GJ, Whittle S, Allen NB, Lubman DI, Simmons JG, Yücel M. Cognitive control as a moderator of temperamental motivations toward adolescent risk-taking behavior. Child Development. 2016;87:395–404. doi: 10.1111/cdev.12480. dx.doi.org/10.1111/cdev.12480. [DOI] [PubMed] [Google Scholar]