Introduction
Adherence to cancer screening is critical for early detection and treatment of several types of cancer, and a lack of screening is associated with late-stage diagnosis and lower survival rates. As recommended by current preventive health guidelines2, several screening tools have proven effective in reducing the burden of various cancers, including cytology (Pap smear) for cervical cancer (1), mammography for breast cancer (2), and fecal occult blood testing (FOBT), sigmoidoscopy, colonoscopy, and fecal immunochemical test (FIT) for colorectal cancer (3, 4), and, more recently, low dose helical computed tomography (LDCT) for lung cancer (5). Despite benefits, cancer screening continues to be underutilized in the United States and worldwide (6, 7). Given the ability of screening tests to reduce cancer morbidity and mortality, improving adherence to cancer screening is of critical importance to public health.
Many factors such as health literacy (8), risk perception (9), lack of health insurance (10) and social influence (11) are associated with cancer screening rates. However, provider-patient communication regarding screening tests may play one of the strongest modifiable roles in cancer screening behavior (12). Physicians and other primary health care providers can serve as a key health information source by assessing patient screening eligibility, negotiating a course of action, and helping to coordinate screening tests and follow-up care (13). The impact of provider recommendation on cancer screening behaviors was recently emphasized in a consensus statement released by the National Institutes of Health (12). Moreover, the U.S. Department of Health and Human Services included increasing provider counseling about screening tests as a main objective in the Healthy People 2020 goals (14).
Until recently, most research examining the impact of a primary care provider recommendation on cancer screening has used simple, narrow questions (e.g., “Did your physician recommend a screening test?”). More recent work has suggested that the presence or absence of a provider recommendation alone may not be sufficient and has focused on the content and quality of the provider-patient communication surrounding screening tests (13). While several screening tools have been developed and adapted to investigate the content of provider-patient conversations about screening tests (e.g., investigator-created informed-decision making scales), these studies have not been systematically reviewed. In addition, several interventions have been proposed to increase and improve provider-patient communication about screening. These interventions have focused mainly on improving patient reminders (15), conducting communication skills training for physicians (16) and using “patient navigators” (12) to increase screening rates.
In this paper, we aimed to systematically review studies that focused on the role of provider-patient communication in screening behavior. We included studies that assessed provider recommendation alone, studies that explored the quality and content of provider-patient discussions about screening, and interventions designed to improve provider-patient communication about screening and subsequent screening behaviors.
We chose to focus this review solely on screening tests that currently hold a “B” recommendation or higher for the general population from the U.S. Preventative Services Task Force (USPSTF), including mammograms, Pap smears and colorectal cancer screening. Other common cancer screening tests, such as the prostate specific antigen (PSA), were excluded from analysis due to the high risk of false-positive tests and low grade from the USPSTF. Despite its B rating, lung cancer screening was excluded from analysis as these recommendations are relatively new (2013), and therefore a parallel literature on provider-patient communication is not yet adequate for systematic review.
Method
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for this review (17).
Literature Search
A comprehensive electronic literature search of articles published between January 1, 1992 and June 10, 2016 was conducted in the following databases: PubMed, PsycINFO (Psychological Abstracts) via OVID, Cochrane via Wiley, and EMBASE provided by Elsevier. All languages and publication types were included in the search strategy. Controlled vocabulary (MeSH, EMTREE and PsycINFO Subject Headings) and keywords were used. Three broad concept categories were searched, and results were combined using the appropriate Boolean operators (AND, OR). The broad categories included breast, colorectal or cervical cancers; screening tests; and the healthcare provider-patient communication relationship. Table 1 contains the full search strategy.
Table 1.
Search Strategies and Terms Used
| Medical Subject Headings (MeSH) | Keyword terms |
|---|---|
| (“Breast Neoplasms” [MeSH] OR “Uterine Cervical Neoplasms”[MeSH] OR “Colorectal Neoplasms” [MeSH]) AND (“Mammography” [MeSH] OR “Ultrasonography, Mammary” [MeSH] OR “Sigmoidoscopy” [MeSH] OR “Colonoscopy” [MeSH] OR “Colonography, Computed Tomographic” [MeSH] OR “Vaginal Smears” [MeSH]) AND “Communication” [MeSH] | (breast cancer OR cervical cancer OR colorectal cancer) AND (cancer screening OR mammography OR mammogram OR sigmoidoscopy OR colonoscopy OR fecal occult blood test OR FOBT OR fecal immunochemical testing OR pap test OR pap smears) AND (patient physician communication OR patient clinician communication OR patient doctor communication OR patient provider communication OR communication OR speak OR speaking OR talk OR talking) |
Review and Abstraction Process
Studies were screened for inclusion in three phases. In the first phase, two authors independently reviewed titles for duplicates and poor fit with focus of this systematic review. If at least one author coded the title to continue to the next round, four authors then independently reviewed abstracts and classified the articles based on the eligibility (inclusion/exclusion) criteria. If at least two authors selected to include the study at this stage, the full text of the article was recommended for full data extraction. Discrepancies were discussed at the full text review stage until a consensus was reached.
Data Extraction
Three authors independently extracted data from all eligible studies and discrepancies were reconciled as necessary. Authors extracted the following items from the studies: sample characteristics (sample size, mean age and sex); the type of study and sampling technique; how the communication item(s) were defined, measured and operationalized; how the adherence item(s) were measured and operationalized, and main findings of the study.
Inclusion/Exclusion Criteria
Peer-reviewed quantitative studies were included in the study if the recommendation was from a primary care provider (physician, nurse practitioner, or physician’s assistant) and interpersonal communication (including face-to-face or telephone) was included as an independent variable. Exclusion criteria included only written communication (e.g., letter or brochure) studied, a healthcare professional other than a primary care provider as the communicator (e.g., peer navigator, clinical nurse), and articles that included standardized patients only. Articles were also excluded from the study when the quality of communication was measured only generally and not specific to the cancer screening discussion (e.g., (18–23)), and when only non-adherers were surveyed and asked about barriers to screening (e.g., (24)). Intervention studies were included if at least one of the following criteria was met: (1) the article measured both screening recommendation and adherence; or (2) the intervention was communication focused (e.g., teaching doctors how to best talk to patients).
Results
Summary of Included Articles
The systematic search resulted in 3,252 records to be searched. Figure 1 contains the PRISMA flow chart for this review. A total of 35 articles were considered suitable for inclusion in the review. All but six of the articles were from the United States–other represented countries included Canada (25, 26), Singapore (27), Israel (28), France (29) and Italy (30). Many articles focused on unique populations, including ethnic minorities, rural and urban residents, and veterans.
Figure 1.
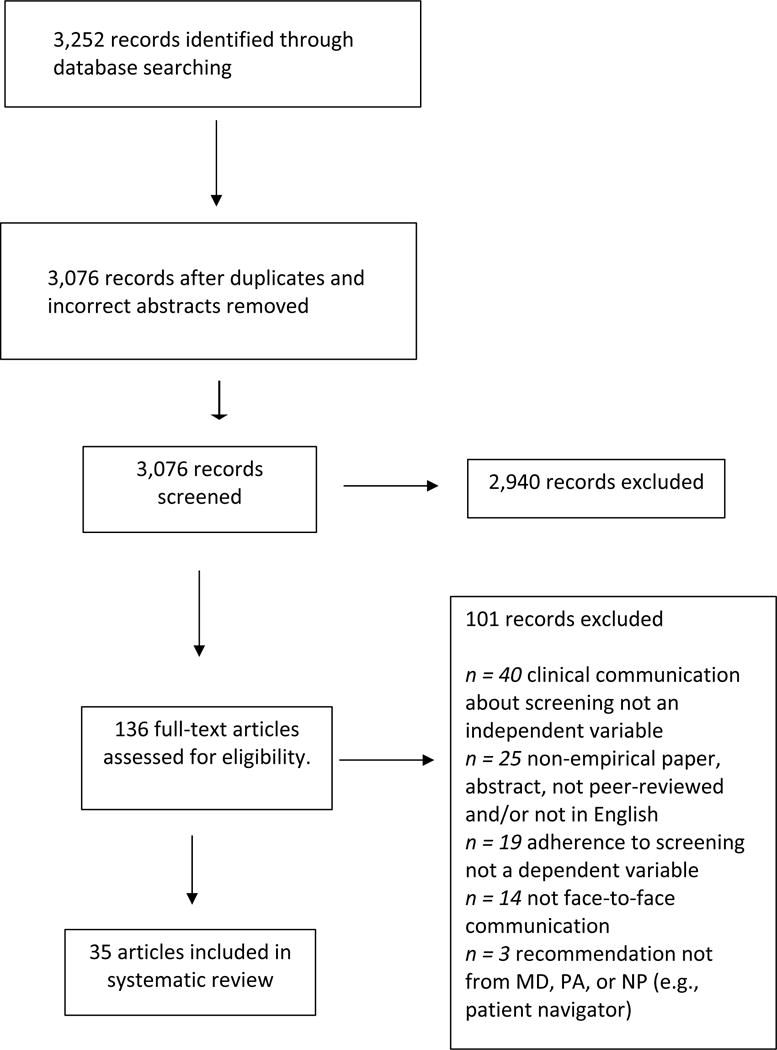
PRISMA Flow Chart.
Tables 2–4 summarize the included 35 articles, with each table displaying information about findings in one of three categories of study: recommendation, quality of communication and intervention. Each table is then sub-divided by cancer type. Four of the articles reported findings on two or more different cancer types (16, 26, 31, 32) and one reported findings on two different categories: recommendation and quality of communication (13). Thus, the total number of findings reported in the tables equals 41.
Table 2.
Characteristics of included recommendation studies (n=24 findings; 22 unique papers)
| Author (year); Country/population | Sample size, (% female, mean/mode age) | Study design; Sampling procedure | Screening test | Communication operationalization, definition; measurement | Total percent adherent; measurement; operationalization | Main findings |
|---|---|---|---|---|---|---|
| Breast Cancer Screening (n=5) | ||||||
| Cruz et al. (2008) Chamorro living in San Diego (63) |
N = 110 (100%, Mo = ≥60) | Cross-sectional Convenience sample |
Mammography | Provider recommendation Patient self-report |
75.9% screened in past 2 years Patient self-report |
Provider recommendation was associated with receipt of mammogram (p = .002). |
| Magai et al. (2004) USA (64) |
N = 1,364 (100%, M = 59.3 [SD = 6.5]) | Cross-sectional Representative sample |
Mammography | Doctor recommendation Patient self-report |
Mean number of mammograms in the past 10 years was 5.3 [SD = 3.9] Patient self-report |
Women with physicians who recommended screening were more likely to have a mammogram. (OR = 2.29, 95% CI = 1.42–3.69). |
| Mah & Bryant (1997) Canada (25) |
N = 1,211 (100%, NR) | Cross-sectional Representative sample | Mammogram | Doctor recommendation Patient self-report | 30% screened in past 2 years Patient self-report |
Screeners (on schedule): 92.0% reported recommendation Intenders (plan to get screened in next 2 years): 45.7% report recommendation Non-Screeners: 21.8% reported recommendation. |
| Roman et al. (2014) Black, Latina and Arab women living in Detroit (32) |
N = 514 (100%, NR) | Cross-sectional Convenience sample |
Mammography | Doctor recommendation Patient self-report |
66.5% on schedule (past year) Patient self-report |
Mammography: A lack of doctor recommendation was significantly associated with lower odds of screening among Latinas (OR = .01, 95% CI = .002, .12) and Arab women (OR = .25, 95% CI = .10, .61) but not significant for Black women. |
| Todd, Harvey and Hoffman-Goetz (2011) Chinese immigrant women living in Canada (26) |
N = 103 (63.61) | Cross-sectional Convenience sample |
Mammography | Ever had doctor recommendation Patient self-report |
92% ever screened and 79% on schedule Patient self-report | Physician recommendation was a strong predictor of screening with only 9% of never screeners reporting receipt of a physician recommendation in the past 5 years compared with 91% of ever users. |
| Cervical Cancer Screening (n=5) | ||||||
| Del Maso (2010) HIV+ women in Northern Italy (30) |
N = 1,002 (100%, NR) | Cross-sectional Representative sample |
Pap | Doctor recommendation Patient self-report |
61% screened in past year Patient self-report |
Receiving screening advice from a gynecologist rather than an infectivologist was associated with better screening participation (OR = 0.6, 95% CI = 0.4, 0.9). |
| Roman et al. (2014) Black, Latina and Arab women living in Detroit (32) |
N = 514 (100%, NR) | Cross-sectional Convenience sample |
Pap | Doctor recommendation Patient self-report |
66.5% on schedule (past 3 years) Patient self-report |
Pap: A lack of doctor recommendation was significantly associated with lower odds of screening among Latinas (OR = .09, 95% CI = .02, .42) and Arab women (OR = .26, 95% CI = .12, .54) but not significant for Black women. |
| Taylor et al. (2004) Vietnamese American living in Seattle (65) |
N = 352 (100%, Mo = ≥50) | Cross-sectional Representative sample |
Pap | Ever had doctor recommendation, Patient self-report | 68% screened in past 3 years Patient self-report |
Women who reported a physician recommendation had a nearly 7.0 higher odds of having been screening for cervical cancer in the preceding 3 years. |
| Taylor et al (2009) Vietnamese American living in Seattle (66) |
N = 1,516 (100%, NR) | Cross-sectional Representative sample |
Pap | Ever had doctor recommendation Patient self-report |
93% ever screened, 81% screened in past 3 years Patient self-report |
Multivariate analysis indicated that doctor recommendation for Pap testing was independently associated with cervical cancer screening (OR = 2.7, 95% CI = 1.8–3.9, p < .001). |
| Tracey et al. (2013) Lesbians living in USA (67) |
N = 1,006 (100%, M = 44) | Cross-sectional Representative sample |
Pap | Provider recommendation Patient self-report |
62% on schedule1 Patient self-report |
Women whose healthcare providers had recommended screening were more likely to become routine screeners (Adjusted OR = 2.04, 95% CI = 1.32, 3.15). |
| Colorectal Cancer Screening (n=14) | ||||||
| Cairns and Viswanath (2006) HINTS (68) |
N = 1,253 (62%, 50–64 only) | Cross-sectional Representative sample | Colonoscopy, sigmoidoscopy, or FOBT | Ever had provider recommendation Patient self-report |
71% ever screened 44% on schedule for FOBT Patient self-report |
Individuals without a recommendation were significantly less likely to be screened, for both uninsured (95% CI = 0.003–0.083) and insured (95% CI = .054-.0119) individuals. |
| Christie et al. (2005) African-American and Hispanic living in East Harlem (62) |
N = 81 (100%, Mo = ≥65) | Cross-sectional Convenience sample |
Colonoscopy or sigmoidoscopy | Ever received doctor recommendation Patient self-report |
49% ever screened Patient self-report |
Doctor recommendation was significantly associated with screening behavior (p <.0001). |
| Halbert (2016) African Americans living in Philadelphia (69) |
N = 262 (56%, 57.2 [SD = 5.0]) | Cross-sectional Convenience sample |
Colonoscopy or sigmoidoscopy | Ever received provider recommendation Patient self-report |
57% ever screened Patient self-report |
Participants who reported that they had discussed colorectal cancer screening with their health care provider had a 10-times greater likelihood of screening compared to those who did not report provider communication about screening (OR = 10.78, 95% CI = 4.85, 29.94, p < .001). |
| Honda (2004) Japanese living in USA (70) |
N = 305 (39%, 52.3 [SD = 15.3]) | Cross-sectional Representative sample | Colonoscopy, sigmoidoscopy, or FOBT | Doctor recommendation, patient self-report, | 37% FOBT in past 2 years and 26% colonoscopy or sigmoidoscopy in past 5 years Patient self-report |
Those with physician recommendation had higher odds of reporting FOBT screening (OR 3.6, 95% CI = 1.8–6.9) and colonoscopy/sigmoidoscopy screening (OR 13.7, 95% CI = 6.1–30.6). |
| Jo et al (2008) Korean Americans living in Los Angeles (42) |
N = 151 (68%, M = 54.2) | Cross-sectional Convenience sample |
Colonoscopy, sigmoidoscopy, or FOBT | Doctor recommendation Patient self-report |
17% received either FOBT in past year or sigmoidoscopy or colonoscopy within 5 years Patient self-report |
Respondents who received a recommendation were significantly more likely to be screened than those who did not receive a recommendation (45% v. 6%, p<.0001). |
| Katz et al. (2011)2 Ohio Appalachia (71) |
N = 170 (62%, ≥50 only) | Cross-sectional Convenience sample |
Colonoscopy, sigmoidoscopy, or FOBT | Doctor recommendation Patient self-report |
29% on schedule3 Patient self-report |
Adjusted odds ratio for being on schedule was significantly higher for adult with a doctor recommendation (OR=6.09; 95% CI=2.80, 13.21, p<.0001). |
| Katz et al. (2004) African Americans living in rural North Carolina (72) |
N = 397 (74%, M = 63) | Cross-sectional Convenience sample |
Colonoscopy, sigmoidoscopy, or FOBT | Doctor recommendation Patient self-report |
31% on schedule for col or sig or FOBT Patient self-report |
Of the individuals who reported being screened, 65% received recommendation, compared to 36% recommendation for those who reported not being screened. |
| Lafata et al. (2014) Patients in Detroit Integrated Delivery System4 (13) |
N = 443 (65%, M = 59.1) | Longitudinal Convenience sample | FOBT, colonoscopy, sigmoidoscopy, double contrast barium enema | Doctor recommendation during study Outside Coder |
53% screened in past year Chart review |
When physicians made a clear recommendation about screening (Advise step), participants were significantly more likely to be screened (OR = 4.31, CI = 1.75, 10.59). |
| Laiyemo et al. (2014) HINTS (73) |
N = 4,383 (54%, M = 63.6) | Cross-sectional Representative sample | Colonoscopy, sigmoidoscopy, or FOBT | Provider recommendation Patient self-report |
62.6% on schedule Patient self-report |
A specific recommendation was associated with a 13-fold increased odds of being compliant with screening (OR = 12.11; 95% CI = 9.41–15.60). |
| Modiri et al. (2013) California Health Inventory Survey (74) |
N = 30,875 (53.4%, M = 63.3) | Cross-sectional Representative sample |
Colonoscopy | Doctor recommendation Patient self-report |
44.5% screened in past 5 years Patient self-report |
Participants who underwent a colonoscopy were significantly more likely to receive a recommendation for this test from their physician (67.3% vs. 39.6%). |
| Paskett et al. (2013) Ohio Appalachia (11) |
N = 1,085 (58.6%, M = 61.4) | Cross-sectional Representative sample |
Colonoscopy, sigmoidoscopy, or FOBT | Ever had doctor recommendation Patient self-report |
49.5% on schedule Chart review |
Having a doctor’s recommendation to be screened resulted in significantly higher screening rates (OR = 9.09, 95% CI = 5.52, 14.97). |
| Thompson et al. (2014) African Americans (75) |
N = 1,021 (67%, M = 63.1 [SD = 7.7]) | Cross-sectional Representative sample |
Colonoscopy, sigmoidoscopy, or FOBT | Provider recommendation Patient self-report |
60% on schedule Chart review |
Individuals with no recommendation were less likely to be adherent (OR = .23, 95% CI = .16, .32). |
| Todd, Harvey and Hoffman-Goetz (2011) Chinese immigrant women living in Canada (26) |
N = 103 (63.61) | Cross-sectional Convenience sample |
Colonoscopy, sigmoidoscopy, or FOBT | Ever had doctor recommendation Patient self-report |
78% ever screened and 58% on schedule Patient self-report |
Physician recommendation significantly explained the variation in both ever vs. never and current vs. non-current colon cancer screening behaviors. |
| Wong et al. (2013) Singapore (27) |
N = 1,743 (60.2%, M = 62.1) | Cross-sectional Representative sample |
Colonoscopy, sigmoidoscopy, or FOBT | Doctor recommendation Patient self-report |
26.7% on schedule Patient self-report |
Recommendation was a strong predictor of positive screening behavior in both men (Adjusted OR = 3.5, 95% CI = 2.33, 5.27) and women (Adjusted OR = 2.35, 95% CI = 1.71, 3.22). |
On schedule based on ACS screening guidelines which recommends a Fecal Occult Blood Test (FOBT) annually, a flexible sigmoidoscopy (FS) every 5 years, or a colonoscopy every 10 years.
Manuscript included intervention, but analysis of interest was contained in baseline, cross-sectional data only.
Article also included in quality studies section.
Table 4.
Characteristics of included intervention studies (n=8 findings; 6 unique papers)
| Author (year); Country/population | Sample size, (% female, mean/mode age) | Study design; Sampling procedure | Screening test | Description of intervention | Total percent adherent measurement; operationalization | Main findings |
|---|---|---|---|---|---|---|
| Breast Cancer Screening (n=2) | ||||||
| Giveon & Kahan (2000) Women living in Israel (28) |
N = 218 (100%, not reported) | Non-random intervention Convenience sample |
Mammography | Physicians were asked to talk to all female patients about screening, including importance, reasons, and discussing patient fears or lack of knowledge | 32% (I)/13% (C) Screened in the past 2 years Patient self-report |
More women (32%) in the intervention group than in the control group (13%) reported that they had started to undergo regular breast examinations. This difference was statistically significant (chi-square 10.71, p < .001). |
| Price-Haywood, Harden-Barrios, & Cooper (2014) Low health literacy patients living in New Orleans (16) |
168 (78.5%, 58.5) | RCT Representative sample |
Mammography | Physicians received communication skills training with standardized patients | 36.6% (average for all three cancer types) Screened in the past year Patient self-report |
Mammography screening rates were significantly higher for intervention physicians’ patients. |
| Cervical Cancer Screening (n=1) | ||||||
| Price-Haywood, Harden-Barrios, & Cooper (2014) Low health literacy patients living in New Orleans (16) |
168 (78.5%, 58.5) | RCT Representative sample |
Pap | Physicians received communication skills training with standardized patients | 36.6% (average for all three cancer types) Screened in the past year Patient self-report |
Mammography screening rates were significantly higher for intervention physicians’ patients. There was no difference for rates of Pap colorectal cancer testing. |
| Colorectal Cancer Screening (n=5) | ||||||
| Aubin-Auger (2015) France (29) |
N = at least 477 in each group (control group: 45.1%, 59.1; intervention group 51.5%, 60.9) | RCT Representative sample |
FOBT | Physicians received patient-centered communication skills training through video and interactive methods. | 36.7% (I)/24.5% (C) in past 2 years (extrapolated from 7-month study period) Chart review |
More patients in the intervention group than in the control group were screened (p = .03). |
| Ferreira et al. (2005) Male veterans living in Chicago (39) |
N = 1,978 (0%, 67.8) | RCT Convenience sample |
Colonoscopy, sigmoidoscopy, or FOBT | Physician communication skills training. Included initial 2-hour workshop and 1 hour feedback sessions every 4–6 months | 43.1% (I)/32.4% (C) Chart review |
Intervention group patients were more likely to receive recommendation to undergo screening than control group patients (76% v. 69.4%, p = .02). Intervention group patients were also more likely to be screened than control group patients (p = .003). |
| Khankari et al. (2007) Patients at an urban FQHC (40) |
N = 154 (68%, 60.1) | Non-random intervention Convenience sample |
Colonoscopy, sigmoidoscopy, or FOBT | Physician communication skills training, establishing a clinic “feedback loop,” and providing patients with education materials prior to the visit. | 27.9% screened in past year Chart review |
Rates of recommendation increased nearly threefold (92.9%) from baseline, and patient screening completion significantly increased (p < .001) from baseline screening rate (11.5%). |
| Myers et al (2004) FOBT+ patients living in the northeast (41) |
N = 2992 (NR, NR) | RCT Representative sample |
Complete diagnostic evaluation for FOBT+ patients (colonoscopy or sigmoidoscopy) | Physician-oriented reminder-feedback mailed form and personalized educational outreach |
Total percent screened not reported Chart Review |
Physicians in the intervention group were significantly more likely to recommend CDE than physicians in the control group (OR = 2.28; 95% CI: 1.37, 3.78). At endpoint, CDE performance rates were greater for the intervention group as compared to the control group (OR = 1.63, 95% CI: 1.06, 2.50). |
| Price-Haywood, Harden-Barrios, & Cooper (2014) Low health literacy patients living in New Orleans (16) |
168 (78.5%, 58.5) | RCT Representative sample |
Colonoscopy, sigmoidoscopy, or FOBT | Physicians received communication skills training with standardized patients | 36.6% (average for all three cancer types) Screened in the past year Patient self-report |
There was no difference for rates of colorectal cancer testing. |
Of the findings included in the review, most (n=24) examined provider recommendation (Table 1). Nine studies analyzed the quality of the provider-patient discussion about cancer screening (Table 2), and eight assessed an intervention to improve provider-patient communication and subsequent screening behaviors (Table 3).
Table 3.
Characteristics of included communication quality studies (n=9 findings; 7 unique papers)
| Author (year); Country/population | Sample size, (% female, mean/mode age) | Study design; Sampling procedure | Screening test | Communication quality definition; measurement | Total percent adherent; measurement; operationalization | Main findings |
|---|---|---|---|---|---|---|
| Breast Cancer Screening (n=3) | ||||||
| Fox, Siu, & Stein (1994) Women living in Los Angeles (33) |
N = 972 (reported in ranges) | Cross-sectional, representative sample | Mammography | Investigator-created scale with items on mammography discussion and doctors “having enthusiasm” for mammography; Patient self-report |
24.1% screened in past year by patient self-report | Physician talking about mammography was significantly related to recent mammogram utilization (p = .002). Additionally, women whose physicians had a great deal of enthusiasm for mammography were 4.5 more likely to complete screening. |
| Lauver et al. (2003) Three groups women (including urban and lesbian) living in the mid-west (35) |
N = 797 (64.4) | Longitudinal, Recruitment included both representative and convenience samples |
Mammography | Investigator-created scale with items on encouragement and explanation Patient self-report |
50.2% screened at second follow-up (16–22 months) Combination of patient self-report and chart review |
Participants with higher mammography-specific communication scores were significantly more likely to have had mammograms. Further, each item from the mammography-specific communication scale was significantly correlated with mammography at the second follow-up interview. |
| Politi et al. (2008) Unmarried women in the US (31) |
N = 605 (53) | Cross-sectional, Convenience sample |
Mammography | Investigator-created scale with items on clearly explaining the test and amount of time spent on discussion. Patient self-report |
71% on schedule by patient self-report | Women who reported that their providers communicated about screening tests were more likely to be on schedule than women who reported that their providers did not communicate about screening tests. |
| Cervical Cancer Screening (n = 1) | ||||||
| Politi et al. (2008) Unmarried women in the US (31) |
N = 605 (53) | Cross-sectional, Convenience sample |
Pap |
Investigator-created scale with items on clearly explaining the test and amount of time spent on discussion. Patient self-report |
77% on schedule by patient self-report | Women who reported that their providers communicated about screening tests were more likely to be on schedule than women who reported that their providers did not communicate about screening tests. |
| Colorectal Cancer Screening (n=5) | ||||||
| Lafata (2014), Patients in Detroit Integrated Delivery System (13) | N = 443 (65%, M = 59.1) | Longitudinal Convenience sample | FOBT, colonoscopy, sigmoidoscopy, double contrast barium enema | 5A steps (assessing, advising, agreeing, assisting, arranging) Outside coder of audio recording |
53% screened in past year Chart review |
Likelihood of screening increased with the more 5A steps the discussion included. (3 steps vs. 0 had an OR of 4.98, p<.001. 1–2 steps vs. 0 had an OR of 2.96, p<.05. 3 or more vs. 1 or 2 had OR = 1.7, p<.05.). |
| Lafata (2015) Patients in Detroit Integrated Delivery System (38) |
N = 414 visits (64%, NR) | Cross-sectional Convenience sample |
Colonoscopy, sigmoidoscopy, or FOBT | Physician use of persuasive techniques Outside coder of audio recording and patient self-report |
56% screened in past year Chart review |
There was no statistically significant relationship observed between the type(s) of persuasion technique used by the doctor and CRC screening adherence. There was also no statistically significant association between patients reporting that their doctor tried to persuade them and their subsequent CRC screening adherence. |
| Ling et al. (2008), Veterans Administration (VA) clinic in Pittsburgh, PA (34) |
N = 91 (0%, all patients 50–74 yo) | Cross-sectional, convenience sample | Colonoscopy, sigmoidoscopy, or FOBT | Informed Decision-Making (IDM) Model Outside coder of audio tapes |
40% screened Chart review |
Provider assessing patient understanding was positively associated with CRC screening completion (p = .002). Discussion of pros-cons was negatively associated with CRC screening completion (p = .01). Eliciting patient preferences was negatively associated with CRC screening completion (p = .001). |
| Mosen et al. (2013) Members of HMO in the Northwest (36) |
N = 883 (58.3%, 59.1 [SD = 5.2]) | Cross-sectional1, convenience sample | Colonoscopy, sigmoidoscopy, or FIT | Informed decision-making (Braddock) Patient self-report |
Total percent adherent not reported, past 9 months Patient self-report |
Patients’ perceptions of the comprehensiveness of colorectal cancer screening discussions were associated with screening in both unadjusted (OR=1.84, 95% CI = 1.20–2.81, p = 0.005) and adjusted (OR=1.51, 95% CI = 1.03–2.21, p = 0.035) models. |
| Napoles et al. (2014) Latinos living in California (37) |
N = 505 (69%, 61 [SD = 8.4]) | Cross-sectional, representative sample | Colonoscopy, sigmoidoscopy, or FOBT | Colorectal Cancer Screening Counseling Survey Patient self-report |
59% on schedule Patient self-report |
Four scales/items were significantly associated with endoscopy/any screening completion: (1) explanations of CRC risks/tests, (2) elicitation of patient’s CRC screening barriers, (3) responsiveness to patient’s CRC screening concerns, and (4) patient’s perceived level of encouragement. |
Study was intervention, but our analysis of interest was baseline cross-sectional data.
Across all the tables, colorectal cancer was the most common type of cancer screening studied (n = 24 findings). Of these, most included several types of screening (colonoscopy, sigmoidoscopy, FOBT). Ten findings examined mammography, and seven focused on Pap smears.
Provider Recommendation
The majority of the provider recommendation findings were focused on colorectal cancer screening (n=14) with five findings about breast cancer screening and five findings about cervical cancer screening.
There was relative uniformity in the study designs and methods of the recommendation studies. Twenty-three of the 24 findings assessed provider recommendation by patient self-report; only one study used an outside coder of audio recordings (13). Most studies also relied on patient self-report for adherence, with only three findings using chart review.
Across all 24 findings examining provider recommendation, there is overwhelming evidence that provider recommendation significantly improves cancer screening rates. This holds true among a variety of populations (e.g., urban and rural, different geographic region, and various ethnicities). Only one study (32) reported negative findings, and in this study it was only for one of three racial/ethnic groups studied. Specifically, the study found that physician screening was not significantly associated with screening among African-American women living in Detroit, but was significantly associated with Latina and Arab women.
Provider-Patient Communication Quality
Studies focusing on quality of communication were fewer in number than those focusing solely on whether a recommendation was made, with only nine findings. Because we use the term quality to capture varying characteristics of communication, it is more difficult to synthesize these studies. Operationalization of screening behavior also varied between studies, such as “on schedule” (e.g. (31)), in the past year (e.g., (33)) and not reported (e.g., (34)).
However, there are common themes and similarities among the three findings on breast cancer screening (31, 33, 35) and one analysis on cervical cancer screening (31), which was part of one of the breast cancer studies. All of these studies used investigator-created, non-validated, patient self-report measures to evaluate aspects of communication, including how clearly the discussion happened. Some studies also measured enthusiasm (33) and encouragement (35). Across the breast cancer and Pap screening studies, adherence was improved with: simply talking about it, enthusiasm, explanations, elicitation of barriers, and responsiveness to patient concerns.
There are also similarities among the five studies on colorectal cancer screening (13, 34, 36–38). These articles were generally more recent, with four of the five articles published between 2013 and 2015. Three (13, 34, 38) audio-recorded provider-patient encounters and then coded this data using a previously published coding system or framework and measured adherence through chart review. The other two studies (36, 37) used patient self-report to measure the communication and screening adherence.
In the colorectal cancer screening analyses, findings that focused on provider encouragement and on shared and informed decision making components were generally positively correlated with screening. One study presented negative findings and reported that discussions of pros and cons and eliciting patient preferences were negatively associated with screening (34). In addition, one study found no significant difference between physicians’ use of various persuasive techniques and subsequent adherence (38).
Intervention
Intervention studies are shown in Table 3. This section included five findings for colorectal cancer screening, two for mammography, and one for Pap. All interventions included some form of communication skills training or screening education for providers. The communication skills trainings varied substantially in the complexity and content of the interventions. One intervention was relatively simple, consisting of instructing physicians to spend a few minutes discussing the importance of screening with patients (28), while others were more complex and included team workshops, video training and multiple feedback sessions (29, 39, 40). Another article with three findings included in the review relied primarily on training with standardized patients (16). Two findings additionally focused on the practice-facilitation workflow surrounding the communication, either by tracking patients and mailing patients a physician letter/brochure prior to the visit (40) or sending reminder-feedback forms for the provider after a patient’s FOBT+ result (41).
There were two types of outcomes of interest for intervention studies included in the review: first, if the intervention improved provider recommendations; and second, if the improved provider counseling subsequently led to increased screening rates for patients. The outcomes were again generally positive. Both mammography interventions reported that significantly more women in the intervention group were screened than women in the control group (16, 28). Additionally, four of the five colorectal screening findings found that patients included in the intervention arm were more likely to both receive a provider recommendation and be screened than the control group or at baseline (29, 39–41). However, one colorectal cancer screening analysis, and the Pap analysis from the same article, found no difference in the control and intervention arm for patient screening rates (16).
Discussion
There should be no doubt that provider recommendations are important to patient adherence to cancer screening. A positive association between provider recommendation and patient screening adherence was present in nearly every study and across many different types of populations and types of cancer screened. However, a dichotomous provider recommendation measure explains only part the variance in screening behavior. For example, in one study of Korean Americans living in California, screening rates were less than 50% even with a physician recommendation (42). One conclusion from our systematic review of the extant literature is that a simple provider recommendation is necessary but not sufficient for optimal adherence to cancer screening guidelines. Provider-patient communication is more nuanced than just a simple recommendation, and the quality and content of the discussion surrounding the recommendation may have an additional and important bearing on a patient’s decision to get screened.
Rather than continue to examine the relationships of recommendation to screening behavior, we advise that cancer prevention and control researchers expand their communication focus to better understand the quality and content of provider-patient communication about screening. Eight articles in this systematic review are noteworthy for examining more granular elements of provider-patient communication about cancer screening (13, 31, 33–38). From these few heterogeneous studies, one of the strongest indicators of screening adherence was the amount of provider enthusiasm and encouragement perceived by patients (33, 35, 37). These findings underscore the importance of providers enthusiastically endorsing and recommending appropriate cancer screening tests. The effect of provider enthusiasm and encouragement has demonstrated great potential for improved screening adherence and should receive further study. Other communication techniques that correlated with positive screening adherence were addressing patient barriers and clearly and thoroughly explaining screening procedures. Interestingly, providers’ use of persuasion techniques alone was not significantly associated with CRC screening adherence, although such techniques have not yet been studied in combination with other measures of quality of communication during screening discussions (38).
Despite preliminary insight found in the communication quality literature, the included studies varied greatly in the aspects of the interaction were studied, and do not systematically further the body of knowledge on what features of patient-provider communication most strongly correlate with screening adherence. In particular, only four of the analyses (13, 34, 36, 37) in the communication quality studies examined patient engagement measures central to shared decision making (SDM), which is essential to a patient-centered approach to medical care (43). SDM is thought to be strongly correlated with positive health-related outcomes for patients, although few studies have explicitly attempted to relate participation and health outcomes using validated instruments (44, 45). The findings in this review suggest positive correlations between SDM measurements and screening adherence, although SDM was measured in a variety of ways across the studies.
However, one study from a Veterans Administration (VA) clinic (34) found that some components of SDM were negatively correlated with screening adherence, including discussing pros and cons, and eliciting patient preferences. One explanation for this finding could be that patients who were more resistive to screening may have received more detailed persuasive screening discussions. Most research on SDM has occurred in the context of diagnosis and treatment decisions (46). Future research should continue to evaluate the impact and nuances of shared decision-making such as screening adherence.
There is also some evidence that the quality of communication cancer screening decisions may be influenced by providers’ own biases and expectations about a patient’s likely adherence, as has been suggested in other clinical contexts (47, 48). Continuing to conduct more research about quality of communication in low-literacy or underserved populations, and trying to better understand how these biases impact communication would be a useful avenue for future research.
The intervention studies give insight as to the most effective tools for improving the content and quality of provider recommendations. Formal communication skills training and education was a successful tool for improving both provider recommendations and patient screening in several articles. The use of standardized patients for training was not associated with increased screenings for two of the three cancer types studied, but this data should be interpreted with caution, as only one included article utilized standardized patients.
For the articles overall, there seemed to be a shift in the literature, with more recent articles focusing primarily on colorectal cancer screening. For the years 2013–2016, 13 of the 18 findings included in the review examined colorectal cancer screening. One possible explanation for this shift is that recommendations for breast cancer and cervical screening have become controversial in recent years (49) while colorectal cancer screening has become more accepted and utilized (50). Within the last decade, there have been programs introduced to increase colorectal cancer screening, as it has historically lagged behind rates for other types of cancer screening (51). These efforts to increase colorectal cancer screening may have contributed to the relative increased publication rates regarding patient-provider communication for colorectal cancer screening as compared to publications for screening for other cancers, as found in this review.
The implications of SDM in cancer screening discussions may continue to be more pronounced as cancer screening decisions grow more complex (52) and personalized (53). Clinical discussions about cancer screening may include issues related to the potential harms of screening, choosing between multiple options for screening (e.g., sigmoidoscopy, colonoscopy, FOBT, or FIT for CRC screening) (54), and personalized risk factors (53). For example, the recent controversy and uncertainty over breast cancer screening highlights the need for more studies examining the quality of communication in provider-patient consultations about screening when the guidelines are complicated and controversial (55–57). In such cases, Sepucha suggests that adherence alone may not be the best outcome measure because it does not necessarily consider the needs, values and expressed preferences of the patient (58). Instead, researchers may want to examine outcomes such as satisfaction with decision (59), decisional conflict (60), and the extent to which patients are informed and receive screening tests that fulfill their goals (54).
There are limitations to the body of research reviewed here. As discussed earlier, most of the articles relied on patient self-report to report adherence outcome measures. Previous work has suggested that self-report measures may be inaccurate (61). When possible, future studies should include chart review to replace or corroborate self-report items. Another limitation of the body of research was inconsistent or missing operationalization measures. Some studies operationalized adherence as being within a certain amount of time (e.g., 1 year), with others framing adherence as being “on schedule” (depending on guidelines at the time of article publication and as described in articles). Still other studies considered “ever” having been screened as being adherent (e.g., (62)). Additionally, it may be that the positive findings are overstated due to publication bias. Lastly, as the provider-patient communication literature on lung cancer screening matures, these findings should be reviewed and compared with the literature from breast, cervical and colorectal cancer screening.
This systematic review has reinforced the importance of provider-patient communication in individuals’ adherence to leading cancer screening guidelines and provides a foundation for future work. As research in this area becomes more sophisticated, it is our hope that important elements of the quality of provider communication can be better identified in order to improve cancer screening adherence.
Acknowledgments
This publication was supported in part by the National Institutes of Health (NIH K07 CA140778 (PI: Bylund)). Its contents are solely the responsibility of the authors and do not necessarily reflect the official views of the National Institutes of Health. Conflicts of interest: none.
Footnotes
The U.S. Preventive Services Task Force is currently finalizing new colorectal cancer screening recommendations.
Contributor Information
Emily B. Peterson, George Mason University, 4400 University Drive, MSN 3D6, Fairfax VA 22031.
Jamie S. Ostroff, Memorial Sloan Kettering Cancer Center, 641 Lexington Ave, 7th Floor, New York, NY 10022
Katherine N. DuHamel, Memorial Sloan Kettering Cancer Center, 641 Lexington Ave, 7th Floor, New York, NY 10022
Thomas A. D’Agostino, Memorial Sloan Kettering Cancer Center, 641 Lexington Ave, 7th Floor, New York, NY 10022
Marisol Hernandez, Memorial Sloan Kettering Cancer Center, 641 Lexington Ave, 7th Floor, New York, NY 10022.
Mollie R. Canzona, Wake Forest University, P.O. Box 7347, Winston-Salem, NC 27109
Carma L. Bylund, Hamad Medical Corporation, Doha, Qatar
References
- 1.Whitlock EP, Vesco KK, Eder M, Lin JS, Senger CA, Burda BU. Liquid-Based Cytology and Human Papillomavirus Testing to Screen for Cervical Cancer: A Systematic Review for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155(10) doi: 10.7326/0003-4819-155-10-201111150-00376. 687-W-215. [DOI] [PubMed] [Google Scholar]
- 2.Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L. Screening for Breast Cancer: An Update for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151(10) doi: 10.1059/0003-4819-151-10-200911170-00009. 727-W.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levin B, Lieberman DA, McFarland B, Smith RA, Brooks D, Andrews KS, et al. Screening and Surveillance for the Early Detection of Colorectal Cancer and Adenomatous Polyps, 2008: A Joint Guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology CA. Cancer J Clin. 2008;58(3):130–60. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 4.Whitlock EP, Lin JS, Liles E, Beil TL, Rongwei F. Screening for Colorectal Cancer: A Targeted, Updated Systematic Review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149(9):638–58. doi: 10.7326/0003-4819-149-9-200811040-00245. [DOI] [PubMed] [Google Scholar]
- 5.Moyer VA. Screening for Lung Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2014;160(5):330–8. doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 6.Han MA, Choi KS, Jun JK, Kim Y, Park EC, Lee HY. Factors associated with the intention to have colorectal cancer screening in Korean adults. Eur J Cancer Care. 2011;20(4):475–82. doi: 10.1111/j.1365-2354.2010.01209.x. [DOI] [PubMed] [Google Scholar]
- 7.Tsai M-H, Xirasagar S, Li Y-J, de Groen PC. Colonoscopy Screening Among US Adults Aged 40 or Older With a Family History of Colorectal Cancer. Prev Chronic Dis. 2015;12:E80. doi: 10.5888/pcd12.140533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis TC, Williams MV, Marin E, Parker RM, Glass J. Health Literacy and Cancer Communication. CA Cancer J Clin. 2002;52(3):134–49. doi: 10.3322/canjclin.52.3.134. [DOI] [PubMed] [Google Scholar]
- 9.Atkinson TM, Salz T, Touza KK, Li Y, Hay JL. Does colorectal cancer risk perception predict screening behavior? A systematic review and meta-analysis. J Behav Med. 2015;38(6):837–50. doi: 10.1007/s10865-015-9668-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collazo TH, Jandorf L, Thelemaque L, Lee K, Itzkowitz SH. Screening Colonoscopy among Uninsured and Underinsured Urban Minorities. Gut Liver. 2015;9(4):502–8. doi: 10.5009/gnl14039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paskett ED, Llanos AA, Young GS, Pennell ML, Lee CJ, Katz ML. Correlates of colorectal cancer screening among residents of Ohio Appalachia. J Commun Health. 2013;38(4):609–18. doi: 10.1007/s10900-013-9683-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinwachs D, Allen JD, Barlow WE, Duncan RP, Egede LE, Friedman LS, et al., editors. National Institutes of Health State-of-the-Science Conference Statement: Enhancing Use and Quality of Colorectal Cancer Screening. Ann Intern Med. 2010;152(10):663–7. doi: 10.7326/0003-4819-152-10-201005180-00237. [DOI] [PubMed] [Google Scholar]
- 13.Lafata JE, Cooper G, Divine G, Oja-Tebbe N, Flocke SA. Patient-physician colorectal cancer screening discussion content and patients’ use of colorectal cancer screening. Patient Educ Couns. 2014;94(1):76–82. doi: 10.1016/j.pec.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DHHS. Health People 2020 Topics & Objectives 2016. 2016 Feb 18; Available from: http://www.healthypeople.gov/2020/topics-objectives/topic/cancer/objectives.
- 15.Baker DW, Brown T, Buchanan DR, Weil J, Balsley K, Ranalli L, et al. Comparative effectiveness of a multifaceted intervention to improve adherence to annual colorectal cancer screening in community health centers: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1235–41. doi: 10.1001/jamainternmed.2014.2352. [DOI] [PubMed] [Google Scholar]
- 16.Price-Haywood EG, Harden-Barrios J, Cooper LA. Comparative effectiveness of audit-feedback versus additional physician communication training to improve cancer screening for patients with limited health literacy. J Gen Intern Med. 2014;29(8):1113–21. doi: 10.1007/s11606-014-2782-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J Clin Epidemiol. 2009;62(10):1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Carcaise-Edinboro P, Bradley CJ. Influence of patient-provider communication on colorectal cancer screening. Med Care. 2008;46(7):738–45. doi: 10.1097/MLR.0b013e318178935a. [DOI] [PubMed] [Google Scholar]
- 19.Ciampa PJ, Osborn CY, Peterson NB, Rothman RL. Patient numeracy, perceptions of provider communication, and colorectal cancer screening utilization. J Health Commun. 2010;15(Suppl 3):157–68. doi: 10.1080/10810730.2010.522699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ling BS, Klein WM, Dang Q. Relationship of communication and information measures to colorectal cancer screening utilization: results from HINTS. J Health Commun. 2006;11(Suppl 1):181–90. doi: 10.1080/10810730600639190. [DOI] [PubMed] [Google Scholar]
- 21.Tan ASL, Moldovan-Johnson M, Gray SW, Hornik RC, Armstrong K. An Analysis of the Association between Cancer-Related Information Seeking and Adherence to Breast Cancer Surveillance Procedures. Cancer Epidem Biomar. 2013;22(1):167–74. doi: 10.1158/1055-9965.EPI-12-0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tessaro I, Mangone C, Parkar I, Pawar V. Knowledge, barriers, and predictors of colorectal cancer screening in an Appalachian church population. Prev Chronic Dis. 2006;3(4):A123. [PMC free article] [PubMed] [Google Scholar]
- 23.Underhill ML, Kiviniemi MT. The Association of Perceived Provider-Patient Communication and Relationship Quality With Colorectal Cancer Screening. Health Educ Behav. 2012;39(5):555–63. doi: 10.1177/1090198111421800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berkowitz Z, Hawkins NA, Peipins LA, White MC, Nadel MR. Beliefs, risk perceptions, and gaps in knowledge as barriers to colorectal cancer screening in older adults. J Am Geriatr Soc. 2008;56(2):307–14. doi: 10.1111/j.1532-5415.2007.01547.x. [DOI] [PubMed] [Google Scholar]
- 25.Mah Z, Bryant HE. The role of past mammography and future intentions in screening mammography usage. Cancer Detect Prev. 1997;21(3):213–20. [PubMed] [Google Scholar]
- 26.Todd L, Harvey E, Hoffman-Goetz L. Predicting Breast and Colon Cancer Screening Among English-as-a-Second-Language Older Chinese Immigrant Women to Canada. J Cancer Educ. 2011;26(1):161–9. doi: 10.1007/s13187-010-0141-7. [DOI] [PubMed] [Google Scholar]
- 27.Wong RK, Wong ML, Chan YH, Feng Z, Wai CT, Yeoh KG. Gender differences in predictors of colorectal cancer screening uptake: a national cross sectional study based on the health belief model. BMC Public Health. 2013;13:677. doi: 10.1186/1471-2458-13-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giveon S, Kahan E. Patient adherence to family practitioners’ recommendations for breast cancer screening: a historical cohort study. Fam Pract. 2000;17(1):42–5. doi: 10.1093/fampra/17.1.42. [DOI] [PubMed] [Google Scholar]
- 29.Aubin-Auger I, Laouénan C, Le Bel J, Mercier A, Baruch D, Lebeau JP, et al. Efficacy of communication skills training on colorectal cancer screening by GPs: a cluster randomised controlled trial. Eur J Cancer Care. 2016;25(1):18–26. doi: 10.1111/ecc.12310. [DOI] [PubMed] [Google Scholar]
- 30.Dal Maso L, Franceschi S, Lise M, de’ Bianchi PS, Polesel J, Ghinelli F, et al. Self-reported history of Pap-smear in HIV-positive women in Northern Italy: A cross-sectional study. BMC Cancer. 2010;10 doi: 10.1186/1471-2407-10-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Politi MC, Clark MA, Rogers ML, McGarry K, Sciamanna CN. Patient–provider communication and cancer screening among unmarried women. Patient Educ Couns. 2008;73(2):251–5. doi: 10.1016/j.pec.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roman L, Meghea C, Ford S, Penner L, Hamade H, Estes T, et al. Individual, provider, and system risk factors for breast and cervical cancer screening among underserved Black, Latina, and Arab women. J Womens Health (Larchmt) 2014;23(1):57–64. doi: 10.1089/jwh.2013.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fox SA, Siu AL, Stein JA. The importance of physician communication on breast cancer screening of older women. Arch Intern Med. 1994;154(18):2058–68. [PubMed] [Google Scholar]
- 34.Ling BS, Trauth JM, Fine MJ, Mor MK, Resnick A, Braddock CH, et al. Informed decision-making and colorectal cancer screening: is it occurring in primary care? Med Care. 2008;46(9 Suppl 1):S23–9. doi: 10.1097/MLR.0b013e31817dc496. [DOI] [PubMed] [Google Scholar]
- 35.Lauver DR, Owen B, Egan J, Lovejoy LS, Henriques JB. Relationships of practitioner communications and characteristics with women’s mammography use. Patient Educ Couns. 2003;51(1):65–74. doi: 10.1016/s0738-3991(02)00166-0. [DOI] [PubMed] [Google Scholar]
- 36.Mosen DM, Feldstein AC, Perrin NA, Rosales AG, Smith DH, Liles EG, et al. More Comprehensive Discussion of CRC Screening Associated With Higher Screening. The American J Manag Care. 2013;19(4):265–71. [PMC free article] [PubMed] [Google Scholar]
- 37.Napoles AM, Santoyo-Olsson J, Stewart AL, Olmstead J, Gregorich SE, Farren G, et al. Physician Counseling on Colorectal Cancer Screening and Receipt of Screening among Latino Patients. J Gen Intern Med. 2014 doi: 10.1007/s11606-014-3126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lafata JE, Wunderlich T, Flocke SA, Oja-Tebbe N, Dyer KE, Siminoff LA. Physician use of persuasion and colorectal cancer screening. Translational Behavioral Medicine. 2015;5(1):87–93. doi: 10.1007/s13142-014-0284-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferreira MR, Dolan NC, Fitzgibbon ML, Davis TC, Gorby N, Ladewski L, et al. Health care provider-directed intervention to increase colorectal cancer screening among veterans: results of a randomized controlled trial. J Clin Oncol. 2005;23(7):1548–54. doi: 10.1200/JCO.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 40.Khankari K, Eder M, Osborn CY, Makoul G, Clayman M, Skripkauskas S, et al. Improving colorectal cancer screening among the medically underserved: a pilot study within a federally qualified health center. J Gen Intern Med. 2007;22(10):1410–4. doi: 10.1007/s11606-007-0295-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Myers RE, Turner B, Weinberg D, Hyslop T, Hauck WW, Brigham T, et al. Impact of a physician-oriented intervention on follow-up in colorectal cancer screening. Prev Med. 2004;38(4):375–81. doi: 10.1016/j.ypmed.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 42.Jo AM, Maxwell AE, Wong WK, Bastani R. Colorectal cancer screening among underserved Korean Americans in Los Angeles County. J Immigr Minor Health. 2008;10(2):119–26. doi: 10.1007/s10903-007-9066-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arora NK, Street RL, Jr, Epstein RM, Butow PN. Facilitating patient-centered cancer communication: A road map. Patient Edu Couns. 2009;77(3):319–21. doi: 10.1016/j.pec.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 44.Clayman ML, Bylund CL, Chewning B, Makoul G. The Impact of Patient Participation in Health Decisions Within Medical Encounters: A Systematic Review. Med Decis Making. 2015 doi: 10.1177/0272989X15613530. [DOI] [PubMed] [Google Scholar]
- 45.Shay LA, Lafata JE. Where is the evidence? A systematic review of shared decision making and patient outcomes. Med Decis Making. 2015;35(1):114–31. doi: 10.1177/0272989X14551638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chewning B, Bylund CL, Shah B, Arora NK, Gueguen JA, Makoul G. Patient preferences for shared decisions: A systematic review. Patient Edu Couns. 2012;86(1):9–18. doi: 10.1016/j.pec.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huizinga MM, Bleich SN, Beach MC, Clark JM, Cooper LA. Disparity in Physician Perception of Patients’ Adherence to Medications by Obesity Status. Obesity. 2010;18(10):1932–7. doi: 10.1038/oby.2010.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Ryn M, Burke J. The effect of patient race and socio-economic status on physicians’ perceptions of patients. Social Science & Medicine. 2000;50(6):813–28. doi: 10.1016/s0277-9536(99)00338-x. [DOI] [PubMed] [Google Scholar]
- 49.Haas JS, Sprague BL, Klabunde CN, Tosteson ANA, Chen JS, Bitton A, et al. Provider Attitudes and Screening Practices Following Changes in Breast and Cervical Cancer Screening Guidelines. J Gen Intern Med. 2015;31(1):52–9. doi: 10.1007/s11606-015-3449-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang DX, Gross CP, Soulos PR, Yu JB. Estimating the magnitude of colorectal cancers prevented during the era of screening: 1976 to 2009. Cancer. 2014;120(18):2893–901. doi: 10.1002/cncr.28794. [DOI] [PubMed] [Google Scholar]
- 51.Itzkowitz SH, Winawer SJ, Krauskopf M, Carlesimo M, Schnoll-Sussman FH, Huang K, et al. New York Citywide Colon Cancer Control Coalition: A public health effort to increase colon cancer screening and address health disparities. Cancer. 2016;122(2):269–77. doi: 10.1002/cncr.29595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jimbo M, Rana GK, Hawley S, Holmes-Rovner M, Kelly-Blake K, Nease DE, et al. What is lacking in current decision aids on cancer screening? CA Cancer J Clin. 2013;63(3):193–214. doi: 10.3322/caac.21180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caverly TJ, Kerr EA, Saini SD. Delivering Patient-Centered Cancer Screening. Am J Prev Med. 50(1):118–21. doi: 10.1016/j.amepre.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 54.Sepucha KR, Feibelmann S, Cosenza C, Levin CA, Pignone M. Development and evaluation of a new survey instrument to measure the quality of colorectal cancer screening decisions. BMC Medical Informatics Decis Making. 2014;14(1):1–9. doi: 10.1186/1472-6947-14-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alvarado M, Ozanne E, Esserman L. Overdiagnosis and overtreatment of breast cancer. Am Soc Clin Oncol Educ Book. 2012:e40–5. doi: 10.14694/EdBook_AM.2012.32.301. [DOI] [PubMed] [Google Scholar]
- 56.Esserman LJ, Thompson IM, Reid B. Overdiagnosis and overtreatment in cancer: An opportunity for improvement. JAMA. 2013;310(8):797–8. doi: 10.1001/jama.2013.108415. [DOI] [PubMed] [Google Scholar]
- 57.Vickers AJ, Eastham JA, Scardino PT, Lilja H. The Memorial Sloan Kettering Cancer Center Recommendations for Prostate Cancer Screening. Urology. 2016 doi: 10.1016/j.urology.2015.12.054. ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sepucha K, Folwer FJ, Mulley AG. Policy support for patient-centered care: The need for measurable improvements in decision quality. Health Affairs. 2004 doi: 10.1377/hlthaff.var.54. [DOI] [PubMed] [Google Scholar]
- 59.Holmes-Rovner M, Kroll J, Schmitt N, Rovner DR, Breer ML, Rothert ML, et al. Patient Satisfaction with Health Care Decisions: The Satisfaction with Decision Scale. Med Decis Making. 1996;16(1):58–64. doi: 10.1177/0272989X9601600114. [DOI] [PubMed] [Google Scholar]
- 60.O’Connor AM. Decisional conflict. In: McFarland GK, McFarlane EA, editors. Nursing diagnosis and interventions. St Louis: The CV Mosby Co; 1993. pp. 486–96. [Google Scholar]
- 61.McGovern PhD PG, Lurie Md MN, Margolis Md MPHKL, Slater PhD JS. Accuracy of Self-Report of Mammography and Pap Smear in a Low-Income Urban Population. Am J Prev Med. 1998;14(3):201–8. doi: 10.1016/s0749-3797(97)00076-7. [DOI] [PubMed] [Google Scholar]
- 62.Christie J, Hooper C, Redd WH, Winkel G, DuHamel C, Itzkowitz S, et al. Predictors of Endoscopy in Minority Women. J Natl Med Assoc. 2005;97(10):1361–8. [PMC free article] [PubMed] [Google Scholar]
- 63.Cruz ALH, Chung W, Huh J, Blas LA, Cruz LAC, Hubbell FA, et al. Breast cancer screening among Chamorro women in California. Cancer Detect Prev. 2008;32(1 SUPPL):16–22. doi: 10.1016/j.cdp.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Magai C, Consedine N, Conway F, Neugut A, Culver C. Diversity matters: Unique populations of women and breast cancer screening. Cancer. 2004;100(11):2300–7. doi: 10.1002/cncr.20278. [DOI] [PubMed] [Google Scholar]
- 65.Taylor VM, Yasui Y, Burke N, Nguyen T, Acorda E, Thai H, et al. Pap testing adherence among Vietnamese American women. Cancer Epidemiol Biomarkers Prev. 2004;13(4):613–9. [PubMed] [Google Scholar]
- 66.Taylor VM, Yasui Y, Nguyen TT, Woodall E, Do HH, Acorda E, et al. Pap smear receipt among Vietnamese immigrants: the importance of health care factors. Ethn Health. 2009;14(6):575–89. doi: 10.1080/13557850903111589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tracy JK, Schluterman NH, Greenberg DR. Understanding cervical cancer screening among lesbians: a national survey. BMC Public Health. 2013;13:442. doi: 10.1186/1471-2458-13-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cairns CP, Viswanath K. Communication and colorectal cancer screening among the uninsured: data from the Health Information National Trends Survey (United States) Cancer Cause Control. 2006;17(9):1115–25. doi: 10.1007/s10552-006-0046-2. [DOI] [PubMed] [Google Scholar]
- 69.Halbert CH, Melvin C, Briggs V, Delmoor E, Rice LJ, Lynch C, et al. Neighborhood Satisfaction and Colorectal Cancer Screening in a Community Sample of African Americans. J Comm Health. 2016;41(1):38–45. doi: 10.1007/s10900-015-0062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Honda K. Factors associated with colorectal cancer screening among the US urban Japanese population. Am J Public Health. 2004;94(5):815–22. doi: 10.2105/ajph.94.5.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Katz ML, Reiter P, Fickle D, Heaner S, Sim C, Lehman A, et al. Community involvement in the development and feedback about a colorectal cancer screening media campaign in Ohio Appalachia. Health Promot Pract. 2011;12(4):589–99. doi: 10.1177/1524839909353736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Katz ML, Ruzek SB, Miller SM, Legos P. Gender differences in patients needs and concerns to diagnostic tests for possible cancer. J Cancer Educ. 2004;19(4):227–31. doi: 10.1207/s15430154jce1904_10. [DOI] [PubMed] [Google Scholar]
- 73.Laiyemo AO, Adebogun AO, Doubeni CA, Ricks-Santi L, McDonald-Pinkett S, Young PE, et al. Influence of provider discussion and specific recommendation on colorectal cancer screening uptake among U.S. adults. Prev Med. 2014;67:1–5. doi: 10.1016/j.ypmed.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Modiri A, Makipour K, Gomez J, Friedenberg F. Predictors of colorectal cancer testing using the California Health Inventory Survey. World J Gastroenterol. 2013;19(8):1247–55. doi: 10.3748/wjg.v19.i8.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thompson VL, Lander S, Xu S, Shyu CR. Identifying key variables in African American adherence to colorectal cancer screening: the application of data mining. BMC Public Health. 2014;14(1):1173. doi: 10.1186/1471-2458-14-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]


