Abstract
Rationale
Mutations in ACTA2, encoding the smooth muscle isoform of α-actin (SM α-actin), cause thoracic aortic aneurysms, acute aortic dissections, and occlusive vascular diseases.
Objective
We sought to identify the mechanism by which loss of SM α-actin causes aortic disease.
Methods and Results
Acta2−/− mice have an increased number of elastic lamellae in the ascending aorta and progressive aortic root dilation as assessed by echocardiography that can be attenuated by treatment with losartan, an angiotensin II (AngII) type 1 receptor blocker. AngII levels are not increased in Acta2−/− aortas or kidneys. Aortic tissue and explanted smooth muscle cells (SMCs) from Acta2−/− aortas show increased production of reactive oxygen radicals (ROS) and increased basal NF-κB signaling, leading to an increase in the expression of the AngII receptor type I (Agtr1a) and activation of signaling at 100-fold lower levels of AngII in the mutant compared to wild-type cells. Furthermore, disruption of SM α-actin filaments in wildtype SMCs by various mechanisms activates NF-κB signaling and increases expression of Agtr1a.
Conclusions
These findings reveal that disruption of SM α-actin filaments in SMCs increases ROS levels, activates NF-κB signaling and increases Agtr1a expression, thus potentiating AngII signaling in vascular SMCs without an increase in the exogenous levels of AngII.
Keywords: Smooth muscle alpha actin, smooth muscle cells, reactive oxygen species, angiotensin
Subject Terms: Aneurysm, ACE/Angiotensin Receptors, Renin Angiotensin System, Animal Models of Human Disease, Cell Signaling/Signal Transduction, Vascular Biology
INTRODUCTION
Vascular smooth muscle cells (SMCs) contract in response to neurostimulation and biomechanical forces of pulsatile blood flow, a process that involves cyclic interactions of the thick and thin filaments of the actomyosin system. Thin filaments are composed of polymers of the smooth muscle specific isoform of α-actin (SM α-actin), which accounts for 40% of total cellular protein in SMCs. Heterozygous mutations in the corresponding gene (ACTA2) predispose to thoracic aortic aneurysms, acute aortic dissections, and occlusive vascular diseases, such as premature onset stroke and coronary artery disease and moyamoya disease (1–3). The majority of ACTA2 mutations leading to familial thoracic aortic disease are missense mutations predicted to produce a mutant α-actin monomer. SMCs explanted from patients with ACTA2 missense mutations have fewer filaments of SM α-actin than wildtype SMCs, suggesting a dominant negative effect of the mutant protein on filament formation (1). More detailed in vitro studies assessing the effects of a recurrent ACTA2 missense mutation, R258C, indicate the mutation alters filament formation and stability and impairs interactions with myosin, all of which are predicted to decrease the number of SM α-actin filaments and force generation by SMCs (4).
Mouse models have been used extensively to understand the link between a gene mutation and progressive thoracic aortic aneurysm formation. The majority of studies have used an established mouse model of Marfan syndrome heterozygous for a missense mutation, Fbn1C1039G/+ (5). Fbn1 encodes an extracellular matrix protein, fibrillin-1, that links the SMC contractile unit to the elastin fibers to focal adhesions on the cell membrane of SMCs (6). Initial studies indicated that blocking TGF-β signaling prevents aneurysm growth in this mouse model (5) and more recent studies have suggested that TGF-β activation of ERK1/2 signaling also contributes to disease (7). Both Smad2 and ERK1/2 can be activated by either TGF-β or angiotensin II (AngII) signaling, and the fact that an AngII type I receptor (Agtr1a) blocking agent, losartan, rescues aneurysms formation in the Marfan mouse model suggests that Agtr1a activation contributes to aneurysm formation. Other mouse models support this hypothesis, including the Fbln4 SMC-specific knock out (Fbln4SMKO) mouse model and mouse models of Loeys-Dietz syndrome with either Tgfbr1 or Tgfbr2 missense mutations (8, 9). Although increased tissue levels of AngII in the aortas of Fbln4SMKO mouse are proposed as the source of AngII signaling in the Fbln4SMKO mouse model, the etiology of the excessive Agtr1a activity has not been identified in the Marfan or Loeys-Dietz mouse models.
In this study, Acta2−/− mice are used to explore how loss of SM α-actin leads to thoracic aortic enlargement. Acta2−/− mice have normal vascular development but are hypotensive and have decreased aortic contraction in response to agonist stimulation (10). Previous studies determined that loss of SM α-actin increases SMC proliferation in vitro and in vivo through increased focal adhesion kinase (FAK) activity driving increased reactive oxygen species (ROS) levels and platelet-derived growth factor (PDGF) receptor β signaling (11). We describe here Agtr1a-dependent aortic root enlargement in the Acta2−/− mouse with no evidence of increased levels of AngII in the Acta2−/− aortic or renal tissues. Instead, we demonstrate that loss of SM α-actin in SMCs increases cellular ROS levels, which increases NF-κB signaling, leading to increased expression of Agtr1a. These cellular changes result in increased AngII signaling in mutant aortic SMCs, which contributes to aortic root enlargement without increased AngII levels.
METHODS
Mouse studies
All mouse experiments were performed in accordance with institutional guidelines set forth by The University of Texas Health Science Center at Houston Animal Welfare Committee. Standard methods were used to rederive Acta2−/− mice (C57/Bl6 background) from frozen embryos obtained from Dr. Warren Zimmer at Texas A&M University. Agtr1a−/− mice were purchased from The Jackson Laboratory (strain B6.129P2-Agtr1atm1Unc/J.).
For all animal treatment trials, sample sizes were determined using a statistical power calculator based on the initial observed effect size. Doses and timepoints for drug treatments in animal models were based on published protocols. All mice underwent echocardiography at 4 weeks of age, then were randomly assigned to control or treatment groups. Losartan (Santa Cruz Biotechnology, Cat#: sc-204796A) was administered at 0.6g/L in the drinking water for six months, with echocardiography performed every 8 weeks, and aortas harvested for histology at the end of the study. Shorter treatments were used for RNA and protein analysis to eliminate possible secondary effects. NAC (Sigma, Cat#: A9165) was administered at 500 mg/kg/day in drinking water. For all protein and mRNA analyses, only ascending aortic tissue was used.
SMC isolation and culture
SMCs were explanted from the ascending aortas of age and gender matched Acta2−/− and wild-type (WT) littermates as previously described (11). Compounds used in cell culture: AngII (Sigma, 1μM for 20 minutes except where noted), Losartan (Sigma, 10μM for 24 hr), C3 (Millipore, 4μg/mL for 24 hr), NAC (Sigma, 5mM for 16 hr), VCC588646 (Vichem Chemie, 10uM for 12 hr), Helenalin (Cayman Chemicals, 2uM for 6 hours), Anatabine (Cayman Chemicals, 120ug/mL or 150ug/mL for 12 hours) and Latrunculin B (Cayman Chemicals, 0.5 and 1μM for 1 hr for images and 24 hr for blot).
Statistical analysis
For all mouse studies, nonparametric statistical tests were conducted. For two groups, an unpaired Mann-Whitney analysis was performed. For three or more groups, the Kruskal-Wallis analysis was performed with Dunn post-tests to compare between specific groups. Error bars on all mouse data represent standard error. Outliers for mouse data were identified but still included in the final dataset. For aortic diameter data determined by echocardiography, the measurement of the diameter in millimeters was normalized to the weight of the mouse in grams (mm/g units where noted in the figures). Survival curve data was analyzed by Log-Rank (Mantel-Cox) test. Cell culture data were analyzed using Student’s t-tests or two-way ANOVA, both two-tailed and unpaired. Error bars on cell culture graphs represent standard deviation. For all experiments p≤0.05 was considered statistically significant. Throughout the figures, we represented significance as *p<0.05 and **p<0.01.
Additional methods are available in the online supplemental material.
RESULTS
Morphological characterization of the postnatal ascending thoracic aorta in the Acta2−/− mice
We found that the Acta2−/− aortas have increased elastin lamellae when compared to WT aortas (Figure 1A,B), which is similar to the increase in elastic lamellae layers observed in the Eln+/− mice (12). At 4 weeks of age, the density of cells staining positive for a SMC marker, calponin, in the medial layer was also higher in the Acta2−/− aortas (Figure 1A,B). Despite evidence for increased SMC density, the levels of several contractile proteins in Acta2−/− aortas were similar to WT aortas, including smooth muscle myosin heavy chain (SM-MHC), calponin, and SM22α (Figure 1C, Online Figure IA). Although SM α-actin is absent, immunoblot with a pan-actin antibody found no difference in total actin levels between Acta2−/− and WT aortas, suggesting compensation by another actin isoform. Using 2-dimensional gel analysis, we confirmed the ectopic expression of an actin isoform at the same isoelectric point with SM α-actin (Online Figure IB,C). Mass spectrometry analysis of the additional actin expressed in the Acta2−/− SMCs failed to distinguish between cardiac and skeletal muscle α-actin, but qPCR determined increased expression of Acta1 (Online Figure ID). Thus, skeletal α-actin expression compensates for the loss of SM α-actin in Acta2−/− SMCs.
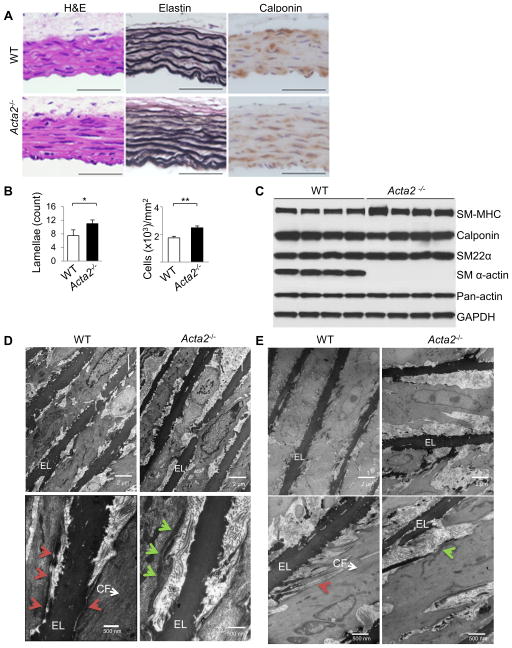
Figure 1. Ultrastructural defects in Acta2−/− mouse aortas.
(A,B) Histology from WT and Acta2−/− aortas at 4 weeks of age shows increased elastin layers and increased density of SMCs marked by calponin staining (n=3 per genotype, *p<0.05, **p<0.01). (C) Western blot analysis of 4 individual WT and Acta2−/− mice shows comparable levels of aortic SMC contractile protein expression at 4 weeks of age. SM α-actin is absent in Acta2−/− aortas as expected, but another actin isoform is expressed to compensate based on similar pan-actin levels for both genotypes. (D,E) Electron microscopy analysis of Acta2−/− aortas at 4 weeks (D) and 6 months (E) of age shows a lack of contractile filaments (CF) within SMCs, aberrant connections between the cells and the elastin fibers (EL), and submembranous dense plaques (green arrows) rather than focal adhesions (red arrows) (n=2 mice per genotype per timepoint).
Electron microscopic (EM) analysis of the WT aorta showed oblique elastin extensions that link to the intracellular contractile filaments in the SMCs through dense plaques on the cell surface, as previously described (red arrows, Figure 1D) (6). In contrast, the Acta2−/− aortas had increased space and fewer connections between the SMCs and elastin fibers, no contractile filaments within the cell, and improper orientation of the SMCs to the elastin fibers (Figure 1D). The focal adhesions linking the microfibrils at the periphery of the elastin fibers to the contractile units were greatly decreased in the Acta2−/− aortas. At the same time, there was the appearance of submembranous dense aggregates in the SMCs that were not present in the WT aortas (green arrows, Figure 1D). At 6 months of age, EM of the Acta2−/− mouse aortas continued to show a lack of connections between the abnormal-appearing SMCs and elastin fibers with increased extracellular matrix material built up in the space between the cells and the elastin fibers (Figure 1E). Taken together, the postnatal Acta2−/− aortas are morphologically characterized by increased numbers of elastin lamellae and SMCs, and there are ultrastructural abnormalities that include decreased connections to the elastin lamellae, loss of intracellular contractile filaments and the appearance of submembranous dense structures.
Aortic root dilation is attenuated by losartan in the Acta2−/− mice
Echocardiography showed no difference between 4 week old WT and Acta2−/− mice in the root or ascending aortic diameters (Online Figure IIA). However, by 6 months of age the Acta2−/− mice had significant enlargement of the aortic root when compared with WT mice despite significant hypotension in the Acta2−/− mice (Figure 2A,D, Online Figure IIB,C). Similar to the Marfan mouse model, survival studies demonstrated no difference in mortality rates between Acta2−/− mice and WT littermates, suggesting that the aortic enlargement does not progress to aortic dissection (Online Figure IID). Additionally, the area of the medial layer in Acta2−/− aortas increased with age and was significantly greater than the WT by 12 months of age (Figure 2B). Despite early differences, by 3 months of age medial cell densities were comparable between the Acta2−/− and WT aortas and the densities remain similar up to 12 months of age (Figure 2C).
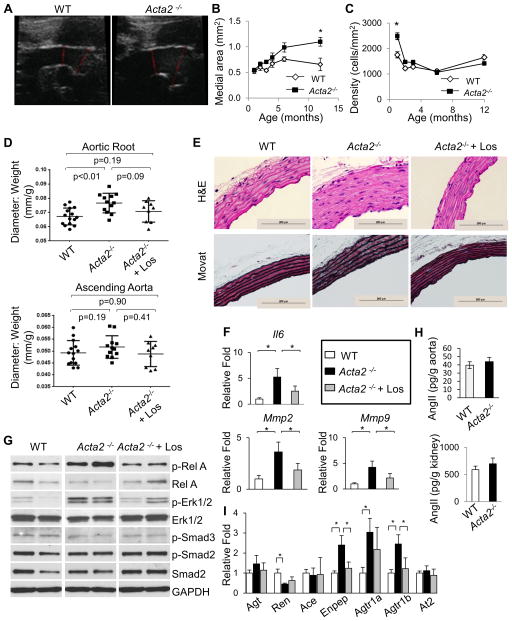
Figure 2. Aortic root enlargement in Acta2−/− aortas is partially attenuated by losartan.
(A) Echocardiography reveals significant aortic root dilation in Acta2−/− aortas compared with WT at 6 months of age. (B) Acta2−/− aortas have increasing medial area over time, with the medial area significantly larger than WT by 12 months of age. (C) Cell density in Acta2−/− aortas is significantly increased at 4 weeks of age when compared to WT aortas, but then normalizes and remains equal to cell density to the WT aorta from 8 weeks to 12 months of age. Medial area and cell density were quantified from H&E stained histology slides (n=3 or more per genotype, *p<0.05, error bars represent s.e.m.). (D) Treatment with losartan attenuates aortic root dilation of Acta2−/− mice as measured by echocardiography. Losartan treatment was initiated at 4 weeks of age and continued for 6 months. (n=15 WT, n=12 Acta2−/−, n=9 Acta2−/− + losartan). (E) Histologic stains of Acta2−/− aortas show minimal medial degeneration at 7 months of age (n=7 or more mice per group). (F) Losartan treatment significantly reduced the increased expression of the Il6, Mmp2, and Mmp9 genes in Acta2−/− aortas. (G) Western blot of Acta2−/− aortas shows increased pRelA and increased pERK1/2 signaling relative to WT, but no change in Smad signaling. Losartan treatment reduces pRelA levels, but not pERK1/2 levels. (H) Levels of AngII in Acta2−/− aortas and kidneys are not significantly different from WT. Measurements were made at 3 months of age (n=20 aortas per genotype, 4 kidneys per genotype). (I) Several components of the renin-angiotensin system are differentially expressed in Acta2−/− aortas, including upregulation of Enpep, Agtr1a, and Agtr1b, and decreased expression of Renin. Only Enpep and Agtr1b were reversed by losartan treatment. For 2F and 2I, lysates were harvested at 8 weeks of age, losartan treatment initiated at 4 weeks, n=3 aortas per group.
We sought to determine if treatment with losartan would block aortic dilation in the Acta2−/− mice. Losartan treatment was initiated at one month of age and continued for six months. Aortic growth was attenuated in the Acta2−/− mice based on the fact that the aortic root diameter in the treated mice is no longer significantly different than the WT mice, whereas the untreated Acta2−/− aortic root diameters are significantly enlarged (Figure 2D). Pathologic analysis at 7 months of age showed minimal medial degeneration with significantly increased deposition of proteoglycans in the mutant mice, which is decreased by losartan (Figure 2E, Online Figure IIIA).
Canonical TGF-β signaling and ERK1/2 activation were increased in the thoracic aortas of the Marfan mouse model, and this activation is reversed with losartan (5,7). We sought to determine if these same pathways were altered in the Acta2−/− aortas but also wanted a marker for AngII signaling. With exposure to AngII, SMCs rapidly phosphorylate RelA (pRelA) at serine 536 through a Rho-dependent pathway; thus, pRelA was also assessed as a marker of NF-κB activation due to AngII (13). Lysates from the Acta2−/− aortas showed increased levels of pRelA and pERK, but only pRelA levels were attenuated with losartan treatment; the Acta2−/− aortas had no increase in pSmad2/3 (Figure 2G, Online Figure IIIB). Expression of several pathologic markers, including Il6, Mmp2 and Mmp9, were increased in Acta2−/− aortic tissue, and the expression of these genes are significantly decreased with losartan treatment (Figure 2F).
Since losartan attenuates aortic dilation, AngII levels and expression of the genes encoding proteins involved in AngII production were assessed in these mice. Although Fbln4SMKO mice had increased AngII in the aortic tissue, AngII levels in Acta2−/− mice were not increased compared with WT in either the aorta or kidneys (Figure 2H). Instead, we found that Agtr1a expression was increased in the Acta2−/− aortas and was not significantly decreased by losartan treatment (Figure 2I). Agtr1b expression levels are 8-fold less than Agtr1a levels (comparison not shown) but were also increased in the mutant aortas and decreased by losartan.
Increased blood pressure augments aortic enlargement in Acta2−/− mice
As previously reported, Acta2−/− mice are hypotensive (WT mice, systolic 114.4 ± 9.5, versus Acta2−/− mice, 82.2 ± 6.4 mmHg) (10). To determine if hypotension in the Acta2−/− mice limits aortic enlargement, blood pressure was raised to WT levels by feeding 4 week old Acta2−/− mice mice a nitric oxide synthesis inhibitor, L-NG-nitroarginine methyl ester (L-NAME), and a high-salt diet (Figure 6F). Raising the blood pressure significantly accelerated the growth of both the aortic root and ascending aorta in the Acta2−/− mice by 3 months of age, suggesting that aortic dilatation in the mutant mice is dependent on the biomechanical forces on the aorta (Figure 6G). We also attempted to increase the hemodynamic forces on the aorta in the Acta2−/− mice through thoracic aortic constriction but the hypotensive mice died with the induction of anesthesia.
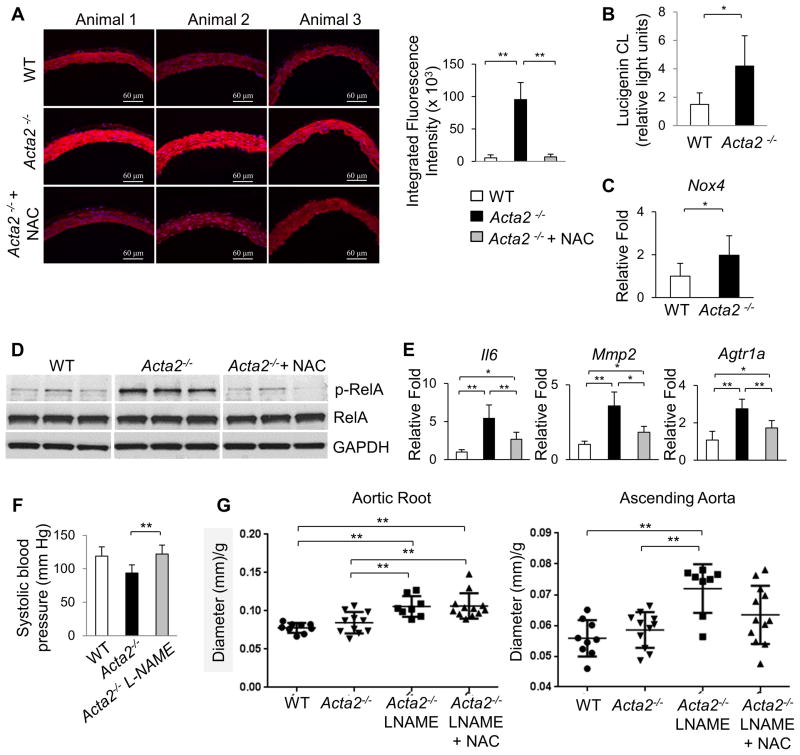
Figure 6. Acta2−/− aortas have increased ROS and molecular changes are ameliorated by NAC treatment.
(A) DHE staining of frozen aortic sections shows increased ROS in Acta2−/− aortas, which is reversed by 3 weeks of treatment with NAC (initiated at 4 weeks of age). (B) NADPH oxidase activity assay shows higher ROS generation in Acta2−/− aortas than WT. (C) Acta2−/− aortas have increased expression of Nox4 (4 weeks of age, n=3 per group). (D) pRelA levels in Acta2−/− aortas were also reduced by NAC treatment (n=5 per group). (E) NAC treatment decreases the increased expression of Il6, and Agtr1a (n=3 per group). (F) Acta2−/− mice have significantly lower systolic blood pressure than WT; treatment with L-NAME and high salt diet increases blood pressure in Acta2−/− mice to WT levels (n=6 or more mice per group). (G) Treatment with L-NAME and high salt diet significantly increases aortic root dilation by 3 months of age. Co-treatment with NAC reduces ascending aortic enlargement (p=0.057 for L-NAME vs. L-NAME + NAC groups) but does not prevent aortic root enlargement (n=9 WT, n=12 Acta2−/−, n=8 Acta2−/− + L-NAME, n=12 Acta2−/− + L-NAME + NAC).
Acta2−/− SMCs have increased ROS levels and basal NF-κB signaling, and increased sensitivity to exogenous AngII
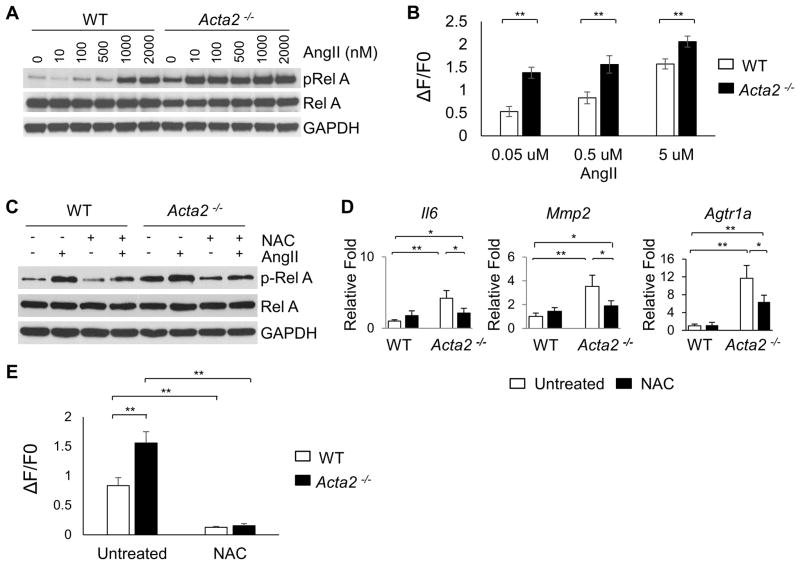
To investigate the increased signaling through the Agtr1a in Acta2−/− mice, SMCs were explanted from the ascending aorta. Our previous studies determined that Acta2−/− SMCs have increased levels of cellular ROS and ERK1/2 signaling (11). To determine if Acta2−/− SMCs are more responsive to exogenous AngII, we assessed pRelA levels. Surprisingly, Acta2−/− SMCs show activated pRelA in the absence of AngII, whereas WT SMCs do not (Figure 3A), and this increased pRelA signaling correlates with increased Il6 expression in the Acta2−/− SMCs (Online Figure VC). Furthermore, the Acta2−/− SMCs show increased sensitivity to AngII and prolonged signaling. In the Acta2−/− SMCs, pRelA levels increase at 10nM AngII, whereas pRelA levels do not increase in WT SMCs until a 100-fold higher dose of AngII is used (1000nM, Figure 3A, Online Figure IVA). The Acta2−/− SMCs are also more sensitive than WT SMCs to exogeneous AngII based on intracellular Ca++ assays (Figure 3B). The Acta2−/− SMCs show earlier and more prolonged increases in pRelA levels than WT SMCs when exposed to 1μM AngII (Online Figure IVB,C). Similar to the Acta2−/− aortas, expression of Agtr1a is increased greater than 8-fold in the Acta2−/− SMCs (Figure 3D). For all cellular studies, Agtr1b expression followed the same pattern as Agtr1a but is approximately 10-fold lower (data not shown).
Figure 3. Increased sensitivity to AngII in Acta2−/− SMCs is driven by ROS.
(A) Dose-response curve after 20 minutes of AngII treatment shows that Acta2−/− SMCs have increased baseline pRelA, which increase with exposure to AngII concentrations as low as 10nM, compared with 1000nM in WT SMCs. (B) Immediately after stimulation with various doses of AngII, Acta2−/− SMCs have increased intracellular Ca++ levels when compared with WT cells. At least 30 cells were measured for each group. (C) Treatment with NAC reduces baseline pRelA in Acta2−/− SMCs and reduces AngII-induced phosphorylation of RelA in both genotypes. (D) Acta2−/− SMCs, like the aortic tissue, have increased expression of Il6, Agtr1a, and Mmp2. NAC treatment significantly reduces expression of all three genes but not to WT levels. (E) Pretreatment with NAC for 12 hours abolishes the increase in intracellular Ca++ levels after stimulation with 0.5uM AngII in both WT and Acta2−/− SMCs.
We sought to determine the signaling pathway responsible for increased Agtr1a expression and AngII sensitivity in the Acta2−/− SMCs. Inhibition of the Agtr1a using losartan effectively blocks the increase of pRelA in response to exogenous AngII but does not decrease the basal level of pRelA in Acta2−/− SMCs; Il6 expression with these treatments correlates with pRelA levels (Online Figure VA–C). AngII–induced NF-κB activation in SMCs is mediated by RhoA/ROCK phosphorylation of Ser536 of RelA (13). Therefore, Acta2−/− SMCs were exposed to Clostridium botulinum exoenzyme C3 exotoxin to block Rho activation, which blocks AngII–inducible pRelA as expected, but does not decrease baseline activation of RelA in the Acta2−/− SMCs (Online Figure VD,E). ROS is a known activator of NF-κB signaling and we previously found increased cellular ROS levels in the Acta2−/− SMCs (11). Blocking cellular ROS using N-acetyl cysteine (NAC) decreases the baseline pRelA levels in the Acta2−/− SMCs and reduces the sensitivity of these cells to exogenous AngII (Figure 3C, Online Figure VF). NAC treatment also decreases the expression of Agtr1a, Il6 and Mmp2 in the mutant SMcs to levels similar to WT SMCs (Figure 3D). Finally, pretreatment of cells with NAC abolishes the increase in intracellular calcium in response to exogenous AngII in both WT and Acta2−/− SMCs (Figure 3E).
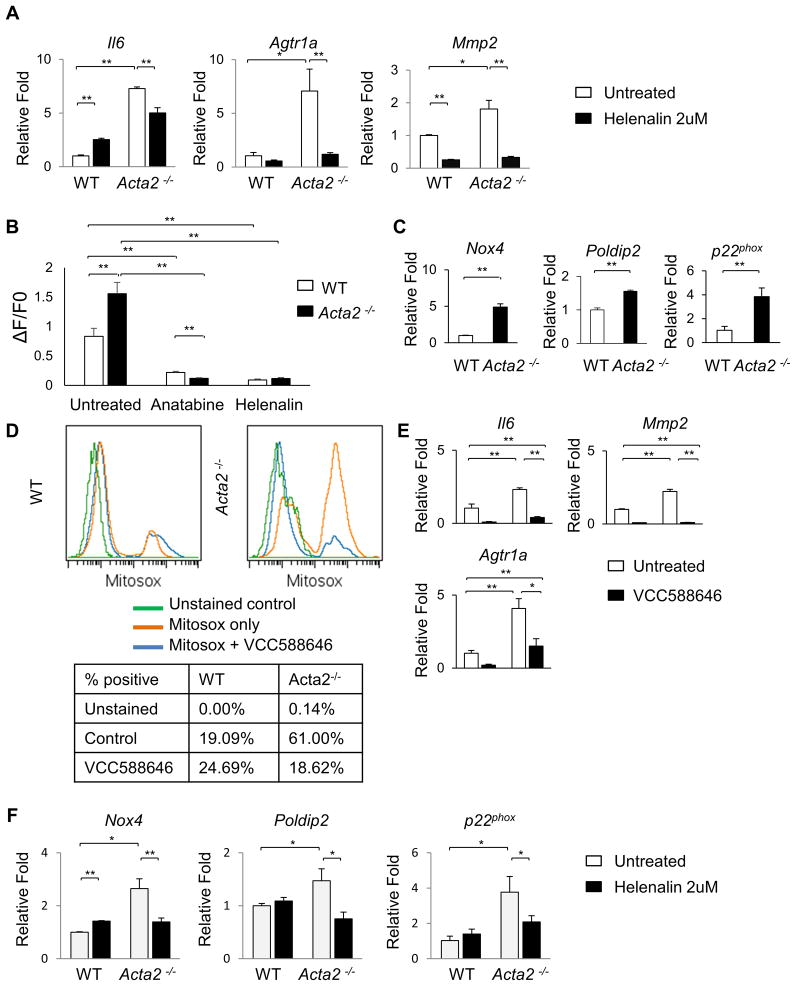
To confirm NF-κB signaling is responsible for the hypersensity of the Acta2−/− SMCs, these cells were treated with an NF-κB inhibitor anatabine, which partially blocked phosphorylation of RelA and also reduced expression of Il6, Agtr1a, and Mmp2 to levels similar to WT cells (Online Figure VIA,B) (14). Helenalin, an inhibitor of NF-κB DNA binding activity, which does not affect RelA phosphorylation, also significantly blocks expression of Il6, Agtr1a, and Mmp2 (Figure 4A) (15,16). Additionally, pretreatment of cells with either anatabine or helenalin abolishes the increase in intracellular calcium in response to exogenous AngII in both WT and Acta2−/− SMCs (Figure 4B).
Figure 4. Inhibition of either NF-κB signaling or Nox4 reduces Agtr1a expression and decreases sensitivity to AngII in Acta2−/− SMCs.
(A) Helenalin, an inhibitor of NF-κB signaling reduces expression of Il6, Mmp2, and Agtr1aafter 6 hours of treatment. (B) 12 hour pretreatment with helenalin or a second NF-κB inhibitor anatabine abolishes the increase in intracellular Ca++ with 0.5uM AngII stimulation in both WT and Acta2−/− SMCs. (C) Acta2−/− SMCs have increased expression of Nox4, Poldip2, and p22phox when compared to WT SMCs. (D) A specific inhibitor of Nox4, VCC588646, reduces ROS in Acta2−/− SMCs to WT levels. (E) VCC588646 treatment reduces expression of Il6, Mmp2, and Agtr1a in WT and Acta2−/− SMCs. (F) Treatment with the NF-κB inhibitor helenalin for 6 hours reduces expression of Nox4, Poldip2, and p22phox in Acta2−/− SMCs.
To identify the source of increased ROS, the expression of the NADPH oxidases were assessed, and expression of Nox4 and p22phox, along with a known activator of Nox4, Poldip2, are all significantly increased in the Acta2−/− SMCs (Figure 4C) (17,18). There is no significant change in expression of Nox1 or Nox2 (Online Figure VIC). Furthermore, a specific inhibitor of Nox4, VCC588646, reduces ROS levels in Acta2−/− SMCs and also significantly decreases expression of Il6, Mmp2, and Agtr1a (Figure 4D,E) (19). NF-κB has been previously shown to directly induce Nox4 expression (20), and treatment with helenalin significantly reduces expression of Nox4, Poldip2, and p22phox in Acta2−/− SMCs. Taken together, our data suggests a feedback loop in which increased ROS levels drive NF-κB signaling that increases Nox4, p22phox, and Poldip2 expression and ROS accumulation. This feedback loop increases expression of Agtr1a leading to increased AngII sensitivity in the aortic SMCs.
Disruption of SM α-actin filaments in wildtype SMCs increases expression of the Agtr1a and sensititivy to exogenous AngII
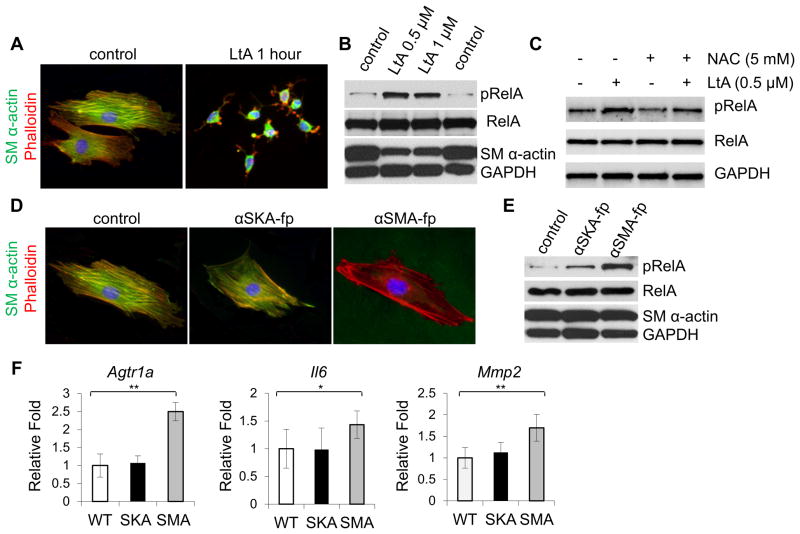
Previous studies showed that loss of another contractile protein in mouse SMCs, SM22α, also increased ROS and NF-κB signaling in SMCs (21). To further explore if disrupting the SMC cytoskeletal/contractile structure increases NF-κB signaling in SMCs, SMCs were treated with latrunculin B, a drug that rapidly and specifically disrupts the cytoskeleton by inhibiting actin polymerization. Latrunculin effectively disrupts F actin formation detected by phalloidin staining in WT SMCs, and increases pRelA levels (Figure 5A,B, Online Figure VIIA). Co-treatment with NAC blunts the increase in pRelA with latrunculin staining, suggesting the mechanism identified in Acta2−/− cells is also applicable here (Figure 5C, Online Figure VIIB).
Figure 5. Loss of α-actin filaments causes increases pRelA levels in WT SMCs.
(A) Treatment with 0.5uM latrunculin rapidly causes depolymerization of α-actin in WT SMCs. (B) After 24hr of latrunculin treatment, pRelA is increased in WT SMCs. (C) Treatment of WT SMCs with NAC for the final 12hr of the 24hr latrunculin treatment blunts the increase in pRelA. (D) Twenty-four hour treatment with aSMA-fp but not aSKA-fp disrupts α-actin filaments in WT SMCs. (E) aSMA-fp causes a dramatic increase in pRelA levels, while aSKA-fp, does not. (F) aSMA-fp but not aSKA-fp significantly increases expression of Il6, Mmp2, and Agtr1a in WT SMCs.
To further study activation of NF-κB signaling with loss of SM α-actin, a cell permeable peptide that disrupts SM α-actin filaments (aSMA-fp) in WT SMCs was obtained, along with a control peptide directed against α-skeletal actin (aSKAfp) that minimally disrupts SM α-actin filaments (Figure 5D) (22). The aSMA-fp peptide treatment in WT SMCs, but not aSKA-fp, significantly increases pRelA levels in WT SMCs, providing further support that specific disruption of α-actin filaments activates NF-κB signaling in SMCs (Figure 5E, Online Figure VIIC). Similar to the Acta2−/− SMCs, activation of NF-κB signaling with aSMA-fp treatment in WT SMCs increases expression of Agtr1a, Il6, and Mmp2 but the aSKA-fp peptide does not (Figure 5F). Taken together, these results indicate that disruption of SM α-actin drives NF-κB signaling and increases Agtr1a expression.
Increased ROS in Acta2−/− aortas
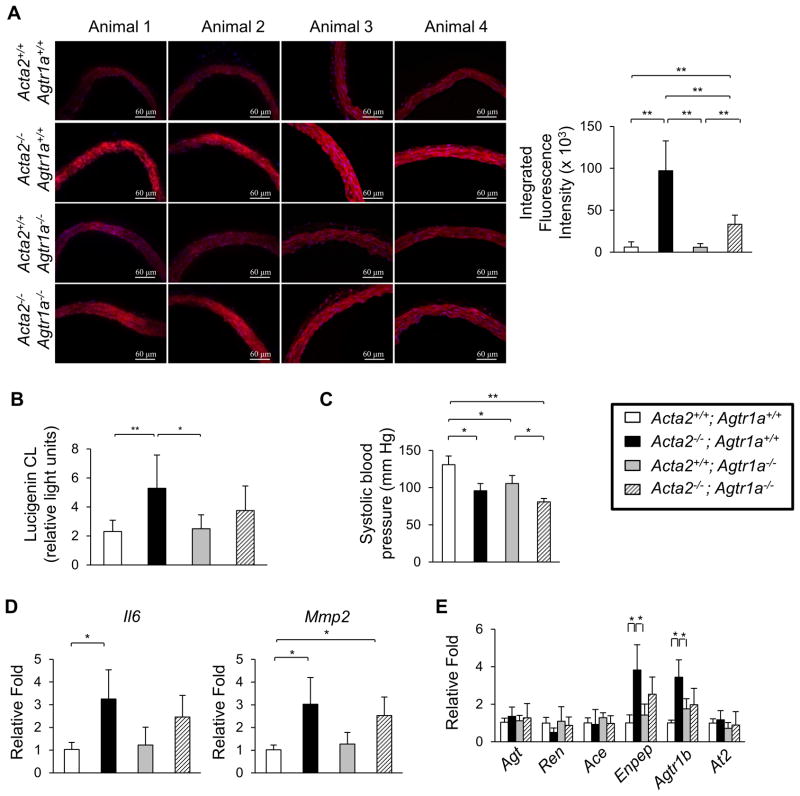
Based on the increased ROS and NF-κB signaling in the Acta2−/− SMCs in vitro, we sought to determine if ROS was increased in the Acta2−/− aortas using dihydroethidium (DHE) staining (23). Acta2−/− aortas have increased DHE staining when compared with WT aortas, and this increased staining was decreased when the Acta2−/− mice were treated with NAC for 3 weeks (Figure 6A). Additionally, NADPH oxidase activity was significantly increased in the mutant aortas (Figure 6B). Similar to the explanted Acta2−/− SMCs, the Acta2−/− aortas have increased expression of Nox4 (Figure 6C), but the expression of the other NADPH oxidase subunits were not significantly changed (Online Figure VIIIA). To confirm the role of ROS in driving signaling changes in the Acta2−/− aortas, we treated Acta2−/− mice with NAC for three weeks starting at four weeks of age. The expression of Il6 and Agtr1a are both significantly reduced after NAC treatment (Figure 6E). Furthermore, treatment with NAC blocks the increase in pRelA, but not the increased phosphorylation of ERK1/2 (Figure 6D, Online Figure VIIIB,C). We used Acta2−/− mice treated with L-NAME and high salt diet toassess whether NAC treatment would prevent aortic dilation. Acta2−/− mice co-treated with NAC and L-NAME with high salt diet no longer had significant dilation of the ascending aorta at three months of age, but the growth of the aortic root was not reversed by NAC treatment (Figure 6G). Since AngII signaling increases superoxide production by activation of NADPH oxidases in SMCs (24), we crossed the Acta2−/− mice into the Agtr1a−/− mice to determine if there is increased ROS in the Acta2−/− aortas in the absence of AngII signaling through the Agtr1a receptor. The Acta2−/−Agtr1a−/− mice are born in the expected Mendelian ratios but have lower blood pressure than the Acta2−/− (Figure 7C). DHE staining indicates that ROS is partially decreased in the Acta2−/−Agtr1a−/− aortas when compared to Acta2−/− levels, but is still significantly higher than WT levels (Figure 7A). Similarly, NADPH oxidase activity in Acta2−/−Agtr1a−/− aortas is not significantly different from either Acta2−/− or WT aortas, suggesting that the addition of the Agtra1 knockout partially but incompletely blocks ROS production (Figure 7B). Furthermore, Mmp2 expression remains significantly higher in the double knockout compared with WT aortas (Figure 7D). It is important to note that the Agtr1b expression in the Acta2−/−Agtr1a−/− aorta is similar to the levels observed in the Agtr1a−/− aortas (Figure 7E). These data support the conclusion that ROS is elevated in the Acta2−/− mice even in the absence of AngII signaling through the Agtr1a receptor.
Figure 7. Knockout of Agtr1a in Acta2−/− mice does not completely prevent increased ROS.
(A) DHE staining of frozen aortic sections shows levels of ROS are lower in Acta2−/− Agtr1a−/− mice than Acta2−/− mice but are still higher than WT mice. (B) NADPH oxidase activity in Acta2−/− Agtr1a−/− is not significantly different from WT or Acta2−/− mice. (C) Acta2−/− Agtr1a−/− mice have significantly lower systolic blood pressure than Acta2−/− mice (6 weeks of age, n=6 or more mice per group). (D) Expression of Mmp2 is elevated in Acta2−/−Agtr1a−/− mice when compared WT mice (2 months of age, n=3 per group). (E) Expression of renin-angiotensin system components is not significantly affected by loss of Agtr1a (2 months of age, n=3 per group).
DISCUSSION
The studies reported here found that loss of SM α-actin in vascular SMCs increases ROS and activates NF-κB signaling, leading to increased expression of Il6, Agtr1a, and Mmp2. Increased Agtr1a expression in Acta2−/− SMCs is associated with activation of AngII signaling at 100-fold lower doses of AngII than required for WT SMCs, as assessed by increases in pRelA and intracellular Ca++ levels. Similarly, Acta2−/− aortas have increased levels of ROS and phosphorylated RelA, and increased expression of Il6, Agtr1a, and Mmp2. ROS levels remain elevated with knockout of the Agtr1a receptor in the Acta2−/− mice, suggesting increases in ROS and downstream molecular changes are not dependent on exogenous AngII signaling through Agtr1a, but exogenous AngII further augments both ROS and NFκB signaling in mutant aortas. Thus, Acta2−/− aortic SMCs in vivo have elevated ROS and NF-κB signaling at baseline that sensitizes these cells to exogenous AngII, driving a feedback loop resulting in further increases in ROS and NF-κB signaling (Online Figure IX). Previous studies found when ROS is increased in SMCs through the overexpression of p22phox, AngII-driven aortic SMC hypertrophy is potentiated (25). Our data suggest that the increased cellular ROS in the p22phox-overexpressing SMCs may activate NF-κB signaling and increase expression of Agtr1a, thus potentiating AngII signaling in aortic SMCs.
SM22α plays a critical role in regulating and stabilizing the actin cytoskeleton in SMCs, and loss of SM22α in SMCs increases ROS and triggers NFκB signaling (21,26). Similar to our results, loss of SM22α enhanced AngII-signaling but the etiology of this enhanced signaling was not identified (27). Based on our data, increased Agtr1a expression driven by NF-κB signaling should be responsible for the enhanced AngII signaling in these cells. Taken together with our data, loss of cytoskeletal/contractile elements in vascular SMCs can lead to enhanced AngII signaling. The etiology of the excessive Agtr1a signaling in the aortas of the Marfan and Loeys-Dietz mouse models has not been identified. ROS levels are increased in both the aortas and explanted SMCs of Marfan mouse models (28,29). Once again, this increase of ROS may lead to NF-κB-dependent increase of Agtr1a expression and contribute to aneurysm formation in the Marfan mouse model.
We observed increased expression of Nox4, p22phox, and a specific activator of Nox4 Polydip2, in the Acta2−/− SMCs, and inhibition of NF-κB signaling rescues this overexpression. Our data supports a feedback loop where increased ROS leads to increased NF-κB activation, which then drives expression of Nox4, p22phox, Polydip2 and further increases ROS production. NF-κB has been previously shown to bind to the Nox4 promoter to drive its expression directly (20). Like NF-κB inhibition, a specific inhibitor of Nox4 used short term (12 hours) decreased cellular ROS and decreased expression of Il6, Mmp2, and Agtr1a (19), suggesting that inhibiting the feedback loop at either point can successfully break the cycle and prevent the downstream effects. However, conclusively confirming the role of Nox4 activity in ROS production is complicated by the fact that loss of Nox4 leads to de-differentiation of SMCs (30). We cannot rule out that loss of SM α-actin activates other pathways that contribute to elevated ROS. Previous studies found that loss of SM22α in SMCs leads to actin filament de-polymerization and activation of the PKCδ-p47phox axis, driving ROS production (27), and this pathway could potentially be the source of the initial increase in ROS driving NF-κB signaling in the Acta2−/− SMCs.
The Acta2−/− mice have dilation of the aortic root by 6 months of age, similar to the enlargement of the aortic root observed in patients with ACTA2 mutations (31), and aortic root dilation is attenuated but not reversed with losartan, an Agtr1a blocking agent. Blocking the Agtr1a through treatment with losartan attenuates pRelA signaling but not ERK1/2 signaling. Additionally, ROS levels remain elevated with knockout of the Agtr1a receptor in the Acta2−/− mice, suggesting increases in ROS and downstream molecular changes are independent of exogenous AngII signaling through the Agtr1a in the Acta2−/− aortic SMCs. Thus, increased ROS and ERK1/2 signaling may be responsible for the incomplete rescue of aortic enlargement with losartan treatment. It is important to note that the significant hypotension of Acta2−/− mice limits aortic enlargement based on the observation that there is increased rate of growth of the aorta when the blood pressure is raised to normal levels using L-NAME and a high salt diet. It is notable that the current data suggest that blocking nitric oxide (NO) signaling protects the aorta from enlargement in mouse models of aneurysms (32), and excessive signaling through a downstream target of NO signaling, type I cGMP-dependent protein kinase, can drive thoracic aortic disease (33). Therefore, there is no evidence that L-NAME contributes to aortic growth; rather the increased blood pressure drives more rapid growth of the aorta.
In the Acta2−/− postnatal aorta, we found increased elastin lamellar units and a greater number of SMCs. A similar increase in lamellar units is found in mice hemizygous for elastin (Eln+/−) (12). Furthermore, humans with ELN hemizygosity also show an increase in elastin lamellae associated with supravalvular aortic stenosis (34,35). The number of lamellar units is species-specific and fixed during development. Based on anatomic and physiologic studies showing that the relationship between wall stress and the number of lamellar units in an artery, the ratio is remarkably constant across species despite variation in arterial diameter, supporting that the number of elastin lamellae in different species is evolutionarily dictated by the wall stress (36). Therefore, the increased lamellar units in the Eln+/− and Acta2−/− mice may be due to altered wall stress resulting from underlying deficiencies during development. The Eln+/− mice are hypertensive, which may contribute to the increased wall stress and therefore the increased numbers of elastin lamellae (37). However, the Acta2−/− mice are hypotensive, and the source of the wall stress is not known (10). Acta2−/− SMCs are stiffer in culture than WT SMC (11), suggesting that cellular stiffness rather than global wall stress may be responsible for the increased lamellar units in the Acta2−/− aortas.
By electron microscopy, the SMCs in the Acta2−/− aortas have dense submembranous structures of unknown etiology that are not present in the WT SMCs. SMC contraction requires two independent processes; polymerization of actin filaments and actomyosin cross-bridge cycling (38). With SMC contraction, a small pool of submembranous actin at ECM/cytoskeletal junctions polymerizes and fortifies the connection between the membrane integrin receptors and the actin in the contractile thin filaments, which remain stably polymerized. The cortical actin and its associated cytoskeletal network provides a rigid structure for transmission of tension generated by the actomyosin cross-bridge cycling in the contractile filaments extending across the cell. The SMCs in the Acta2−/− aortae show little to no filaments crossing the cell body, suggesting other actin isoforms cannot substitute for SM α-actin for contractile thin filament formation in aortic SMCs. Although the etiology of the subcortical structures is not known, they could result from excessive polymerization of subcortical actin in an attempt to generate cellular tension in the absence of SM α-actin filaments.
In summary, we have shown that disruption of SM α-actin filaments activates NF-κB signaling and increases expression of Agtr1a, thus potentiating signaling in the Acta2−/− aortic SMCs with exposure to AngII. These results demonstrate a mechanism by which pathways downstream of the Agtr1a, which are established to drive aortic aneurysm formation, can be activated in the absence of increased AngII levels. Our findings suggest that losartan may attenuate aneurysm growth in patients with ACTA2 mutations, but also highlight that blocking NF-κB signaling or decreasing ROs levels in aortic SMCs may also prevent aneurysm growth. Additionally, the studies reported here shed light on how AngII signaling in SMCs, and potentially other cell types, can be increased even in the absence of a rise in exogenous levels of AngII.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Mutations in ACTA2 cause thoracic aortic aneurysms and dissections.
An angiotensin II (AngII) type 1 receptor blocker (losartan) reduces or prevents aortic disease in multiple mouse models of thoracic aortic aneurysms.
Smooth muscle cells (SMCs) explanted from the ascending aorta of Acta2−/− mice have increased cellular levels of reactive oxygen species (ROS).
What New Information Does This Article Contribute?
Losartan treatment attenuates aortic root enlargement in Acta2−/− mice, but there is no evidence of increased AngII levels in the aorta or kidneys.
The higher ROS levels in Acta2−/− SMCs drives increased expression of the AngII type I receptor (Agtr1a) via NF-κB signaling, leading to activation of AngII signaling in aortic SMCs at 100-fold lower levels of AngII than required to activate signaling in wild-type SMCs.
This study was initiated to investigate the mechanism of thoracic aortic aneurysm formation associated with mutations in ACTA2, the gene encoding the smooth muscle isoform of α-actin (SM α-actin). We show that Acta2−/− mice have aortic root dilatation, which is attenuated by treatment with an angiotensin II (AngII) type 1 receptor blocker (losartan). We did not find increased levels of AngII in the aorta or kidney of Acta2−/− mice. Instead, we show that Acta2−/− SMCs activate signaling at 100-fold lower levels of AngII when compared to wild-type cells due to increased expression of AngII type I receptor (Agtr1a). We go on to show that the increase in cellular reactive oxygen species (ROS) in Acta2−/− SMCs activates NF-κB signaling, which drives expression of both Agtr1a and Nox4, further increasing ROS generation. Importantly, we show that this signaling mechanism is initiated in wild-type SMCs when SM α-actin filaments are disrupted by other methods. Taken together, our results show a mechanism by which increased AngII type I receptor signaling in aortic SMCs can drive aortic aneurysm formation without an increase in exogenous AngII levels, and this mechanism may underlie aneurysm formation due to other causes. Further, inhibition of ROS or NF-κB signaling may be a viable therapeutic strategy for preventing aneurysm formation.
Acknowledgments
We would like to thank Christine Chaponnier and Giulio Gabbiani (University of Geneva) for giving us the αSKA-fp and αSMA-fp peptides.
SOURCES OF FUNDING
The following sources provided funding for these studies: RO1 HL109942, P01HL110869-01, UL1 RR024148, John Ritter Foundation, Vivian L. Smith Foundation, and Richard T. Pisani Funds (all to D.M.M.); the National Marfan Foundation Victor A. McKusick Fellowship (C.L.P.); the National Center for Advancing Translational Sciences of the National Institutes of Health TL1TR000369 and UL1TR000371 (A.M.P.).
Nonstandard Abbreviations and Acronyms
- AngII
angiotensin II
- Agtr1a
angiotensin receptor, type 1 a
- EM
electron microscopy
- FAK
focal adhesion kinase
- NAC
N-acetyl cysteine
- PDGF
platelet derived growth factor
- ROS
reactive oxygen species
- SM α-actin
smooth muscle specific α-actin
- SMC
smooth muscle cell
- SM-MHC
smooth muscle myosin heavy chain
- WT
wildtype
Footnotes
DISCLOSURES
The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
J.C., A.M.P., C.S.K., C.L.P., C.V., S.W., K.L.B., J.C., and L.G. designed and performed the cellular and mouse studies. S.M. did the two dimensional gels to analyze cellular actins. L.-J.R. and E.C.D. did the electron microscopic analyses. J.X. and M.X.Z. did the calcium imaging studies. R.M., P.K., Y.-J.G., and S.K.P. performed and analyzed the echocardiographic data. C.S.K. and D.M.M. designed the studies and wrote and assembled the manuscript. All authors read and approved the manuscript in its final form.
References
- 1.Guo DC, Pannu H, Papke CL, Yu RK, Avidan N, Bourgeois S, Estrera AL, Safi HJ, Sparks E, Amor D, Ades L, McConnell V, Willoughby CE, Abuelo D, Willing M, Lewis RA, Kim DH, Scherer S, Tung PP, Ahn C, Buja LM, Raman CS, Shete S, Milewicz DM. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet. 2007;39:1488–93. doi: 10.1038/ng.2007.6. [DOI] [PubMed] [Google Scholar]
- 2.Guo DC, Papke CL, Tran-Fadulu V, Regalado ES, Avidan N, Johnson RJ, Kim DH, Pannu H, Willing MC, Sparks E, Pyeritz RE, Singh MN, Dalman RL, Grotta JC, Marian AJ, Boerwinkle EA, Frazier LQ, LeMaire SA, Coselli JS, Estrera AL, Safi HJ, Veeraraghavan S, Muzny DM, Wheeler DA, Willerson JT, Yu RK, Shete SS, Scherer SE, Raman CS, Buja LM, Milewicz DM. Mutations in smooth muscle alpha-actin (ACTA2) cause coronary artery disease, stroke, and moyamoya disease, along with thoracic aortic disease. Am J Hum Genet. 2009;84:617–27. doi: 10.1016/j.ajhg.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milewicz DM, Ostergaard JR, la-Kokko LM, Khan N, Grange DK, Mendoza-Londono R, Bradley TJ, Olney AH, Ades L, Maher JF, Guo D, Buja LM, Kim D, Hyland JC, Regalado ES. De novo ACTA2 mutation causes a novel syndrome of multisystemic smooth muscle dysfunction. Am J Med Genet A. 2010;152A:2437–43. doi: 10.1002/ajmg.a.33657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu H, Fagnant PM, Bookwalter CS, Joel P, Trybus KM. Vascular disease-causing mutation R258C in ACTA2 disrupts actin dynamics and interaction with myosin. Proc Natl Acad Sci U S A. 2015;112:E4168–E4177. doi: 10.1073/pnas.1507587112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, Podowski M, Neptune ER, Halushka MK, Bedja D, Gabrielson K, Rifkin DB, Carta L, Ramirez F, Huso DL, Dietz HC. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–21. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis EC. Smooth muscle cell to elastic lamina connections in developing mouse aorta. Role in aortic medial organization. Lab Invest. 1993;68:89–99. [PubMed] [Google Scholar]
- 7.Habashi JP, Doyle JJ, Holm TM, Aziz H, Schoenhoff F, Bedja D, Chen Y, Modiri AN, Judge DP, Dietz HC. Angiotensin II type 2 receptor signaling attenuates aortic aneurysm in mice through ERK antagonism. Science. 2011;332:361–5. doi: 10.1126/science.1192152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang J, Yamashiro Y, Papke CL, Ikeda Y, Lin Y, Patel M, Inagami T, Le VP, Wagenseil JE, Yanagisawa H. Angiotensin-converting enzyme-induced activation of local angiotensin signaling is required for ascending aortic aneurysms in fibulin-4-deficient mice. Sci Transl Med. 2013;5:183ra58. doi: 10.1126/scitranslmed.3005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallo EM, Loch DC, Habashi JP, Calderon JF, Chen Y, Bedja D, Van EC, Gerber EE, Parker SJ, Sauls K, Judge DP, Cooke SK, Lindsay ME, Rouf R, Myers L, ap Rhys CM, Kent KC, Norris RA, Huso DL, Dietz HC. Angiotensin II-dependent TGF-beta signaling contributes to Loeys-Dietz syndrome vascular pathogenesis. J Clin Invest. 2014;124:448–60. doi: 10.1172/JCI69666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schildmeyer LA, Braun R, Taffet G, Debiasi M, Burns AE, Bradley A, Schwartz RJ. Impaired vascular contractility and blood pressure homeostasis in the smooth muscle alpha-actin null mouse. FASEB J. 2000;14:2213–20. doi: 10.1096/fj.99-0927com. [DOI] [PubMed] [Google Scholar]
- 11.Papke CL, Cao J, Kwartler CS, Villamizar C, Byanova KL, Lim SM, Sreenivasappa H, Fischer G, Pham J, Rees M, Wang M, Chaponnier C, Gabbiani G, Khakoo AY, Chandra J, Trache A, Zimmer W, Milewicz DM. Smooth muscle hyperplasia due to loss of smooth muscle alpha-actin is driven by activation of focal adhesion kinase, altered p53 localization and increased levels of platelet-derived growth factor receptor-beta. Hum Mol Genet. 2013 doi: 10.1093/hmg/ddt167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li DY, Faury G, Taylor DG, Davis EC, Boyle WA, Mecham RP, Stenzel P, Boak B, Keating MT. Novel arterial pathology in mice and humans hemizygous for elastin. J Clin Invest. 1998;102:1783–7. doi: 10.1172/JCI4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui R, Tieu B, Recinos A, Tilton RG, Brasier AR. RhoA mediates angiotensin II-induced phospho-Ser536 nuclear factor kappaB/RelA subunit exchange on the interleukin-6 promoter in VSMCs. Circ Res. 2006;99:723–30. doi: 10.1161/01.RES.0000244015.10655.3f. [DOI] [PubMed] [Google Scholar]
- 14.Paris D, Beaulieu-Abdelahad D, Bachmeier C, Reed J, Ait-Ghezala G, Bishop A, Chao J, Mathura V, Crawford F, Mullan M. Anatabine lowers Alzheimer’s Abeta production in vitro and in vivo. Eur J Pharmacol. 2011;670:384–91. doi: 10.1016/j.ejphar.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 15.Lyss G, Schmidt TJ, Merfort I, Pahl HL. Helenalin, an anti-inflammatory sesquiterpene lactone from Arnica, selectively inhibits transcription factor NF-kappaB. Biol Chem. 1997;378:951–61. doi: 10.1515/bchm.1997.378.9.951. [DOI] [PubMed] [Google Scholar]
- 16.Lyss G, Knorre A, Schmidt TJ, Pahl HL, Merfort I. The anti-inflammatory sesquiterpene lactone helenalin inhibits the transcription factor NF-kappaB by directly targeting p65. J Biol Chem. 1998;273:33508–16. doi: 10.1074/jbc.273.50.33508. [DOI] [PubMed] [Google Scholar]
- 17.Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassegue B, Griendling KK. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res. 2009;105:249–59. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Datla SR, McGrail DJ, Vukelic S, Huff LP, Lyle AN, Pounkova L, Lee M, Seidel-Rogol B, Khalil MK, Hilenski LL, Terada LS, Dawson MR, Lassegue B, Griendling KK. Poldip2 controls vascular smooth muscle cell migration by regulating focal adhesion turnover and force polarization. Am J Physiol Heart Circ Physiol. 2014;307:H945–H957. doi: 10.1152/ajpheart.00918.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barman SA, Chen F, Su Y, Dimitropoulou C, Wang Y, Catravas JD, Han W, Orfi L, Szantai-Kis C, Keri G, Szabadkai I, Barabutis N, Rafikova O, Rafikov R, Black SM, Jonigk D, Giannis A, Asmis R, Stepp DW, Ramesh G, Fulton DJ. NADPH oxidase 4 is expressed in pulmonary artery adventitia and contributes to hypertensive vascular remodeling. Arterioscler Thromb Vasc Biol. 2014;34:1704–15. doi: 10.1161/ATVBAHA.114.303848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manea A, Tanase LI, Raicu M, Simionescu M. Transcriptional regulation of NADPH oxidase isoforms, Nox1 and Nox4, by nuclear factor-kappaB in human aortic smooth muscle cells. Biochem Biophys Res Commun. 2010;396:901–7. doi: 10.1016/j.bbrc.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 21.Shen J, Yang M, Ju D, Jiang H, Zheng JP, Xu Z, Li L. Disruption of SM22 promotes inflammation after artery injury via nuclear factor kappaB activation. Circ Res. 2010;106:1351–62. doi: 10.1161/CIRCRESAHA.109.213900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hinz B, Gabbiani G, Chaponnier C. The NH2-terminal peptide of alpha-smooth muscle actin inhibits force generation by the myofibroblast in vitro and in vivo. J Cell Biol. 2002;157:657–63. doi: 10.1083/jcb.200201049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dikalov S, Griendling KK, Harrison DG. Measurement of reactive oxygen species in cardiovascular studies. Hypertension. 2007;49:717–27. doi: 10.1161/01.HYP.0000258594.87211.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–8. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 25.Weber DS, Rocic P, Mellis AM, Laude K, Lyle AN, Harrison DG, Griendling KK. Angiotensin II-induced hypertrophy is potentiated in mice overexpressing p22phox in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2005;288:H37–H42. doi: 10.1152/ajpheart.00638.2004. [DOI] [PubMed] [Google Scholar]
- 26.Zeidan A, Sward K, Nordstrom I, Ekblad E, Zhang JC, Parmacek MS, Hellstrand P. Ablation of SM22alpha decreases contractility and actin contents of mouse vascular smooth muscle. FEBS Lett. 2004;562:141–6. doi: 10.1016/S0014-5793(04)00220-0. [DOI] [PubMed] [Google Scholar]
- 27.Lv P, Miao SB, Shu YN, Dong LH, Liu G, Xie XL, Gao M, Wang YC, Yin YJ, Wang XJ, Han M. Phosphorylation of smooth muscle 22alpha facilitates angiotensin II-induced ROS production via activation of the PKCdelta-P47phox axis through release of PKCdelta and actin dynamics and is associated with hypertrophy and hyperplasia of vascular smooth muscle cells in vitro and in vivo. Circ Res. 2012;111:697–707. doi: 10.1161/CIRCRESAHA.112.272013. [DOI] [PubMed] [Google Scholar]
- 28.Carta L, Smaldone S, Zilberberg L, Loch D, Dietz HC, Rifkin DB, Ramirez F. MAPKp38 is an early determinant of promiscuous Smad2/3 signaling in the aortas of fibrillin-1 (Fbn1) null mice. J Biol Chem. 2008 doi: 10.1074/jbc.M806962200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang HH, van BC, Chung AW. Vasomotor dysfunction in the thoracic aorta of Marfan syndrome is associated with accumulation of oxidative stress. Vascul Pharmacol. 2010;52:37–45. doi: 10.1016/j.vph.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, Schmidt HH, Lassegue B, Griendling KK. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2007;27:42–8. doi: 10.1161/01.ATV.0000251500.94478.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Regalado E, Guo DC, Prakash S, Bensend TA, Flynn K, Estrera A, Safi H, Liang D, Hyland J, Child A, Arno G, Boileau C, Jondeau G, Braverman A, Moran R, Morisaki H, Pyeritz R, Coselli J, LeMaire S, Milewicz DM Montalcino Aortic Consortium. Aortic Disease Presentation and Outcome Associated with ACTA2 mutations. Circ Cardiovasc Genet. 2015 doi: 10.1161/CIRCGENETICS.114.000943. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oller J, Mendez-Barbero N, Ruiz EJ, Villahoz S, Renard M, Canelas LI, Briones AM, Alberca R, Lozano-Vidal N, Hurle MA, Milewicz D, Evangelista A, Salaices M, Nistal JF, Jimenez-Borreguero LJ, De BJ, Campanero MR, Redondo JM. Nitric oxide mediates aortic disease in mice deficient in the metalloprotease Adamts1 and in a mouse model of Marfan syndrome. Nat Med. 2017 doi: 10.1038/nm.4266. [DOI] [PubMed] [Google Scholar]
- 33.Guo DC, Regalado E, Casteel DE, Santos-Cortez RL, Gong L, Kim JJ, Dyack S, Horne SG, Chang G, Jondeau G, Boileau C, Coselli JS, Li Z, Leal SM, Shendure J, Rieder MJ, Bamshad MJ, Nickerson DA, Kim C, Milewicz DM. Recurrent Gain-of-Function Mutation in PRKG1 Causes Thoracic Aortic Aneurysms and Acute Aortic Dissections. Am J Hum Genet. 2013;93:398–404. doi: 10.1016/j.ajhg.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brooke BS, Bayes-Genis A, Li DY. New insights into elastin and vascular disease. Trends Cardiovasc Med. 2003;13:176–81. doi: 10.1016/s1050-1738(03)00065-3. [DOI] [PubMed] [Google Scholar]
- 35.Pober BR, Johnson M, Urban Z. Mechanisms and treatment of cardiovascular disease in Williams-Beuren syndrome. J Clin Invest. 2008;118:1606–15. doi: 10.1172/JCI35309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolinsky H, Glagov S. A lamellar unit of aortic medial structure and function in mammals. Circ Res. 1967;20:99–111. doi: 10.1161/01.res.20.1.99. [DOI] [PubMed] [Google Scholar]
- 37.Faury G, Pezet M, Knutsen RH, Boyle WA, Heximer SP, McLean SE, Minkes RK, Blumer KJ, Kovacs A, Kelly DP, Li DY, Starcher B, Mecham RP. Developmental adaptation of the mouse cardiovascular system to elastin haploinsufficiency. J Clin Invest. 2003;112:1419–28. doi: 10.1172/JCI19028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gunst SJ, Zhang W. Actin cytoskeletal dynamics in smooth muscle: a new paradigm for the regulation of smooth muscle contraction. Am J Physiol Cell Physiol. 2008;295:C576–C587. doi: 10.1152/ajpcell.00253.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.