Abstract
Agrin is utilized by motor neurons to stimulate the LRP4-MuSK receptor in muscles for neuromuscular junction formation. Recent studies of cancer identify novel functions of the LRP4-MuSK pathway. Agrin may act as a mechanotransduction signal in the extracellular matrix to coordinate the cross talk between the LRP4-MuSK pathway and integrin-focal adhesion pathway. Ensuing YAP activation promotes hepatocellular carcinoma. In this spotlight, we discuss the implications of the converged pathways in NMJ formation and liver cancers.
Keywords: Agrin, LRP4, MuSK, YAP, neuromuscular junction, liver cancer
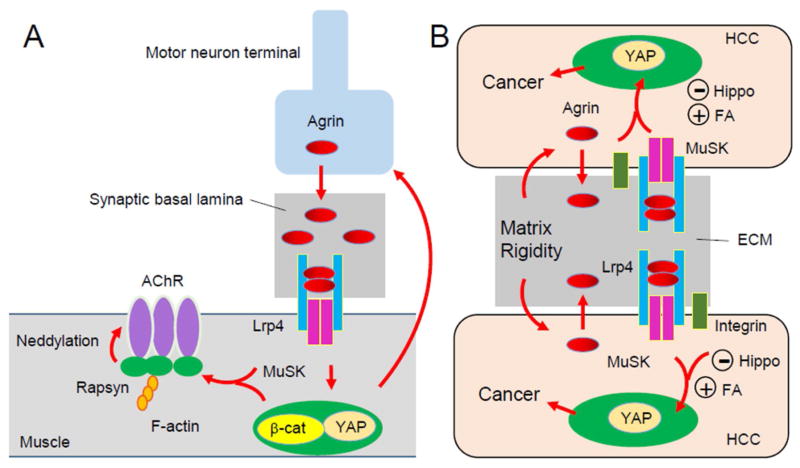
The neuromuscular junction (NMJ) is a synapse formed between motor neurons and skeletal muscle fibers, critical for the control of muscle contraction. The neurotransmitter acetylcholine (ACh) is released from the nerve terminals to activate the ACh receptor (AChR) heavily clustered on the postjunctional membrane, to initiate muscle contraction. In between the nerve terminal and postjunctional membrane is synaptic basal lamina, a form of extracellular matrix (ECM) that is enriched with proteins essential for NMJ formation and maintenance. NMJ formation and maintenance requires Agrin, a large glycoprotein that is released from motor neurons(1). Agrin induces AChR clusters in cultured muscles and direct NMJ formation in vivo. Agrin is enriched in the synaptic basal lamina and acts by binding to the extracellular region of LRP4 (low-density lipoprotein receptor related protein 4), a transmembrane protein of the LDL receptor family(2, 3). The binding leads to the formation of an Agrin-LRP4 heterodimer, and subsequently, two heterodimers form a tetramer that is necessary to activate MuSK (muscle specific kinase)(4). Albeit a type I receptor tyrosine kinase, MuSK does not bind to Agrin directly, but its kinase activity is necessary for AChR clustering and NMJ formation (Figure 1). The role of the Agrin-LRP4-MuSK pathway in NMJ formation is supported by genetic evidence that mutant mice lacking any of the three genes do not form NMJs(1). However, signaling downstream of MuSK is not well understood. AChR clustering requires rapsyn, an adaptor protein thought to bridge the AChR with the cytoskeleton(1). Recent data suggests rapsyn may serve as an E3 ligase to promote the neddylation of the AChR, a step necessary for AChR clustering and NMJ formation(5) (Figure 1).
Figure 1.
Agrin signaling and YAP in neuromuscular junction formation (NMJ) and liver cancer development. A, Agrin is released from motor nerve terminals to induce AChR clusters and to direct NMJ formation. YAP and β-catenin in muscles regulate both post- and pre-synaptic differentiation. B, Agrin serves as a mechanotransduction signal to activate YAP by suppressing the Hippo pathway and by stimulating integrin-focal adhesion (FA) and thus to promote liver cancer development.
Agrin, LRP4 and MuSK are not restricted at the NMJ; rather, they are expressed in a number of tissues or organs and have been implicated in the development and function of the brain, kidney, mammary glands and bones(6, 7). A recent proteomic study by Hong and colleagues discovered that Agrin is overexpressed and secreted in hepatocellular carcinoma (HCC)(8). Agrin enhances cellular proliferation, migration and oncogenic signaling of tumor cells in a manner dependent on LRP4 and MuSK. A follow-up paper recently published in Cell Reports reveals intriguing underlying signaling mechanisms of Agrin in liver cancer development(9). They showed depleting Agrin by antibody or knockdown of Agrin induces phosphorylation and cytoplasmic retention of YAP (Yes-associated protein), a transcription co-activator that has been implicated in tumorigenesis and reduced YAP-TEAD4-dependent transcription. The data suggest that Agrin promotes nuclear YAP accumulation, interaction with TEAD, and expression of YAP target genes. Agrin activation of YAP requires both the LRP4-MuSK signal and integrin-focal adhesion. In fact, in the absence of endogenous Agrin, neither pathway is sufficient to activate YAP, indicating Agrin serves a converging point of the two pathways. Two intracellular mechanisms are shown for YAP activation by Agrin: negating the Hippo pathway (via suppressing Merlin and LATS1/2) and/or activating Rho and ROCK and increasing actin stress fibers. Remarkably, in response to stiff extracellular matrix (ECM), HCC cells express more Agrin, LRP4 and MuSK; conversely, Agrin increases ECM stiffness (indicated by intense fibrillary collagen staining) and collagen contraction and induces actin stress fibers and nuclear YAP localization. Further mechanistic study suggests that YAP’s mechanoresponsiveness to ECM stiffness requires Agrin activation of both MuSK and integrin pathways (such as the focal adhesion kinase FAK). Together, these observations support a model where Agrin may serve as a mechanotransduction signal to activate YAP by suppressing the Hippo pathway and by stimulating integrin-focal adhesion and thus to promote liver cancer development (Figure 1). This model is supported by compelling cell biology data and will inspire future studies of HCC in mutant mice without Agrin, LRP4, or MuSK.
Not surprisingly, many of the pathways or molecules in HCC have been implicated in AChR clustering or NMJ formation including integrin, Rho, and FAK(1). Much like focal adhesions, the NMJ is enriched with synaptic basal lamina in the ECM. The postjunctional muscle membrane enviginate to form junctional folds whose function remains unclear. Perhaps they could promote ECM stiffness to increase signaling events necessary for postjunctional differentiation. Recent evidence indicates that Yap is required for proper formation and regeneration of the NMJ(10). Agrin also suppresses Yap phosphorylation and increases Yap nuclear translocation muscle cells (Zhao et al., data not shown). An interesting question is whether YAP also serves as a sensor of stiff EMC or mechanoresponsiveness at the NMJ. YAP regulation of the NMJ formation seems to involve β-catenin; in particular, muscle YAP and β-catenin direct a retrograde signaling for the development of motor nerve terminals(10). β-catenin is a known oncogenic factor for multiple types of cancers, including liver cancer. It would be of interest to examine if YAP regulation of β-catenin is conserved in liver tumor cells, downstream of the Agrin-LRP4-MuSK pathway.
At the NMJ, Agrin is utilized by motor neurons to direct muscle differentiation(1). In HCC development, it is unclear whether Agrin is produced homogenously in all cancer cells or specifically in a group of cancer cells. Therefore it is unclear whether Agrin acts in autonomous or non-autonomous manner. Is Agrin expressed more in a selected group of cancer cells or cancer stem cells? For therapeutic consideration, it would be ideal to identify the cancer cells that express Agrin or do so in tumor originating stage. The NMJ occupies only 0.01–0.1% of entire muscle surface and Agrin is restricted in the synaptic basal lamina(1); consequently, Agrin-initiated signaling is extremely “local” at the NMJ, compared with cancer cells that are surrounded by ECM. In HCC, Agrin, via the FAK-ILK-PAK1 pathway, confines Hippo signaling to focal adhesions. Interestingly, PAK1 has been implicated in Agrin-induced AChR clustering(1). Finally, LRP4 is a 200 kDa protein, with more than 1700 amino acid residues outside of the cell membrane. Besides Agrin, many molecules have been claimed as ligands of LRP4 including APP, DKK, SOST, and Wnt(6). LRP4 is thought to negatively regulate the canonical Wnt/β-catenin signaling, which suggests that LRP4 may be a tumor suppressor. These observations highlight the need for further investigation of the Agrin-LRP4-MuSK pathway in tumor development as well as NMJ formation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wu H, Xiong WC, Mei L. To build a synapse: signaling pathways in neuromuscular junction assembly. Development. 2010;137(7):1017–33. doi: 10.1242/dev.038711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim N, Stiegler AL, Cameron TO, Hallock PT, Gomez AM, Huang JH, Hubbard SR, Dustin ML, Burden SJ. Lrp4 is a receptor for Agrin and forms a complex with MuSK. Cell. 2008;135(2):334–42. doi: 10.1016/j.cell.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang B, Luo S, Wang Q, Suzuki T, Xiong WC, Mei L. LRP4 serves as a coreceptor of agrin. Neuron. 2008;60(2):285–97. doi: 10.1016/j.neuron.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zong Y, Zhang B, Gu S, Lee K, Zhou J, Yao G, Figueiredo D, Perry K, Mei L, Jin R. Structural basis of agrin-LRP4-MuSK signaling. Genes & development. 2012;26(3):247–58. doi: 10.1101/gad.180885.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li L, Cao Y, Wu H, Ye X, Zhu Z, Xing G, Shen C, Barik A, Zhang B, Xie X, et al. Enzymatic Activity of the Scaffold Protein Rapsyn for Synapse Formation. Neuron. 2016;92(5):1007–19. doi: 10.1016/j.neuron.2016.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen C, Xiong WC, Mei L. LRP4 in neuromuscular junction and bone development and diseases. Bone. 2015;80:101–8. doi: 10.1016/j.bone.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Sun XD, Li L, Liu F, Huang ZH, Bean JC, Jiao HF, Barik A, Kim SM, Wu H, Shen C, et al. Lrp4 in astrocytes modulates glutamatergic transmission. Nature neuroscience. 2016;19(8):1010–8. doi: 10.1038/nn.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakraborty S, Lakshmanan M, Swa HL, Chen J, Zhang X, Ong YS, Loo LS, Akincilar SC, Gunaratne J, Tergaonkar V, et al. An oncogenic role of Agrin in regulating focal adhesion integrity in hepatocellular carcinoma. Nature communications. 2015;6:6184. doi: 10.1038/ncomms7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakraborty S, Njah K, Pobbati AV, Lim YB, Raju A, Lakshmanan M, Tergaonkar V, Lim CT, Hong W. Agrin as a Mechanotransduction Signal Regulating YAP through the Hippo Pathway. Cell reports. 2017;18(10):2464–79. doi: 10.1016/j.celrep.2017.02.041. [DOI] [PubMed] [Google Scholar]
- 10.Zhao K, Shen C, Lu Y, Huang Z, Li L, Rand CD, Pan J, Sun XD, Tan Z, Wang H, et al. Muscle Yap is a Regulator of Neuromuscular Junction Formation and Regeneration. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2017 doi: 10.1523/JNEUROSCI.2934-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]