Abstract
An expression system has been established for the incorporation of selenomethionine into recombinant proteins produced from plasmids in Escherichia coli. Replacement of methionine by selenomethionine is demonstrated at the level of 100% for both T4 and E. coli thioredoxins. The natural recombinant proteins and the selenomethionyl variants of both thioredoxins crystallize isomorphously. Anomalous scattering factors were deduced from synchrotron X-ray absorption measurements of crystals of the selenomethionyl proteins. Taken with reference to experience in the structural analysis of selenobiotinyl streptavidin by the method of multiwavelength anomalous diffraction (MAD), these data indicate that recombinant selenomethionyl proteins analyzed by MAD phasing offer a rather general means for the elucidation of atomic structures.
Full text
PDF

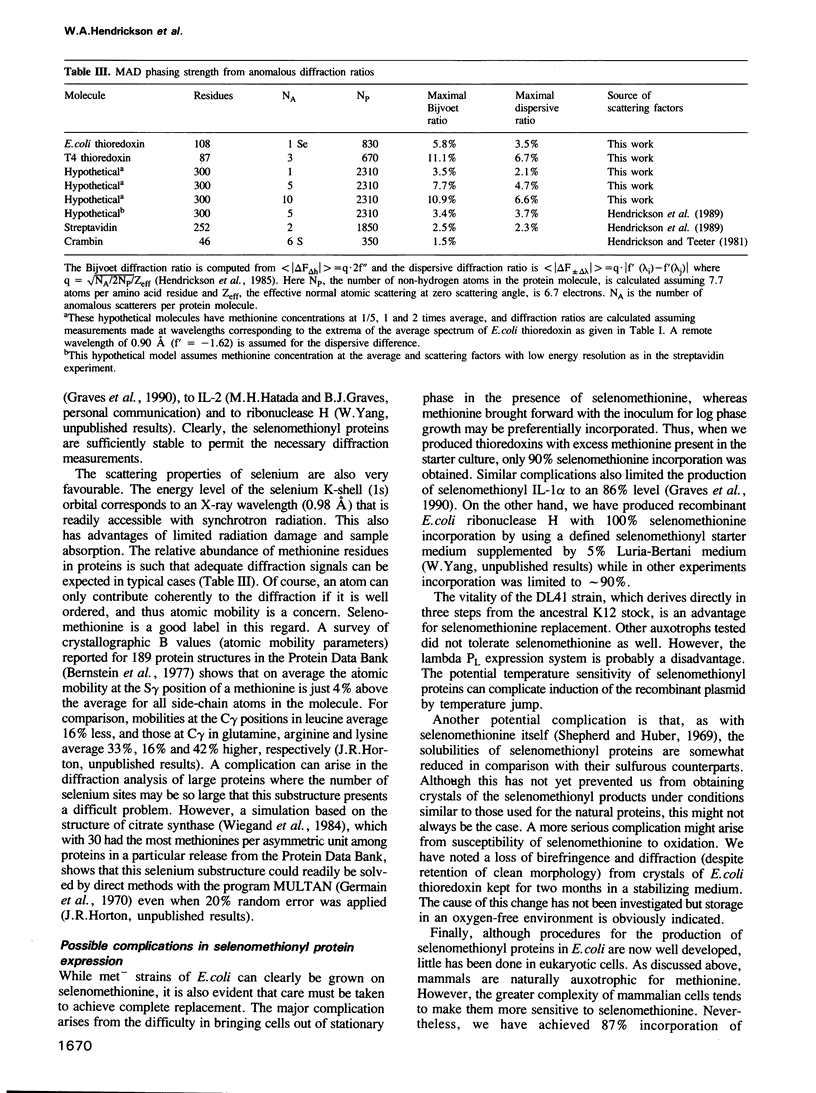


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- COWIE D. B., COHEN G. N. Biosynthesis by Escherichia coli of active altered proteins containing selenium instead of sulfur. Biochim Biophys Acta. 1957 Nov;26(2):252–261. doi: 10.1016/0006-3002(57)90003-3. [DOI] [PubMed] [Google Scholar]
- Chambers I., Frampton J., Goldfarb P., Affara N., McBain W., Harrison P. R. The structure of the mouse glutathione peroxidase gene: the selenocysteine in the active site is encoded by the 'termination' codon, TGA. EMBO J. 1986 Jun;5(6):1221–1227. doi: 10.1002/j.1460-2075.1986.tb04350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen K. C., McDougal J. S., Inacker R., Folena-Wasserman G., Arthos J., Rosenberg J., Maddon P. J., Axel R., Sweet R. W. A soluble form of CD4 (T4) protein inhibits AIDS virus infection. Nature. 1988 Jan 7;331(6151):82–84. doi: 10.1038/331082a0. [DOI] [PubMed] [Google Scholar]
- Epp O., Ladenstein R., Wendel A. The refined structure of the selenoenzyme glutathione peroxidase at 0.2-nm resolution. Eur J Biochem. 1983 Jun 1;133(1):51–69. doi: 10.1111/j.1432-1033.1983.tb07429.x. [DOI] [PubMed] [Google Scholar]
- Frank P., Licht A., Tullius T. D., Hodgson K. O., Pecht I. A selenomethionine-containing azurin from an auxotroph of Pseudomonas aeruginosa. J Biol Chem. 1985 May 10;260(9):5518–5525. [PubMed] [Google Scholar]
- Guss J. M., Merritt E. A., Phizackerley R. P., Hedman B., Murata M., Hodgson K. O., Freeman H. C. Phase determination by multiple-wavelength x-ray diffraction: crystal structure of a basic "blue" copper protein from cucumbers. Science. 1988 Aug 12;241(4867):806–811. doi: 10.1126/science.3406739. [DOI] [PubMed] [Google Scholar]
- Guyer M. S., Reed R. R., Steitz J. A., Low K. B. Identification of a sex-factor-affinity site in E. coli as gamma delta. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):135–140. doi: 10.1101/sqb.1981.045.01.022. [DOI] [PubMed] [Google Scholar]
- Hahn G. A., Brown J. W. Properties of a methionyl-tRNA synthetase from Sarcina lutea. Biochim Biophys Acta. 1967 Sep 12;146(1):264–271. doi: 10.1016/0005-2744(67)90093-9. [DOI] [PubMed] [Google Scholar]
- Hendrickson W. A., Pähler A., Smith J. L., Satow Y., Merritt E. A., Phizackerley R. P. Crystal structure of core streptavidin determined from multiwavelength anomalous diffraction of synchrotron radiation. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2190–2194. doi: 10.1073/pnas.86.7.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson W. A., Smith J. L., Phizackerley R. P., Merritt E. A. Crystallographic structure analysis of lamprey hemoglobin from anomalous dispersion of synchrotron radiation. Proteins. 1988;4(2):77–88. doi: 10.1002/prot.340040202. [DOI] [PubMed] [Google Scholar]
- Hendrickson W. A., Smith J. L., Sheriff S. Direct phase determination based on anomalous scattering. Methods Enzymol. 1985;115:41–55. doi: 10.1016/0076-6879(85)15006-8. [DOI] [PubMed] [Google Scholar]
- Holmgren A., Söderberg B. O., Eklund H., Brändén C. I. Three-dimensional structure of Escherichia coli thioredoxin-S2 to 2.8 A resolution. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2305–2309. doi: 10.1073/pnas.72.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin. 6. The amino acid sequence of the protein from escherichia coli B. Eur J Biochem. 1968 Dec 5;6(4):475–484. doi: 10.1111/j.1432-1033.1968.tb00470.x. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P., Theriot L. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagen products from DNA. Genetics. 1966 Jun;53(6):1119–1136. doi: 10.1093/genetics/53.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R. E., Criddle R. S. The isolation and properties of beta-galactosidase from Escherichia coli grown on sodium selenate. Biochim Biophys Acta. 1967 Aug 29;141(3):587–599. doi: 10.1016/0304-4165(67)90187-0. [DOI] [PubMed] [Google Scholar]
- LeMaster D. M. Nucleotide sequence and protein overproduction of bacteriophage T4 thioredoxin. J Virol. 1986 Sep;59(3):759–760. doi: 10.1128/jvi.59.3.759-760.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMaster D. M., Richards F. M. 1H-15N heteronuclear NMR studies of Escherichia coli thioredoxin in samples isotopically labeled by residue type. Biochemistry. 1985 Dec 3;24(25):7263–7268. doi: 10.1021/bi00346a036. [DOI] [PubMed] [Google Scholar]
- LeMaster D. M., Richards F. M. NMR sequential assignment of Escherichia coli thioredoxin utilizing random fractional deuteriation. Biochemistry. 1988 Jan 12;27(1):142–150. doi: 10.1021/bi00401a022. [DOI] [PubMed] [Google Scholar]
- Leinfelder W., Zehelein E., Mandrand-Berthelot M. A., Böck A. Gene for a novel tRNA species that accepts L-serine and cotranslationally inserts selenocysteine. Nature. 1988 Feb 25;331(6158):723–725. doi: 10.1038/331723a0. [DOI] [PubMed] [Google Scholar]
- Lunn C. A., Kathju S., Wallace B. J., Kushner S. R., Pigiet V. Amplification and purification of plasmid-encoded thioredoxin from Escherichia coli K12. J Biol Chem. 1984 Aug 25;259(16):10469–10474. [PubMed] [Google Scholar]
- MUDD S. H., CANTONI G. L. Selenomethionine in enzymatic transmethylations. Nature. 1957 Nov 16;180(4594):1052–1052. doi: 10.1038/1801052a0. [DOI] [PubMed] [Google Scholar]
- McConnell K. P., Hoffman J. L. Methionine-selenomethionine parallels in rat liver polypeptide chain synthesis. FEBS Lett. 1972 Jul 15;24(1):60–62. doi: 10.1016/0014-5793(72)80826-3. [DOI] [PubMed] [Google Scholar]
- Murthy H. M., Hendrickson W. A., Orme-Johnson W. H., Merritt E. A., Phizackerley R. P. Crystal structure of Clostridium acidi-urici ferredoxin at 5-A resolution based on measurements of anomalous X-ray scattering at multiple wavelengths. J Biol Chem. 1988 Dec 5;263(34):18430–18436. [PubMed] [Google Scholar]
- Normanly J., Masson J. M., Kleina L. G., Abelson J., Miller J. H. Construction of two Escherichia coli amber suppressor genes: tRNAPheCUA and tRNACysCUA. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6548–6552. doi: 10.1073/pnas.83.17.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd L., Huber R. E. Some chemical and biochemical properties of selenomethionine. Can J Biochem. 1969 Sep;47(9):877–881. doi: 10.1139/o69-137. [DOI] [PubMed] [Google Scholar]
- Sjöberg B. M., Holmgren A. Studies on the structure of T4 thioredoxin. II. Amino acid sequence of the protein and comparison with thioredoxin from Escherichia coli. J Biol Chem. 1972 Dec 25;247(24):8063–8068. [PubMed] [Google Scholar]
- Sjöberg B. M., Söderberg B. O. Thioredoxin induced by bacteriophage T4: crystallization and preliminary crystallographic data. J Mol Biol. 1976 Jan 25;100(3):415–419. doi: 10.1016/s0022-2836(76)80072-1. [DOI] [PubMed] [Google Scholar]
- Stadtman T. C. Specific occurrence of selenium in enzymes and amino acid tRNAs. FASEB J. 1987 Nov;1(5):375–379. doi: 10.1096/fasebj.1.5.2445614. [DOI] [PubMed] [Google Scholar]
- Söderberg B. O., Sjöberg B. M., Sonnerstam U., Brändén C. I. Three-dimensional structure of thioredoxin induced by bacteriophage T4. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5827–5830. doi: 10.1073/pnas.75.12.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton L. K., Templeton D. H. Biaxial tensors for anomalous scattering of X-rays in selenolanthionine. Acta Crystallogr A. 1988 Nov 1;44(Pt 6):1045–1051. doi: 10.1107/s0108767388007810. [DOI] [PubMed] [Google Scholar]
- Wiegand G., Remington S., Deisenhofer J., Huber R. Crystal structure analysis and molecular model of a complex of citrate synthase with oxaloacetate and S-acetonyl-coenzyme A. J Mol Biol. 1984 Mar 25;174(1):205–219. doi: 10.1016/0022-2836(84)90373-5. [DOI] [PubMed] [Google Scholar]
- Zinoni F., Birkmann A., Stadtman T. C., Böck A. Nucleotide sequence and expression of the selenocysteine-containing polypeptide of formate dehydrogenase (formate-hydrogen-lyase-linked) from Escherichia coli. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4650–4654. doi: 10.1073/pnas.83.13.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]