Abstract
One of the major immune checkpoints responsible for immune evasion in cancer cells is the interaction between programmed cell death-1 (PD-1) and its ligand (PD-L1). Since human trophoblastic cells display many of the features of malignant cells such as the ability to invade normal tissue including blood vessels and are apparently not eradicated by the host immune system, we undertook the present study to determine whether PD-L1 was upregulated in different types of trophoblastic cells during normal pregnancy and in gestational trophoblastic diseases. Immunohistochemistry using an anti-PD-L1 specific antibody demonstrated that in early and term normal placentas, PD-L1 was highly expressed in syncytiotrophoblast and to a much lower extent in intermediate trophoblastic cells located in the chorion laeve and implantation site. PD-L1 immunoreactivity was undetectable in cytotrophoblastic cells. This staining pattern in normal placenta was recapitulated in various types of gestational trophoblastic disease. PD-L1 was highly expressed by syncytiotrophoblast in complete moles and choriocarcinomas. The intermediate trophoblastic neoplasms, placental site trophoblastic tumors and epithelioid trophoblastic tumors, showed variable PD-L1 immunoreactivity but at a lower intensity than in the neoplastic syncytiotrophoblast in choriocarcinoma. In addition, we observed PD-1 positive lymphocytes located within the implantation site and in trophoblastic tumors. In summary, this study describes a novel mechanism for trophoblastic cells to create a tolerogenic feto-maternal interface by upregulating PD-L1 in syncytiotrophoblast and in intermediate trophoblast. Trophoblastic tumors may also use PD-L1 expression to evade the host immune response thereby promoting their survival.
Keywords: Trophoblast, gestational, choriocarcinoma, PD-L1, PD-1
Introduction
Escape from immune surveillance and prevention of subsequent rejection by the host immune system are fundamental to sustain embryonic and fetal development in the uterus as they express allogeneic paternal antigens. This immunological paradox of pregnancy remains mainly unclear. Since it is the trophoblast that directly interacts with the maternal environment, research has largely focused on elucidating how trophoblastic cells contribute to local immune suppression. For example, lack of MHC class II antigens on trophoblast is thought to facilitate survival of the semi-allogeneic conceptus in the presence of maternal lymphocytes. CCL5 is a chemokine secreted by T cells that modulates the balance of maternal Treg/T effector lymphocytes in promoting maternal tolerance of trophoblast[1]. It has been reported that human leukocyte antigen-G (HLA-G) is highly expressed by the extravillous (intermediate) trophoblastic cells in the trophoblastic columns, implantation site and the chorion leave but not in syncytiotrophoblast or cytotrophoblast[2]. The constitutive expression of HLA-G on these cells plays an important role in inducing immunotolerance by inhibiting cytotoxicity mediated by natural killer (NK) cells [3-6] and T-cells [7-9]. In addition, the finding of decreased protein and mRNA levels of indoleamine 2,3-dioxygenase (IDO) in the placenta and decidua from recurrent spontaneous abortuses suggests an important role for IDO in the maintenance of normal pregnancy [10]. It has also been suggested that during pregnancy the programmed cell death-1 (PD-1) pathway can modify the maternal decidual lymphocyte cytokine secretion facilitating immune suppression[11].
In recent years, immune checkpoints have received significant attention in cancer research, among them, PD-1 is one of the best characterized checkpoint proteins. The interaction between PD-1 and its ligand, PD-L1, triggers signal transduction pathways that result in T-cell suppression, allowing PD-L1 expressing cancer cells to escape eradication by PD-1 positive immune cells. It has been reported that PD-L1 is expressed in various tumor types including non–small cell lung cancer, colorectal carcinoma, renal cell carcinoma, melanoma and hematopoietic malignancies[12,13]. Neutralization of PD-1 pathway activity by antibodies against PD-1 is considered a promising anti-cancer strategy alone or in combination with other immune-based therapy[13,14]. In non-gestational endometrium, the density of PD-1+/CD3+ lymphocytes was lower than in the first trimester placental sites, suggesting that PD-L1 expressing trophoblast may exploit PD-1/PD-L1 mediated immune suppression in normal gestation. In fact, it has been reported that pregnant mice treated with anti-PD-L1 blocking antibody lose their embryos [15] and a deficiency in PD-L1 has been associated with an increased frequency of fetal resorption and decreased fetal survival[7]. In view of these studies showing the functional roles of PD-L1 in mouse models, we undertook the present study to determine the expression pattern of PD-L1 in human placentas at various gestational ages and in various types of gestational trophoblastic disease.
Materials and Methods
Tissues and case selection
The Pathology Diagnosis System was used to identify cases from the Surgical Pathology files and the gynecologic pathology division consultation files in the Department of Pathology, the Johns Hopkins Hospital, Baltimore, Maryland. Hematoxylin-and-eosin stained sections were reviewed from each case to confirm the original diagnosis. Formalin-fixed paraffin-embedded tissue blocks were then retrieved from 16 first trimester abortuses (including 6 showing an exaggerated placental site), 5 normal term placentas 7 complete hydatidiform moles,11 placental site nodules, 35 choriocarcinomas, 6 placental site trophoblastic tumors (PSTTs) and 14 epithelioid trophoblastic tumors (ETTs). Sections of normal breast, small and large intestines, pancreas, salivary glands, gallbladder, brain, tonsils, kidney, lung, lymph node, tonsil and stomach were used as normal controls. Most of specimens were arranged in tissue microarrays to facilitate the immunohistochemistry. All samples were anonymous and collection of the archived paraffin tissues was approved by the Johns Hopkins institutional review board.
Immunohistochemistry
Immunohistochemistry was performed at the Johns Hopkins Oncology Tissue Services using a rabbit monoclonal anti-PD-L1 antibody (Cell Signaling #13684S) and a mouse monoclonal anti-PD-1 (abcam, ab52587) antibody. After deparaffinization and hydration, slides were incubated in a Target Retrieval solution (Dako) and steamed for 45 min. The slides were then blocked with a Dual Blocking solution (Dako) for 5 min followed by incubation with either of primary antibodies as mentioned above at room temperature for 45 min, followed by ploy-HRP anti- mouse or anti-rabbit secondary antibodies for 30 min. 3,3′-Diaminobenzidine (Sigma) was applied onto the slides to develop the immunoreactivity on tissues. For counter staining, Dako Mayers hematoxylin (Dako, 1:5) was applied for one min. After washes, the slides were dehydrated, cleaned and mounted with coverslips using xylene.
Results
The immunostaining pattern of PD-L1 in normal placentas is summarized in Table 1. PD-L1 immunoreactivity was intense on the outer surface of syncytiotrophoblast on chorionic villi in all early and term placentas (Fig. 1A). Interestingly, the inner aspect of the syncytiotrophoblast, facing the chorionic villous stroma, was mostly negative. In contrast to syncytiotrophoblast, the cytotrophoblastic cells on the villi showed undetectable PD-L1 immunoreactivity (Fig. 1A). The intermediate trophoblastic cells in implantation sites from both early and term placentas demonstrated either undetectable or very weak and focal staining (Fig. 1B and 1C). The immunointensity of PD-L1 in exaggerated placental sites was similar to those in normal implantation sites. None of them had an immunointensity comparable to that in syncytiotrophoblast. Likewise, the intermediate trophoblastic cells in the chorion laeve (fetal membrane) showed either very weak or undetectable PD-L1 staining. The pattern of PD-L1 staining was predominantly membranous, with no nuclear or cytoplasmic localization. PD-L1 immunoreactivity was not detectable in normal breast, small and large intestines, pancreas, salivary glands, gallbladder, brain, tonsils, kidney, lung, lymph node, tonsil and stomach which were also included in tissue microarrays.
Table 1. PDL-1 immunoreactivity in different types of trophoblastic cells in early and term human placentas.
| PDL-1 immunoreactivity | Cytotrophoblast (n= 16) | Syncytiotrophoblast (n= 16) | Intermediate trophoblast | |
|---|---|---|---|---|
| Implantation site (n= 16) | Chorion laeve* (n= 5) | |||
| Undetectable | 16 | 0 | 3 | 2 |
| Weak and focal | 0 | 0 | 13 | 3 |
| Intense and diffuse | 0 | 16 | 0 | 0 |
Chorion laeve from term placentas
Fig. 1.
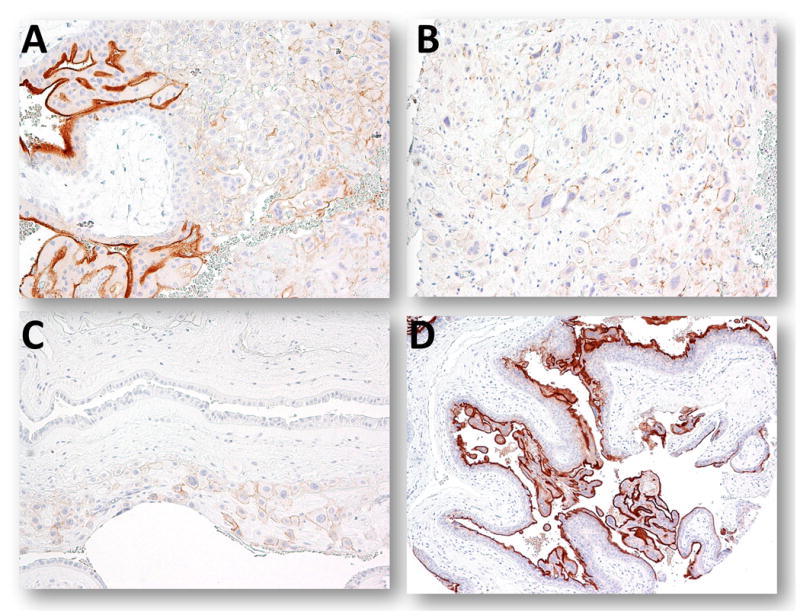
Expression of PD-L1 in placentas. A. An anchoring chorionic villus. B. An implantation site from the first trimester placenta. C. Chorionic leave (fetal membrane) from a term placenta. D. A complete hydatidiform mole.
PD-L1 immunohistochemistry was performed on 68 gestational trophoblastic lesions. The staining pattern in complete hydatidiform moles was identical to the normal placentas. PD-L1 immunoreactivity was intense in syncytiotrophoblast and to a much lesser extent in implantation site intermediate trophoblastic cells, while cytotrophoblastic cells were virtually negative (Fig. 1D). In choriocarcinoma, 22 (73%) of 30 specimens showed intense and diffuse PD-L1 immunoreactivity in syncytiotrophoblast and weaker immunoreactivity in intermediate trophoblastic cells. Three (10%) of choriocarcinomas showed weak staining and 5 choriocarcinomas were negative for PD-L1 immunoreactivity (Table 2 and Fig. 2). In these cases there was a paucity of viable trophoblast, most of the tissue was necrotic. The expression levels of PD-L1 in intermediate trophoblastic tumors including PSTT and ETT varied from undetectable to intense staining (Fig. 3). We did not observe PD-L1 immunoreactivity in any of placental site nodules.
Table 2. PDL-1 immunoreactivity in various types of gestational trophoblastic lesions.
| PDL-1 immunoreactivity | CHM (n= 7) | Placental site nodule (n= 11) | Choriocarcinoma (n= 30) | PSTT (n= 6) | ETT (n= 14) |
|---|---|---|---|---|---|
| Undetectable | 0 | 11 | 5 | 3 | 6 |
| Weak and focal | 0 | 0 | 3 (mainly in ST) | 1 | 4 |
| Intense and diffuse | 7 (only in ST) | 0 | 22 (only in ST) | 2 | 4 |
CHM: complete hydatidiform mole; PSTT: placental site trophoblastic tumor; ETT: epithelioid trophoblastic tumor; ST: syncytiotrophoblast
Fig. 2.
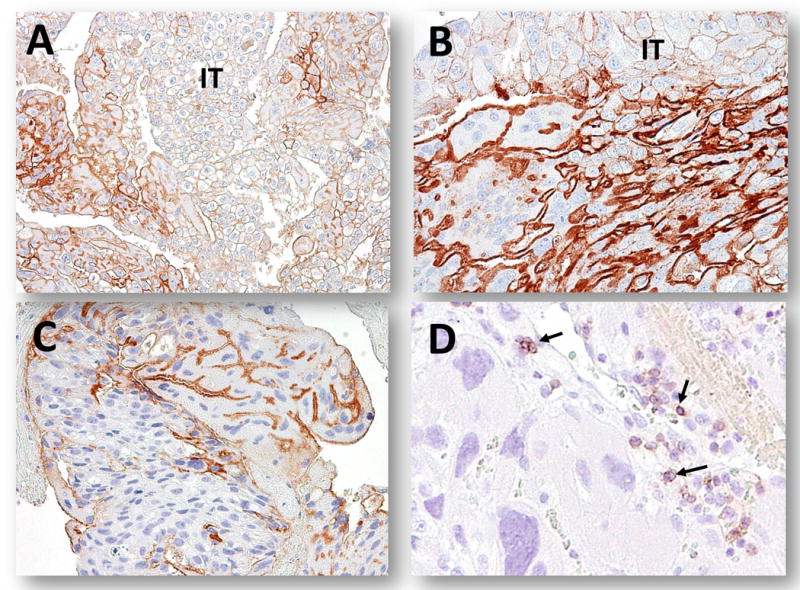
PD-L1 immunoreactivity in choriocarcinomas. A-C. In three individual cases, syncytiotrophoblast showed intense and diffuse PD-L1 immunoreactivity while the intermediate trophoblastic cells (IT) exhibit weaker immunoreactivity. D. PD-1 positive immune cells (arrows) within a choriocarcinoma.
Fig. 3.
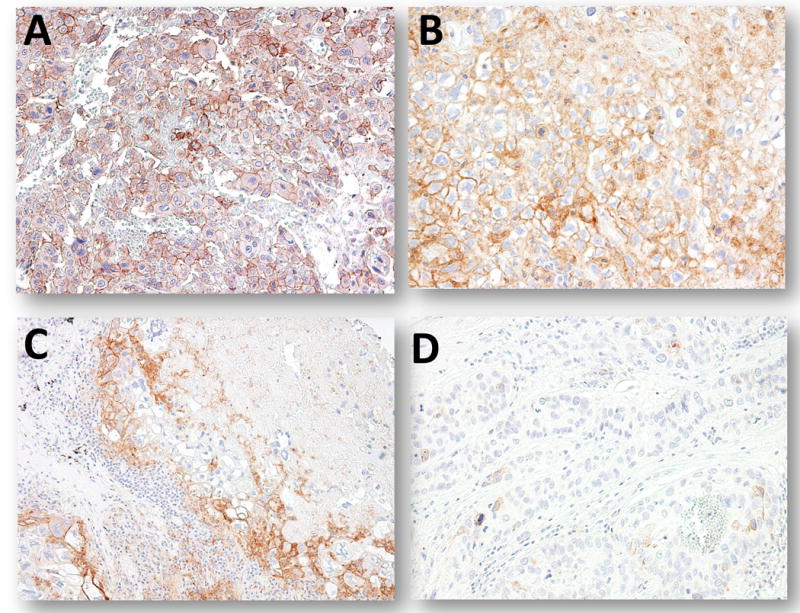
PD-L1 immunoreactivity in placental site trophoblastic tumor (PSTT) and epithelioid trophoblastic tumor (ETT). A and B. Two PSTTs show PD-L1 immunostaining but the intensity is lower than that in syncytiotrophoblast. C. An ETT demonstrates focal and weak PD-L1 staining. D. Another ETT demonstrates an undetectable PD-L1expression level.
We also assessed the expression of PD-1 in immune cells in the same cohort of normal placentas and trophoblastic neoplasms. PD-1 positive immune cells were scattered in the implantation sites of early placentas and the density of positive cells varied from one to 7 per high-power fields (x40) (Fig. 4A and 4B). In gestational trophoblastic tumors, PD-1 positive immune cells were present in association with PSTT, ETT and choriocarcinomas but again their density varied widely from 0 to 5 positive cells per high-power fields (Fig. 4C and 4D). In general, the PD-1 positive immune cells were enriched in the areas with a high density of lymphocytes. PD-1 immunoreactivity was detected in lymphocytes of tonsils and lymph nodes but not in other normal tissues examined including breast, small and large intestines, pancreas, salivary glands, gallbladder, brain, kidney, lung and stomach.
Fig. 4.
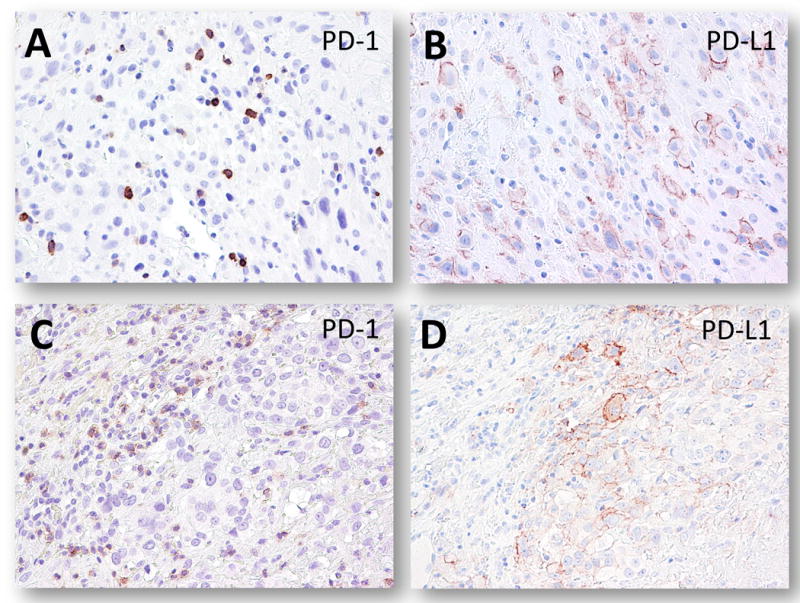
Immunostaining of PD-1 (A and C) and PD-L1 (B and D) in a representative placenta and a PSTT. A and B. An early implantation site. C and D. A PSTT.
Discussion
Survival of the developing embryo and fetus requires immune tolerance by inactivating the maternal immune system at the placental-maternal interface which is thought to be accomplished by trophoblast. In the human placenta, there are different types of trophoblastic cells based on their location, distinct gene expression profiles and specific functions [16]. These include cytotrophoblast which is the germinative component, syncytiotrophoblast which is the terminally differentiated component and responsible for most of the functional activity of trophoblast and intermediate trophoblast (extra-villous trophoblast) which plays an important role in establishing the maternal-fetal blood circulation[16-18]. Among the various trophoblastic subpopulations, syncytiotrophoblast forms a continuous layer of tissue that extends and covers all surfaces of villous trees and part of the inner surface of chorionic and basal plates[17] where it is immersed in the maternal blood in the inter-villous space. In contrast, intermediate trophoblastic cells are in close contact with immune cells either in the implantation site or in fetal membranes. In this study, we found that syncytiotrophoblast highly expresses PD-L1, whereas cytotrophoblast does not and intermediate trophoblast express little or no PD-L1, a result suggesting that syncytiotrophoblast employs PD-L1 as the immune checkpoint to inactivate PD-1 expressing immune cells.
The molecular mechanisms accounting for PD-L1 upregulation in syncytiotrophoblast are unclear but they may involve a complex interaction of regulatory T cells, cytokines, chemokines and growth factors derived from the feto-placental environment which can directly or indirectly upregulate PD-L1 expression[19]. In neoplastic tissues, expression of PD-L1 was augmented in an EGFR- and JAK2/STAT1-dependent manner in head and neck carcinoma[20], and targeting the PD-1/PD-L1 signaling axis using a specific JAK2 inhibitor is able to block PD-L1 upregulation in tumor cells and enhanced their immunogenicity. As EGFR is expressed by both normal and neoplastic syncytiotrophoblast[21], it is possible that the EGF pathway may also participate in regulating PD-L1 expression. IFN-γ is a pro-inflammatory cytokine secreted by uterine natural killer cells in endometrium during early pregnancy[22]. Trophoblastic cells are known to increase IFN-γ expression by a variety of microenvironment stimuli including CD56+ decidual NK cells [23]. IFN-γ, in turn, upregulates PD-L1 because like EGFR, IFN-γ also utilizes Janus kinase 2 (JAK2) as a signaling mediator[24].
The relatively low expression levels of PD-L1 in intermediate trophoblastic cells as compared to syncytiotrophoblast suggest that intermediate trophoblastic cells use other mechanisms to overcome surveillance by the maternal immune system. In fact, up-regulation of HLA-G, a unique non-classical MHC class I molecule, has been reported to be expressed exclusively in the intermediate trophoblastic cells but not in syncytiotrophoblast or cytotrophoblast[2,25,26]. Intermediate trophoblastic cells establish a tolerogenic microenvironment through the interaction with maternal innate and adaptive cells[27]. Specifically, HLA-G can present with nonamer peptides and bind CD8 in an analogous manner to classical HLA-class I proteins, facilitating the escape of immune surveillance (alloreactivity) by host T-lymphocytes and NK-cells[28,29]. Since PD-1 positive lymphocytes were detected in many of gestational trophoblastic disease (GTD) tissues as in a normal placental site [30], it is likely that PD-L1 expressing syncytiotrophoblast works in conjunction with HLA-G expressing intermediate trophoblastic cells to shield the entire gestational sac and fetus from attack by maternal immune cells.
GTD encompasses a heterogeneous group of lesions with specific clinical features, morphological characteristics and pathogenesis[16]. The recent World Health Organization-classification of GTD includes abnormally formed placentas (complete, partial hydatidiform mole and invasive mole), trophoblastic neoplasms (choriocarcinoma, PSTT and ETT) as well as non-neoplastic tumor-like lesions (exaggerated placental site, and placental site nodule)[31]. In this study, we demonstrated high expression levels of PD-L1 in syncytiotrophoblast from complete moles and choriocarcinomas. Moreover, PD-L1 immunoreactivity, although generally weak, was detected in the tumor cells morphologically corresponding to intermediate trophoblastic cells in choriocarcinoma, PSTT and ETT. Placental site nodules were negative for PD-L1. The above findings suggest that the expression pattern of PD-L1 is retained in GTD as in normal placenta.
Although this study reports the PD-L1/PD-1 immune checkpoint in syncytiotrophoblast, other checkpoint pathways should also be studied to determine if they also operate in human trophoblastic cells to suppress the immune response by modulating local immunity. Besides PD-1, the checkpoint molecules known so far include cytotoxic T cell antigen-4 (CTLA-4), T cell immunoglobulin mucin-3 (TIM-3), lymphocyte activation gene-3 (LAG-3), B- and T- cell lymphocyte attenuator (BTLA) which serve as negative regulators of activated cytotoxic T cells[32]. Future studies to demonstrate if there is a positive association between loss of these immune checkpoint molecules and infertility or spontaneous abortion would provide further support that they are important in immune tolerance during pregnancy.
In conclusion, this study provides new data showing that different trophoblastic populations may utilize different immune suppressive mechanisms to ensure tolerance of conceptus. Normal and neoplastic syncytiotrophoblast highly expresses PD-L1, a ligand for PD-1 immune checkpoint receptor, while intermediate trophoblastic cells express much lower PD-L1 and cytotrophoblast do not have detectable PD-L1 immunoreactivity. Thus, the PD-1 and PD-L1 pathway may represent one of the main mechanisms for syncytiotrophoblast that collaborates with HLA-G expressing intermediate trophoblastic cells to inhibit the function of activated T cells, resulting in an immunosuppressive microenvironment that protects the placenta and facilitates GTD tissues from escaping immune surveillance.
Acknowledgments
This study was supported by the Richard W. TeLinde Gynecologic Pathology Program, Department of Gynecology and Obstetrics, Johns Hopkins University.
References
- 1.Fraccaroli L, Alfieri J, Larocca L, et al. A potential tolerogenic immune mechanism in a trophoblast cell line through the activation of chemokine-induced T cell death and regulatory T cell modulation. Human reproduction. 2009;24:166–175. doi: 10.1093/humrep/den344. [DOI] [PubMed] [Google Scholar]
- 2.Singer G, Kurman RJ, McMaster M, et al. HLA-G immunoreactivity is specific for intermediate trophoblast in gestational trophoblastic disease and can serve as a useful marker in differential diagnosis. The American journal of surgical pathology. 2002;26(7):914–920. doi: 10.1097/00000478-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Tilburgs T, Evans JH, Crespo AC, et al. The HLA-G cycle provides for both NK tolerance and immunity at the maternal-fetal interface. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:13312–13317. doi: 10.1073/pnas.1517724112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pazmany L, Mandelboim O, Vales-Gomez M, et al. Protection from natural killer cell-mediated lysis by HLA-G expression on target cells. Science. 1996;274:792–795. doi: 10.1126/science.274.5288.792. [DOI] [PubMed] [Google Scholar]
- 5.Poehlmann TG, Schaumann A, Busch S, et al. Inhibition of term decidual NK cell cytotoxicity by soluble HLA-G1. American journal of reproductive immunology. 2006;56:275–285. doi: 10.1111/j.1600-0897.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 6.Long EO, Kim HS, Liu D, et al. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annual review of immunology. 2013;31:227–258. doi: 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Habicht A, Dada S, Jurewicz M, et al. A link between PDL1 and T regulatory cells in fetomaternal tolerance. Journal of immunology. 2007;179:5211–5219. doi: 10.4049/jimmunol.179.8.5211. [DOI] [PubMed] [Google Scholar]
- 8.Le Gal FA, Riteau B, Sedlik C, et al. HLA-G-mediated inhibition of antigen-specific cytotoxic T lymphocytes. International immunology. 1999;11:1351–1356. doi: 10.1093/intimm/11.8.1351. [DOI] [PubMed] [Google Scholar]
- 9.LeMaoult J, Caumartin J, Daouya M, et al. Immune regulation by pretenders: cell-to-cell transfers of HLA-G make effector T cells act as regulatory cells. Blood. 2007;109:2040–2048. doi: 10.1182/blood-2006-05-024547. [DOI] [PubMed] [Google Scholar]
- 10.Ban Y, Chang Y, Dong B, et al. Indoleamine 2,3-dioxygenase levels at the normal and recurrent spontaneous abortion fetal-maternal interface. The Journal of international medical research. 2013;41:1135–1149. doi: 10.1177/0300060513487642. [DOI] [PubMed] [Google Scholar]
- 11.Taglauer ES, Trikhacheva AS, Slusser JG, et al. Expression and function of PDCD1 at the human maternal-fetal interface. Biology of reproduction. 2008;79:562–569. doi: 10.1095/biolreprod.107.066324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gatalica Z, Snyder C, Maney T, et al. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2014;23:2965–2970. doi: 10.1158/1055-9965.EPI-14-0654. [DOI] [PubMed] [Google Scholar]
- 13.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. The New England journal of medicine. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 14.Patel SP, Kurzrock R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Molecular cancer therapeutics. 2015;14:847–856. doi: 10.1158/1535-7163.MCT-14-0983. [DOI] [PubMed] [Google Scholar]
- 15.D’Addio F, Riella LV, Mfarrej BG, et al. The link between the PDL1 costimulatory pathway and Th17 in fetomaternal tolerance. Journal of immunology. 2011;187:4530–4541. doi: 10.4049/jimmunol.1002031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shih Ie M. Gestational trophoblastic neoplasia--pathogenesis and potential therapeutic targets. The lancet oncology. 2007;8:642–650. doi: 10.1016/S1470-2045(07)70204-8. [DOI] [PubMed] [Google Scholar]
- 17.Benirschke K, Burton GJ, Baergen RN. Pathology of the human placenta. 6th. Springer; Heidelberg: 2012. Basic structure of the villous trees; pp. 55–100. [Google Scholar]
- 18.Shih IM, Mazur M, Kurman RJ. Gestational trophoblastic tumors and related tumro-like lesions. In: Kurman RJ, Ellenson LH, Ronnett BM, editors. Balustein's Pathology of the Female Genital Tract. Springer; Heidelberg: 2011. pp. 1075–1136. [Google Scholar]
- 19.Alijotas-Reig J, Llurba E, Gris JM. Potentiating maternal immune tolerance in pregnancy: a new challenging role for regulatory T cells. Placenta. 2014;35:241–248. doi: 10.1016/j.placenta.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Concha-Benavente F, Srivastava RM, Trivedi S, et al. Identification of the Cell-Intrinsic and -Extrinsic Pathways Downstream of EGFR and IFNgamma That Induce PD-L1 Expression in Head and Neck Cancer. Cancer research. 2016;76:1031–1043. doi: 10.1158/0008-5472.CAN-15-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tuncer ZS, Vegh GL, Fulop V, et al. Expression of epidermal growth factor receptor-related family products in gestational trophoblastic diseases and normal placenta and its relationship with development of postmolar tumor. Gynecologic oncology. 2000;77:389–393. doi: 10.1006/gyno.2000.5777. [DOI] [PubMed] [Google Scholar]
- 22.Murphy SP, Tayade C, Ashkar AA, et al. Interferon gamma in successful pregnancies. Biology of reproduction. 2009;80:848–859. doi: 10.1095/biolreprod.108.073353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sotnikova N, Voronin D, Antsiferova Y, et al. Interaction of decidual CD56+ NK with trophoblast cells during normal pregnancy and recurrent spontaneous abortion at early term of gestation. Scandinavian journal of immunology. 2014;80:198–208. doi: 10.1111/sji.12196. [DOI] [PubMed] [Google Scholar]
- 24.Bellucci R, Martin A, Bommarito D, et al. Interferon-gamma-induced activation of JAK1 and JAK2 suppresses tumor cell susceptibility to NK cells through upregulation of PD-L1 expression. Oncoimmunology. 2015;4:e1008824. doi: 10.1080/2162402X.2015.1008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rabreau M, Rouas-Freiss N, Landi M, et al. HLA-G expression in trophoblast cells is independent of embryonic development. Hum Immunol. 2000;61:1108–1112. doi: 10.1016/s0198-8859(00)00196-8. [DOI] [PubMed] [Google Scholar]
- 26.Sheu JJ, Shih Ie M. Clinical and biological significance of HLA-G expression in ovarian cancer. Semin Cancer Biol. 2007;17:436–443. doi: 10.1016/j.semcancer.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gregori S, Amodio G, Quattrone F, et al. HLA-G Orchestrates the Early Interaction of Human Trophoblasts with the Maternal Niche. Frontiers in immunology. 2015;6:128. doi: 10.3389/fimmu.2015.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajagopalan S, Long EO. A human histocompatibility leukocyte antigen (HLA)-G-specific receptor expressed on all natural killer cells. The Journal of experimental medicine. 1999;189:1093–1100. doi: 10.1084/jem.189.7.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ponte M, Cantoni C, Biassoni R, et al. Inhibitory receptors sensing HLA-G1 molecules in pregnancy: decidua-associated natural killer cells express LIR-1 and CD94/NKG2A and acquire p49, an HLA-G1-specific receptor. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:5674–5679. doi: 10.1073/pnas.96.10.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang SC, Li YH, Piao HL, et al. PD-1 and Tim-3 pathways are associated with regulatory CD8+ T-cell function in decidua and maintenance of normal pregnancy. Cell death & disease. 2015;6:e1738. doi: 10.1038/cddis.2015.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hui P, Baergen R, Cheung ANY, et al. Gestational trophoblastic disease. In: Kurman RJ, Carcangiu ML, Young RH, editors. WHO classification of tumorus of female reproductive organs. WHO; Lyon: 2014. pp. 155–168. [Google Scholar]
- 32.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature reviews Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]


