Abstract
The aim of this study is to assess characteristic impedance (Zc) of the proximal aorta in young and middle-aged individuals with isolated systolic hypertension (ISH). Zc is an index of aortic stiffness relative to aortic size. In the Dallas Heart Study, 2,001 untreated participants 18 to 64 years of age (mean age 42.3years, 44% black race) were divided into the following groups based on office blood pressure (BP) measurements: 1) optimal BP (systolic BP [SBP]<120 and [DBP]<80 mmHg; n=837); 2) prehypertension (SBP 120–139 and/or DBP 80–89 mmHg; n=821); 3) ISH (SBP ≥140 and DBP <90 mmHg; n=121); 4) isolated diastolic hypertension (IDH: SBP<140 and DBP≥90 mmHg; n=44); and 5) systolic-diastolic hypertension (SDH: SBP≥140 and DBP≥90 mmHg; n=178). Zc, aortic arch pulse wave velocity (PWV), and minimum ascending aortic size were quantified using cardiovascular magnetic resonance. In multivariable-adjusted linear models, Zc was highest in the ISH group compared with the optimal BP, IDH, or SDH groups (103.2±4.0 vs. 68.3±2.1, 75.4±6.0, and 88.9±4.8 dyne*sec/cm5, respectively; all P<0.05). The Zc-ISH association did not differ by race. Aortic PWV was highest in the ISH group compared with the optimal BP, IDH, or SDH groups (6.3±0.3 vs. 4.3±0.1, 4.4±0.4 and 5.5±0.3 m/s, respectively; all P<0.05), whereas aortic size was similar across groups (all P > 0.2). Results were similar in a subgroup of 1,551 participants 18 to 49 years of age. In a multiracial population-based sample, we found evidence of a mismatch between proximal aortic stiffness and diameter in young and middle-aged adults with ISH.
Keywords: Isolated systolic hypertension, young and middle-aged adults, characteristic impedance (Zc), and arterial stiffness
Introduction
Isolated systolic hypertension (ISH), defined as systolic blood pressure (SBP) ≥140 mmHg and diastolic BP (DBP) <90 mmHg, is a common hypertension subtype in young and middle-aged adults.1–3 The prevalence has been increasing over the last decade in the United States.1–3 Nevertheless, whether ISH in young and middle-aged adults represents “pseudo” or “spurious” hypertension is debated.4–6 We have recently shown that in Americans under 50 years of age, ISH was associated with an increased risk of cardiovascular disease (CVD) mortality compared with optimal BP.7 However, the pathophysiology of ISH in those under 50 years of age is incompletely elucidated.4–6
An abnormal relationship between aortic flow and pressure has been demonstrated in older adults with high SBP.8,9 Stiffening of the proximal aortic wall contributes to an exaggerated rise in SBP, which may be offset by aortic remodeling, via which the diameter of the aorta increases (so-called outward remodeling). If this outward remodeling does not occur, the characteristic impedance of the aorta (Zc) increases.8–10 Zc is an index of aortic stiffness relative to aortic size. Higher Zc has been reported in older hypertensive adults with wide pulse pressure.8,9,11–14 However, the Zc-ISH association remains to be characterized in young and middle-aged adults.
The Dallas Heart Study (DHS) is a community-based cohort; investigators conducted a comprehensive assessment of aortic structure and function using cardiovascular magnetic resonance (CMR). Using data from the DHS, we assessed Zc in young and middle-aged adults with ISH. We also assessed whether the Zc-ISH association differs between participants 18 to 49 years of age and those 50 to 64 years of age.
Methods
The DHS is a multiracial and multiethnic (black, white, and Hispanic), probability-based population study, recruiting Dallas (Texas, USA) County residents 18 to 64 years of age. Details of the DHS have been described previously.15 Briefly, the DHS phase 1 consisted of three sequential visits between 2000 and 2002, including two home visits and a clinic visit. During the first two visits, a survey was conducted through a face-to-face interview and self-reported ethnicity was obtained. In the third visit, 2,795 participants underwent CMR imaging studies.16 All participants provided written informed consent, and the University of Texas Southwestern Medical Center institutional review board approved the protocol.
BP measurements and BP phenotype definitions
After 5 minutes of rest in seated position, trained medical staff obtained five BP measurements with one minute intervals, using the appropriate cuff size in the office setting. The average of five BP readings was used for the current analyses. An automated oscillometric device (Series #52,000, Welch Allyn, Arden, North Carolina) was used.17 Staff were trained to choose the appropriate cuff size for each participant and wrap the cuff around the arm with the center of the bladder over the brachial artery.
Participants were classified into 5 mutually exclusive categorical BP groups: 1) the optimal BP group (SBP <120 mm Hg and DBP <80 mm Hg); 2) the prehypertension group (SBP 120 to 139 mm Hg and DBP 80 to 89 mm Hg, SBP 120 to 139 mm Hg and DBP <80 mm Hg, or SBP <120 mm Hg and DBP 80 to 89 mm Hg); 3) the ISH group; 4) the isolated diastolic hypertension (IDH) group (SBP <140 mm Hg and DBP ≥90 mm Hg); and 5) the systolic-diastolic hypertension (SDH) group (SBP ≥140 mm Hg and DBP ≥90 mm Hg).18 Mean arterial pressure was defined as (SBP + 2×DBP)/3 and pulse pressure as SBP minus DBP. To exclude confounding influence of antihypertensive drugs on aortic structure and function, participants who received antihypertension medication were excluded in this study.
Variable definitions
Data on smoking, physical activity, medication use, clinical history of CVD, and fasting laboratory values were collected using standardized protocols.15 Physical activity was assessed based upon self-reported frequency and type of leisure-time physical activity, which were converted to metabolic equivalence units (METs) and reported as MET-min/week.19 Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula.20 Details of the assessment of visceral fat using MR imaging are described in the online-only Data Supplement.
Cardiovascular Magnetic Resonance (CMR)
Participants underwent CMR using a 1.5 Tesla whole-body system (Intera, Philips Medical Systems, Best, Netherlands), with a four-element surface array coil. Four BP measurements were obtained during CMR examinations and used to calculate CMR-derived measures (see online-only Data Supplement). Ascending aortic distensibility and aortic arch pulse wave velocity (PWV) were assessed using a breath-hold, velocity-encoded, phase-contrast gradient echo sequence; the images were acquired perpendicular to the course of the ascending aorta 4 cm above the aortic valve plane.21 The ascending aorta was imaged in cross-section with the following conditions: temporal resolution of < 40 ms; 256 × 256 matrix; 34-cm field of view; 20° flip angle; 10 ms repetition time; 5 ms echo time; through-plane velocity encoding of ± 150 cm/sec; and slice thickness 8 mm. The images were acquired using prospective ECG gating. The time-velocity curve was interpolated to a temporal resolution of 10 ms by using a cubic spline for analysis. The aortic arch was also evaluated with an oblique sagittal, double inversion-recovery spin echo image (“candy cane” view) with the following conditions: 33 cm field of view; electrocardiographically gated repetition time; 5.3 ms echo time; 32 echo train length. The images were analyzed using the MASS/FLOW (Medis, Leiden, Netherlands).22 Area contour of the ascending aorta was automatically traced through all phases of the cardiac cycle, and its maximum and minimum cross-sectional areas were measured. Minimum ascending aortic area was defined as aortic size in this study. The operator assessing the area contours was blind to participant’s clinical characteristics. Details of our technique have been described previously.16
Calculation of characteristic impedance (Zc) and aortic arch PWV
Time-velocity flow curves of the ascending and descending aorta were produced using MASS/FLOW (Medis, Leiden, Netherlands). Transit time was calculated as the time difference between the ascending and descending upstroke velocities at half-maximum velocity. Arch distance was determined by drawing a freehand line through the center of the aorta between the ascending and descending aorta position where flow measurements were made.13 Aortic arch PWV (d/t) was calculated by dividing arch distance (d) by transit time (t), with larger velocities indicating greater aortic stiffness.23 Aortic arch PWV was also estimated using the Bramwell-Hill equation.24 The Pearson correlation between flow-based PWV and Bramwell-Hill-based PWV was 0.51 (P<0.0001). Zc is a measure of the opposition of the circulation to oscillatory flow input. We estimated Zc, by using the water hammer equation, as the product of aortic arch PWV and blood density divided by the ascending aortic area in diastole. Blood density was assumed to be fixed at 1.06 gram/cm3 as previously described.13,25 Details of the assessments of ascending aortic distensibility and compliance, stroke volume, and systemic vascular resistance are described in the online-only Data Supplement.
Analytical sample
Of the 2,759 participants, we excluded 196 participants whose CMR image quality was missing or insufficient for interpretation, 166 participants with prevalent CVD, and 396 participants who received antihypertensive medication, leaving a sample of 2,001 participants for analysis.
Statistical analyses
Descriptive statistics are presented as mean and standard deviation (SD) or proportion where appropriate. Multiple pairwise comparisons of the demographic variables and clinical characteristics among the categorical BP groups were conducted using Jonckheere-Terpstra or Fisher’s Exact tests. Multivariable-adjusted linear regression models were used to compare CMR-derived measures (as continuous variables) across the categorical BP groups; P values were obtained by Bonferroni multiple pairwise comparison tests. The primary exposure was ISH (vs. other BP categories). The primary outcome was Zc, and secondary outcomes included other CMR-derived measures, such as aortic arch PWV, aortic distensibility and compliance, aortic size, stroke volume, and systemic vascular resistance. Possible violations of the assumptions of multiple linear regression were examined by visual inspection of the distribution of residuals through both histograms and normal probability plots. We further checked for deviations of linearity and homoscedasticity by visually inspecting scatterplots of standardized residuals by standardized predicted values. In addition, we assessed variance inflation factors to examine the possibility of multicollinearity and values >4 were considered to indicate collinearity. Covariates included demographic variables: age, sex, race, and clinical characteristics: height, weight, heart rate, smoking, physical activity, total cholesterol, fasting glucose, and eGFR. These covariates were selected a priori because they have known correlations with BP levels18 and aortic impedance.26 We also assessed whether the associations between ISH and outcomes were attenuated after adjustment for mean arterial pressure and visceral fat, known potential contributors to cardiovascular risk in persons with ISH.4–6 Next, analyses for heterogeneity of effect between ISH and outcomes by age (<50 or ≥50 years) or race (black or non-black individuals) were performed, with inclusion of additive interaction terms. Lastly, although the categorical BP groups are a clinically relevant classification,18 this approach may be arbitrary. Therefore, we assessed the continuous association between pulse pressure and CMR-derived measures in separate models. All statistical analyses were performed with SAS version 9.4 (SAS Institute, Cary, North Carolina). Statistical significance was defined by a P value <0.05 using 2-sided tests.
Results
Demographic variables and clinical characteristics by the categorical BP groups are shown in Table 1. Of the included 2,001 participants, 6 % (n=121) were classified as the ISH group, 2 % (n=44) as the IDH group, and 9 % (n=178) as the SDH group. In the ISH group, half were women (n=64), 64% were African Americans (n=38), and 36% were 18 to 49 years of age (n=43). Higher age, higher proportion of male sex, black race, and prevalent diabetes, and higher mean visceral fat were observed in the ISH group than the optimal BP group. Resting heart rate was similar between the ISH group and the optimal BP group. Results were similar in the subgroup of 1,551 participants 18 to 49 years of age (Supplementary Table S1 and S2). The mean ± SD of age for participants 18 to 49 years of age was 38.5±6.3. For those 50 to 64 years of age, it was 55.3±3.9.
Table 1.
Clinical characteristics of study cohort by categorical BP groups among participants 18 to 64 years of age (n=2,001)
| Descriptive variable | Optimal BP (n=837) | Prehypertension (n=821) | ISH (n=121) | IDH (n=44) | SDH (n=178) |
|---|---|---|---|---|---|
| Age, years | 39.3±8.6 | 43.2±8.7 *‡|| | 50.9±8.3 *†§|| | 42.4±6.8 ‡ | 46.3±8.7 *†‡ |
| Men, n (%) | 33.8 | 60.1*‡ | 47.1*† | 43.2 | 50.0 * |
| Ethnicity, % | |||||
| Non-Hispanic white | 33.5 | 36.9 | 26.5 | 31.8 | 26.4 |
| African American | 36.9 | 44.8* | 63.6*† | 52.3 | 61.8 *† |
| Hispanic | 26.6 | 16.6* | 9.9* | 6.8* | 10.1 * |
| Other | 3.0 | 1.7 | 0§ | 9.1‡ | 1.7 |
| Height, cm | 165.3±10.1 | 170.0±10.2 * | 168.4±9.4 * | 167.3±9.9 | 168.8±10.7 * |
| Body weight, kg | 76.1±17.8 | 86.2±19.3 *|| | 87.7±20.6 * | 89.8±18.2 * | 91.5±20.7 *† |
| Body mass index, kg/m2 | 27.9±6.2 | 30.0±6.5 *|| | 30.9±6.7 * | 32.1±6.1 * | 32.3±7.9 *† |
| Diabetes mellitus, % | 3.6 | 7.6* | 11.6* | 6.8 | 10.1* |
| Current smoker, % | 26.0 | 27.4 | 26.5 | 31.8 | 36.2† |
| Physical activity, MET-min/week | 477.8±747.3 | 476.4±872.6 | 434.2±618.6 | 292.8±380.7 | 370.7±618.2 |
| Total cholesterol, mg/dL | 174.1±36.8 | 184.0±38.8 * | 182.8±41.5 | 183.3±38.6 | 188.6±42.9 * |
| High-density lipoprotein cholesterol, mg/dL | 51.5±14.2 | 49.0±15.1 * | 50.6±15.6 | 47.2±11.6 | 47.7±14.8 * |
| Fasting glucose, mg/dL | 91.9±21.2 | 100.5±40.2 *|| | 103.6±36.9 * | 103.4±50.7 | 110.5±67.0 *† |
| eGFR, mL/min/1.73 m2 | 103.2±22.0 | 99.1±20.3 * | 100.6±22.0 | 98.6±25.2 | 99.1±26.0 |
| Office BP measurements | |||||
| SBP, mmHg | 110.6±6.4 | 127.7±5.4 *‡§|| | 148.1±7.9 *†§|| | 135.5±3.0 *†‡|| | 155.9±14.0 *†‡§ |
| DBP, mmHg | 70.0±5.5 | 79.7±5.0 *‡§|| | 84.2±4.3 *†§|| | 91.9±1.8 *†‡|| | 96.2±4.7 *†‡§ |
| Mean arterial pressure, mmHg | 83.5±5.3 | 95.7±4.3 *‡§|| | 105.5±3.9 *†|| | 106.5±1.8 *†|| | 116.1±6.7 *†‡§ |
| Pulse pressure, mmHg | 40.7±4.8 | 47.9±5.8 *‡§|| | 63.9±9.0 *†§|| | 43.5±3.0 *†‡|| | 59.7±12.4 *†‡§ |
| Heart rate, bpm | 73.6±10.2 | 76.3±11.2 *§ | 74.2±11.9§ | 83.4±12.1 *†‡|| | 77.5±12.5 *§ |
| BP measurements during MRI scan | |||||
| SBP, mmHg | 115.3±10.0 | 130.1±13.0 *‡§|| | 151.1±17.1 *†§|| | 136.1±10.8 *†‡|| | 155.8±20.2 *†‡§ |
| DBP, mmHg | 75.0±7.0 | 82.6±7.8 *‡§|| | 89.3±8.4 *†§|| | 90.7±6.0 *†‡|| | 96.4±8.7 *†‡§ |
| Mean arterial pressure, mmHg | 88.5±7.5 | 98.4±8.8 *‡§|| | 109.9±9.9 *†|| | 105.8±7.1 *†|| | 116.2±11.6 *†‡§ |
| Pulse pressure, mmHg | 40.3±6.5 | 47.6±9.1 *‡§|| | 61.8±14.3 *† | 45.4±7.3 *‡|| | 59.4±15.0 *†§ |
| Heart rate, bpm | 65.2±9.5 | 67.9±10.5 *§|| | 68.8±10.9 *§ | 75.2±12.3 *†‡|| | 70.3±11.7 *†§ |
| Adiposity variable | |||||
| VAT, kg | 1.73±0.78 | 2.27±0.97 *|| | 2.28±0.90 * | 2.53±0.93 * | 2.50±1.02 *† |
Data are expressed as the means ± SD or percentage. Office BP measurements were calculated from the average of 5 BP measures taken during the in-office visit. Participants were stratified into 5 mutually exclusive categorical BP groups: 1) optimal BP (SBP <120 mm Hg and DBP <80 mm Hg); 2) prehypertension (SBP 120 to 139 mm Hg and DBP 80 to 89 mm Hg, SBP 120 to 139 mm Hg and DBP <80 mm Hg, or SBP <120 mm Hg and DBP 80 to 89 mm Hg); 3) ISH (SBP ≥140 mm Hg and DBP <90 mm Hg); 4) IDH (SBP <140 mm Hg and DBP ≥90 mm Hg); and 5) SDH (SBP ≥140 mm Hg and DBP ≥90 mm Hg). Multiple comparisons among BP categories were conducted using Jonckheere-Terpstra (for continuous variables) or Fisher’s Exact tests (for categorical variables). P values were obtained by Bonferroni multiple pairwise comparison tests, and statistical significance was defined as P <0.05.
P<0.05 vs. optimal BP;
P<0.05 vs. prehypertension;
P<0.05 vs. ISH;
P<0.05 vs. IDH;
P<0.05 vs. SDH.
ISH indicates isolated systolic hypertension; IDH, isolated diastolic hypertension; SDH, systolic and diastolic hypertension; SBP, systolic blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; VAT, visceral adipose tissue.
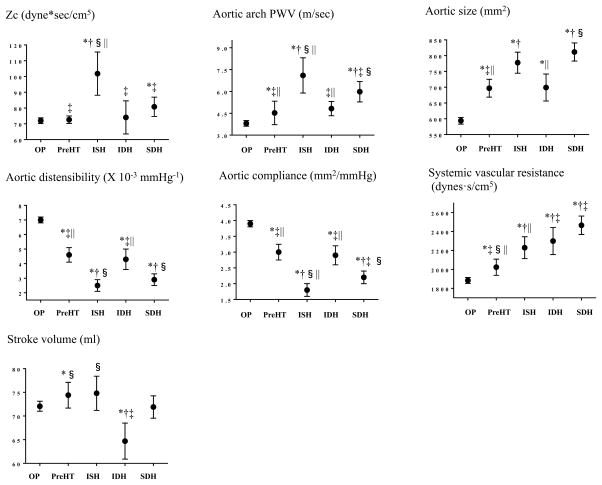
Figure 1 shows non-adjusted CMR-derived measures by the categorical BP groups. Zc and aortic arch PWV were highest in the ISH group. Aortic size was higher, whereas aortic distensibility and compliance were lower in the ISH group compared with the optimal BP group. Systemic vascular resistance was higher in the ISH group compared with the optimal BP group, whereas it was lower when compared to that in the SDH group. Stroke volume was similar between the ISH group and the optimal BP group, whereas it was lowest in the IDH group. Results were consistent across subgroups defined by age (<50 or ≥50 years: Supplementary Figure S2) and race (Figure S3).
Figure 1. Cardiovascular magnetic resonance-derived measures by the categorical BP groups.
Means (95% CIs) in cardiovascular magnetic resonance-derived measures by each BP group are shown. Univariate linear regression models were used and P values were obtained by Bonferroni multiple comparison tests. Statistical significance was defined as P <0.05. *P<0.05 vs. optimal BP; †P<0.05 vs. prehypertension; ‡P<0.05 vs. ISH; §P<0.05 vs. IDH; ||P<0.05 vs. SDH.
OP indicates optimal BP; PreHT, prehypertension; ISH, isolated systolic hypertension; IDH, isolated diastolic hypertension; SDH, systolic and diastolic hypertension.
Table 2 shows the results of linear regression models examining the associations between CMR-derived measures in the ISH group and the other categorical BP groups. With adjustments for covariates, Zc and aortic arch PWV were highest, whereas aortic compliance was lowest in the ISH group (Model 1 in Table 2). Additional adjustments by visceral fat and mean arterial pressure did not change the results (Model 2 and 3 in Table 2). Aortic size in the ISH group was larger than in the optimal BP group, but smaller than in the SDH group (Model 1 in Table 2). After the model was adjusted for mean arterial pressure, the differences in aortic size between the ISH group and other categorical BP groups were no longer significant (Model 3 in Table 2). Results were similar when aortic arch PWV was estimated using the Bramwell-Hill equation (Supplementary Table S3).
Table 2.
CMR-derived measures by categorical BP groups among participants 18 to 64 years of age
| Hemodynamic variables | Model | Optimal BP (n=837) | Prehypertension (n=821) | ISH (n=121) | IDH (n=44) | SDH (n=178) |
|---|---|---|---|---|---|---|
| Proximal aortic characteristic impedance (Zc), dyne* sec/cm5 | 1 | 72.4±13.6 | 74.3±1.3‡ | 97.7±3.4*†§|| | 70.0±5.6‡ | 79.0±28.4‡ |
| 2 | 72.3±13.7 | 74.3±1.3‡ | 98.2±3.5*†§|| | 70.0±5.6‡ | 79.3±2.9‡ | |
| 3 | 68.3±2.1 | 75.4±1.4‡|| | 103.2±4.0*†§|| | 75.4±6.0‡ | 88.9±4.8*†‡ | |
| Aortic compliance, mm2/mmHg | 1 | 3.7±0.03 | 3.1±0.04*‡|| | 2.2±0.1*†§ | 3.0±0.2*‡|| | 2.2±0.1*†§ |
| 2 | 3.7±0.04 | 3.1±0.04*‡|| | 2.2±0.1*†§ | 3.0±0.2*‡|| | 2.4±0.1*†§ | |
| 3 | 3.5±0.1 | 3.1±0.04*‡ | 21.0±1.0*†§ | 3.3±0.2‡|| | 2.8±0.1*§ | |
| Aortic distensibility, × 10−3 mmHg−1 | 1 | 6.3±0.1 | 4.9±0.1*‡|| | 3.7±0.2*† | 4.6±0.3*|| | 3.6±0.2*†§ |
| 2 | 6.3±0.1 | 4.9±0.1*‡|| | 3.7±0.2*† | 4.6±0.3*|| | 3.5±0.2*†§ | |
| 3 | 5.6±0.1 | 5.1±0.1* | 4.5±0.2* | 5.4±0.3 | 5.0±0.3 | |
| Aortic arch pulse wave velocity, m/s | 1 | 4.2±0.1 | 4.6±0.1‡|| | 6.4±0.2*†§|| | 4.5±0.4‡|| | 5.6±0.2*†‡§ |
| 2 | 4.2±0.1 | 4.5±0.4‡|| | 6.4±0.2*†§ | 4.6±0.1‡|| | 5.7±0.2*†§ | |
| 3 | 4.3±0.1 | 4.6±0.1‡|| | 6.3±0.3*†§ | 4.4±0.4‡ | 5.5±0.3*† | |
| Aortic size, mm2 | 1 | 641.7±5.2 | 670.2±5.0*|| | 692.1±13.1*|| | 697.4±21.3 | 759.3±10.8*†‡ |
| 2 | 641.8±5.2 | 670.3±5.0*|| | 689.7±13.1*|| | 698.4±21.3 | 760.8±10.9*†‡ | |
| 3 | 682.9±7.9 | 659.8±5.2 | 638.1±15.0 | 643.3±22.5 | 662.4±17.9 | |
| Left ventricular stroke volume, ml | 1 | 73.4±0.5 | 73.2±0.5§ | 75.4±1.3§ | 66.3±2.1*†‡ | 70.9±1.1 |
| 2 | 73.2±0.5 | 73.3±0.5§ | 75.3±1.3§ | 66.9±2.1*†‡ | 71.1±1.1 | |
| 3 | 73.2±0.8 | 73.3±0.5§ | 75.3±1.5§ | 66.9±2.2†‡ | 71.2±1.8 | |
| Systemic vascular resistance, dynes·s/cm5 | 1 | 1828.2±16.6 | 2061.6±16.0*§|| | 2175.2±42.0*§|| | 2406.3±68.4*†‡ | 2523.2±34.7*†‡ |
| 2 | 1834.1±16.6 | 2061.9±16.0*‡|| | 2177.0±42.0*|| | 2386.2±68.3*† | 2520.1±35.0*†‡ |
Adjusted means (±SE) in CMR-derived measures by each BP group are shown. Each CMR-derived measure was analyzed as the outcome in separate models. Multivariable-adjusted linear regression models were used and multiple pairwise comparisons among the BP groups were conducted. As adjustment factors, Model 1 included demographic variables (age, sex, and race) + clinical characteristics at visit 3 (height, weight, heart rate, smoking, physical activity, total cholesterol, fasting glucose, and eGFR), Model 2 included demographic variables + clinical characteristics at visit 3 +VAT, and Model 3 included demographic variables + clinical characteristics at visit 3 +VAT+ mean arterial pressure. Mean arterial pressure was incorporated in the formula of systemic vascular resistance, and therefore, model 3 in systemic vascular resistance did not exist. P values were obtained by Bonferroni multiple pairwise comparison tests, and statistical significance was defined as P <0.05.
P<0.05 vs. optimal BP;
P<0.05 vs. prehypertension;
P<0.05 vs. ISH;
P<0.05 vs. IDH;
P<0.05 vs. SDH.
CMR indicates cardiovascular magnetic resonance; ISH, isolated systolic hypertension; IDH, isolated diastolic hypertension; SDH, systolic and diastolic hypertension; SE, standard error; SBP, systolic blood pressure; DBP, diastolic blood pressure; VAT, visceral adipose tissue; eGFR, estimated glomerular filtration rate.
There was an interaction between ISH (vs. optimal BP) and age (<50 or ≥50 years) in association with Zc and aortic arch PWV (all P<0.02). The differences in Zc and aortic arch PWV parameters between the ISH group and the optimal BP group were greater in participants 50 to 64 years of age than in those 18 to 49 years of age (Supplementary Table S4 and S5). The interaction term between ISH (vs. optimal BP) and race in association with aortic arch PWV, but not with Zc, was significant (P=0.04). The difference in aortic arch PWV between ISH and optimal BP groups was greater in black compared with non-black individuals (Supplementary Table S6 and S7). When we analyzed the associations of pulse pressure as a continuous variable with CMR-derived measures (Table 3), higher mean arterial pressure, greater aortic arch PWV and left ventricular stroke volume, and smaller aortic size were each associated with higher pulse pressure.
Table 3.
Associations between brachial pulse pressure and CMR-derived measures in participants 18 to 64 years of age (n=2,001)
| CMR-derived measures | β (SE) | P-value | Model R2, % |
|---|---|---|---|
| Mean arterial pressure | 5.5 (0.2) | <0.0001 | 54.3 |
| Aortic arch PWV | 2.4 (1.7) | <0.0001 | |
| Aortic size | −1.1 (1.1) | <0.0001 | |
| Left ventricular stroke volume | 1.8 (0.2) | <0.0001 |
β = unstandardized regression coefficient; model R2 = a measure for the model prediction. The table shows adjusted β coefficient (SE) associated with 1 SD increases of each exposure. The one-SD increment of each exposure is as follows: mean arterial pressure, 11.2 mmHg; aortic arch PWV, 2.6 m/s; aortic size, 180.6 mm2; left ventricular stroke volume, 16.2 ml. Mean arterial pressure, aortic arch PWV, aortic size, and left ventricular stroke volume entered into the same model, and the following variables were used as covariates: demographic variables (age, sex, and race) + clinical characteristics (height, weight, heart rate, smoking, physical activity, total cholesterol, fasting glucose, and eGFR) +VAT. Statistical significance was defined as P <0.05.
Discussion
There are 3 major findings in this study. First, in this community-based multiracial and multiethnic cohort, the ISH group had greater Zc and aortic stiffness than the optimal BP, IDH, and SDH groups. The differences were independent of mean arterial pressure and visceral obesity. The results were similar when analyzed in individual 18 to 49 years of age only. Second, aortic size was similar between the ISH group and the other categorical BP groups after adjustment for mean arterial pressure, implicating higher aortic stiffness as the predominant factor for higher Zc in the ISH group. Third, the higher Zc seen in the ISH group vs. the optimal BP group was more significant among participants 50 to 64 years of age than in those 18 to 49 years of age. The Zc-ISH association did not differ by race.
Hemodynamics have been assessed in young adults with ISH, and findings have been inconsistent.4–6,27,28 A study from the Netherlands demonstrated normal aortic PWV with higher pressure amplification in young adults with ISH (n=57; mean age 28 years), suggesting ISH in the young as a spurious form of hypertension.27 In contrast, a population study from the United Kingdom illustrated higher aortic stiffness and cardiac output in young adults with ISH (n=109; mean age 20 years) compared to normotensive individuals.28 However, these studies have not assessed aortic impedance or size in young adults with ISH. We highlighted that among individuals 18 to 49 years of age, Zc was higher in the ISH group than in the optimal BP, IDH, and SDH groups. Increased Zc has been reportedly associated with adverse cardiovascular outcomes in a community-based cohort, independent of aortic stiffness.29 Further investigation is needed to determine whether higher pulsatile arterial pressure driven by a mismatch between proximal aortic stiffness and diameter contributes to incident CVD events in young adults with ISH.
A univariate analysis revealed that aortic size was greater in the ISH group compared with the optimal BP group. However, the aortic size was similar in the ISH group compared with the optimal BP group after adjustments for distending pressure (i.e., mean arterial pressure). Conversely, greater aortic arch PWV and lower aortic distensibility in the ISH group compared with the optimal BP group persisted even after adjustment for mean arterial pressure. This suggests that greater proximal aortic stiffness may predominantly contribute to ISH in young and middle-aged adults. Outward remodeling of the proximal aorta may limit increases in pulsatile pressure as the aorta stiffens with age.30 This adaptation may be limited in young and middle-aged adults with ISH because of alterations in aortic mechanical properties.31 Previous studies in older hypertensive adults have indicated that higher pulse pressure was associated both higher wall stiffness and smaller aortic diameter.9,13 In our study, higher pulse wave velocity, smaller aortic size, and larger stroke volume was also significantly associated with higher pulse pressure, suggesting a similar pathophysiologic process in elevated pulse pressure between young/middle-aged adults and older adults.
Significant interactions were found between age (<50 or ≥50 years) and ISH (vs. optimal BP) in association with Zc and aortic arch PWV, but not with aortic size. Further investigation is needed to determine whether the pathophysiology of ISH differs by age, or whether ISH develops on a pathophysiological continuum from young to middle adulthood. Those with IDH or SDH were more likely than those with optimal BP to have higher systemic vascular resistance. Zc and aortic stiffness in the SDH group, but not in the IDH group, were higher than in the optimal BP group. Collectively, higher systemic vascular resistance appears to be a major contributor to high DBP, whereas aortic stiffness and a mismatch between aortic stiffness and diameter are major contributors to high SBP. Determining whether ISH in young and middle-aged adults has a unique pathophysiology (e.g., whether a de novo stiffening of the proximal aorta antedates the development of peripheral vascular remodeling) is worthy of further research.
Highest Zc was observed in both black and non-black individuals in the ISH group. Conversely, the difference in aortic arch PWV between the ISH group and the optimal BP group was larger in black compared with non-black individuals. In young and middle-aged adults, black individuals are more likely than whites to have greater aortic stiffness, independent of known cardiovascular risk factors.32,33 In models for aortic arch PWV, the difference in PWV between the ISH group and the optimal BP group remained unchanged in black individuals, even after an adjustment for mean arterial pressure. The difference was, however, attenuated in non-black individuals. High aortic stiffness in black individuals with ISH may be attributable to factors beyond high BP, including genetic, environmental, and neurohumoral factors.34,35
Strengths of this study include the well-characterized, community-based multiethnic cohort, participants who received antihypertensive medication were excluded, the standardized data collection protocols of office BP measurements, and application of a comprehensive standardized CMR test battery. However, there are limitations. First, since this is an observational study, we are unable to determine causality in the findings. Second, the results require careful interpretation because of the small sample size of ISH. Third, we used flow-based PWV derived from CMR, which might overestimate the arrival time of the flow (consequently, underestimating PWV) as the pulse wave reflection at more distal locations within the aorta blunts the upstroke of flow. However, results were similar when PWV was estimated using the Bramwell-Hill equation. Fourth, we used peripheral but not central pulse pressure to estimate aortic distensibility. Therefore, aortic compliance and distensibility may be underestimated in the optimal BP group who are more likely to have lowest central systolic BP and pulse pressure. Fifth, trained technicians obtained 5 BP readings after participants had been seated for 5 minutes in this study. Nevertheless, BP phenotypes defined based upon a single office visit may not be accurate.
Perspective
Among young and middle-aged adults, individuals with ISH have greatest aortic stiffness and mismatch between proximal aortic stiffness and diameter than those in other BP groups. These findings challenge the classic view that ISH in young and middle-aged adults represents “pseudo” or “spurious” hypertension. Further studies are required to determine the biological mechanisms and effective interventions to improve aortic function in young and middle-aged adults with ISH.
Supplementary Material
Novelty and Significance.
1) What Is New
In a community-based multiracial and multiethnic cohort, we found evidence of a mismatch between proximal aortic stiffness and diameter in young and middle-aged adults with isolated systolic hypertension (ISH).
2) What Is Relevant?
ISH is a common hypertension subtype in young and middle-aged adults. Nevertheless, whether ISH in young and middle-aged adults represents “pseudo” or “spurious” hypertension is debated. The pathophysiology of ISH in young and middle-aged adults is also incompletely elucidated.
3) Summary
We highlight that among young and middle-aged adults, individuals with ISH have greatest aortic stiffness and mismatch between proximal aortic stiffness and diameter than those in other BP groups.
Acknowledgments
Funding Sources
The Dallas Heart Study was supported by a grant from the Reynolds Foundation and grant UL1TR001105 from the National Center for Advancing Translational Sciences of the National Institutes of Health. Dr. Yano is supported by the AHA Strategically Focused Research Network (SFRN) Fellow Grant. Dr. Neeland is supported by grant K23DK106520 from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institute of Health and by the Dedman Family Scholarship in Clinical Care from UT Southwestern. Dr. Mitchell is support by a grant (HL126136) from the National Institutes of Health. Dr. Vongpatanasin is supported by R01HL113738, UT Southwestern O’Brien Kidney Center grant P30 DK079328 and Norman & Audrey Kaplan Chair in Hypertension Research. This work is also supported by an AHA SFRN grant to UT Southwestern and to Northwestern University and a grant to Northwestern University from the National Center for Advancing Translational Sciences of the National Institutes of Health.
Footnotes
Disclosures:
Dr Mitchell is the owner of Cardiovascular Engineering, Inc, a company that develops and manufactures devices to measure vascular stiffness, serves as a consultant to and receives honoraria from Novartis, Merck, and Servier, and is funded by research grants from Novartis and the National Institutes of Health. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Franklin SS, Jacobs MJ, Wong ND, L’Italien GJ, Lapuerta P. Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension. 2001;37:869–874. doi: 10.1161/01.hyp.37.3.869. [DOI] [PubMed] [Google Scholar]
- 2.Grebla RC, Rodriguez CJ, Borrell LN, Pickering TG. Prevalence and determinants of isolated systolic hypertension among young adults: the 1999–2004 US National Health And Nutrition Examination Survey. J Hypertens. 2010;28:15–23. doi: 10.1097/HJH.0b013e328331b7ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu X, Rodriguez CJ, Wang K. Prevalence and trends of isolated systolic hypertension among untreated adults in the United States. J Am Soc Hypertens. 2015;9:197–205. doi: 10.1016/j.jash.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McEniery CM, Franklin SS, Cockcroft JR, Wilkinson IB. Isolated Systolic Hypertension in Young People Is Not Spurious and Should Be Treated: Pro Side of the Argument. Hypertension. 2016;68:269–275. doi: 10.1161/HYPERTENSIONAHA.116.06547. [DOI] [PubMed] [Google Scholar]
- 5.Lurbe E, Redon J. Isolated Systolic Hypertension in Young People Is Not Spurious and Should Be Treated: Con Side of the Argument. Hypertension. 2016;68:276–280. doi: 10.1161/HYPERTENSIONAHA.116.06548. [DOI] [PubMed] [Google Scholar]
- 6.Yano Y, Lloyd-Jones DM. Isolated Systolic Hypertension in Young and Middle-aged Adults. Curr Hypertens Rep. 2016;18:78. doi: 10.1007/s11906-016-0686-x. [DOI] [PubMed] [Google Scholar]
- 7.Yano Y, Stamler J, Garside DB, Daviglus ML, Franklin SS, Carnethon MR, Liu K, Greenland P, Lloyd-Jones DM. Isolated systolic hypertension in young and middle-aged adults and 31-year risk for cardiovascular mortality: the Chicago Heart Association Detection Project in Industry study. J Am Coll Cardiol. 2015;65:327–335. doi: 10.1016/j.jacc.2014.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell GF, Conlin PR, Dunlap ME, Lacourcière Y, Arnold JM, Ogilvie RI, Neutel J, Izzo JL, Jr, Pfeffer MA. Aortic diameter, wall stiffness, and wave reflection in systolic hypertension. Hypertension. 2008;51:105–111. doi: 10.1161/HYPERTENSIONAHA.107.099721. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell GF, Lacourcière Y, Ouellet JP, Izzo JL, Jr, Neutel J, Kerwin LJ, Block AJ, Pfeffer MA. Determinants of elevated pulse pressure in middle-aged and older subjects with uncomplicated systolic hypertension: the role of proximal aortic diameter and the aortic pressure-flow relationship. Circulation. 2003;108:1592–1598. doi: 10.1161/01.CIR.0000093435.04334.1F. [DOI] [PubMed] [Google Scholar]
- 10.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316:1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 11.Dujardin JP, Stone DN. Characteristic impedance of the proximal aorta determined in the time and frequency domain: a comparison. Med Biol Eng Comput. 1981;19:565–568. doi: 10.1007/BF02442770. [DOI] [PubMed] [Google Scholar]
- 12.Lucas CL, Wilcox BR, Ha B, Henry GW. Comparison of time domain algorithms for estimating aortic characteristic impedance in humans. IEEE Trans Biomed Eng. 1988;35:62–68. doi: 10.1109/10.1337. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell GF, Gudnason V, Launer LJ, Aspelund T, Harris TB. Hemodynamics of increased pulse pressure in older women in the community-based Age, Gene/Environment Susceptibility-Reykjavik Study. Hypertension. 2008;51:1123–1128. doi: 10.1161/HYPERTENSIONAHA.107.108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Segers P, Rietzschel ER, De Buyzere ML, Vermeersch SJ, De Bacquer D, Van Bortel LM, De Backer G, Gillebert TC, Verdonck PR Asklepios investigators. Noninvasive (input) impedance, pulse wave velocity, and wave reflection in healthy middle-aged men and women. Hypertension. 2007;49:1248–1255. doi: 10.1161/HYPERTENSIONAHA.106.085480. [DOI] [PubMed] [Google Scholar]
- 15.Victor RG, Haley RW, Willett DL, Peshock RM, Vaeth PC, Leonard D, Basit M, Cooper RS, Iannacchione VG, Visscher WA, Staab JM, Hobbs HH Dallas Heart Study Investigators. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93:1473–1480. doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 16.Maroules CD, Khera A, Ayers C, Goel A, Peshock RM, Abbara S, King KS. Cardiovascular outcome associations among cardiovascular magnetic resonance measures of arterial stiffness: the Dallas heart study. J Cardiovasc Magn Reson. 2014;16:33. doi: 10.1186/1532-429X-16-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yano Y, Vongpatanasin W, Ayers C, Turer A, Chandra A, Carnethon MR, Greenland P, de Lemos JA, Neeland IJ. Regional Fat Distribution and Blood Pressure Level and Variability: The Dallas Heart Study. Hypertension. 2016;68:576–583. doi: 10.1161/HYPERTENSIONAHA.116.07876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 19.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O’Brien WL, Bassett DR, Jr, Schmitz KH, Emplaincourt PO, Jacobs DR, Jr, Leon AS. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King KS, Chen KX, Hulsey KM, McColl RW, Weiner MF, Nakonezny PA, Peshock RM. White matter hyperintensities: use of aortic arch pulse wave velocity to predict volume independent of other cardiovascular risk factors. Radiology. 2013;267:709–717. doi: 10.1148/radiol.13121598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Geest RJ, Niezen RA, van der Wall EE, de Roos A, Reiber JH. Automated measurement of volume flow in the ascending aorta using MR velocity maps: evaluation of inter- and intraobserver variability in healthy volunteers. J Comput Assist Tomogr. 1998;22:904–911. doi: 10.1097/00004728-199811000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Dogui A, Redheuil A, Lefort M, DeCesare A, Kachenoura N, Herment A, Mousseaux E. Measurement of aortic arch pulse wave velocity in cardiovascular MR: comparison of transit time estimators and description of a new approach. J Magn Reson Imaging. 2011;33:1321–1329. doi: 10.1002/jmri.22570. [DOI] [PubMed] [Google Scholar]
- 24.Bramwell JC, Hill AV. The velocity of the pulse wave in man. Proc R Soc Lond B. 1922;93:298–306. [Google Scholar]
- 25.Goel A, Maroules CD, Mitchell GF, Peshock R, Ayers C, McColl R, Vongpatanasin W, King KS. Ethnic Difference in Proximal Aortic Stiffness: An Observation from the Dallas Heart Study. JACC Cardiovasc Imaging. 2017;10:54–61. doi: 10.1016/j.jcmg.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T American Heart Association Council on Hypertension. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement From the American Heart Association. Hypertension. 2015;66:698–722. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hulsen HT, Nijdam ME, Bos WJ, Uiterwaal CS, Oren A, Grobbee DE, Bots M. Spurious systolic hypertension in young adults; prevalence of high brachial systolic blood pressure and low central pressure and its determinants. J Hypertens. 2006;24:1027–1033. doi: 10.1097/01.hjh.0000226191.36558.9c. [DOI] [PubMed] [Google Scholar]
- 28.McEniery CM, Yasmin, Wallace S, Maki-Petaja K, McDonnell B, Sharman JE, Retallick C, Franklin SS, Brown MJ, Lloyd RC, Cockcroft JR, Wilkinson IB ENIGMA Study Investigators. Increased stroke volume and aortic stiffness contribute to isolated systolic hypertension in young adults. Hypertension. 2005;46:221–226. doi: 10.1161/01.HYP.0000165310.84801.e0. [DOI] [PubMed] [Google Scholar]
- 29.Cooper LL, Rong J, Benjamin EJ, Larson MG, Levy D, Vita JA, Hamburg NM, Vasan RS, Mitchell GF. Components of hemodynamic load and cardiovascular events: the Framingham Heart Study. Circulation. 2015;131:354–361. doi: 10.1161/CIRCULATIONAHA.114.011357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Virmani R, Avolio AP, Mergner WJ, Robinowitz M, Herderick EE, Cornhill JF, Guo SY, Liu TH, Ou DY, O’Rourke M. Effect of aging on aortic morphology in populations with high and low prevalence of hypertension and atherosclerosis. Comparison between occidental and Chinese communities. Am J Pathol. 1991;139:1119–1129. [PMC free article] [PubMed] [Google Scholar]
- 31.Mitchell GF. Arterial Stiffness and Wave Reflection: Biomarkers of Cardiovascular Risk. Artery Res. 2009;3:56–64. doi: 10.1016/j.artres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhuiyan AR, Li S, Li H, Chen W, Srinivasan SR, Berenson GS. Distribution and correlates of arterial compliance measures in asymptomatic young adults: the Bogalusa Heart Study. Am J Hypertens. 2005;18(5 Pt 1):684–691. doi: 10.1016/j.amjhyper.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell GF. Arterial stiffness and hypertension. Hypertension. 2014;64:13–18. doi: 10.1161/HYPERTENSIONAHA.114.00921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su S, Wang X, Kapuku GK, Treiber FA, Pollock DM, Harshfield GA, McCall WV, Pollock JS. Adverse childhood experiences are associated with detrimental hemodynamics and elevated circulating endothelin-1 in adolescents and young adults. Hypertension. 2014;64:201–207. doi: 10.1161/HYPERTENSIONAHA.113.02755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Avolio AP, Van Bortel LM, Boutouyrie P, Cockcroft JR, McEniery CM, Protogerou AD, Roman MJ, Safar ME, Segers P, Smulyan H. Role of pulse pressure amplification in arterial hypertension: experts’ opinion and review of the data. Hypertension. 2009;54:375–383. doi: 10.1161/HYPERTENSIONAHA.109.134379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.