Abstract
Purpose
To present the long-term and final report of a phase III trial designed to assess dose-response relationship for postoperative radiotherapy (PORT) and pathologic risk groups in head and neck cancer.
Materials/Methods
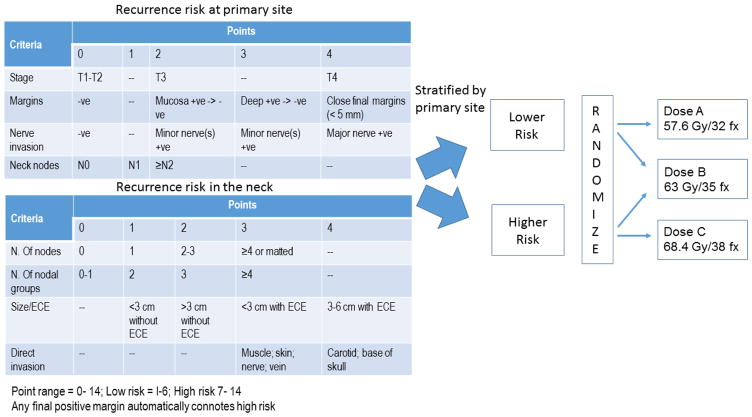
Patients who underwent primary surgery for AJCC stage III or IV squamous cell carcinoma of the oral cavity, oropharynx, hypopharynx, or larynx, and requiring PORT were eligible. Patients’ primary sites and involved necks were independently assigned to higher or lower risk categories based on a cumulative point score representing increasing risk of recurrence. The sites in the lower risk group were randomized to receive 57.6Gy or 63Gy, and in the higher risk group to 63Gy or 68.4Gy, all at 1.8Gy per fraction.
Results
A total of 264 patients were included. The actuarial 5-year locoregional control (LRC) rate was 67%. A second primary cancer was documented in 27%. The 5- and 10-year freedom from distant metastasis rates were 64% and 60% while 5- and 10-year OS rates were 32% and 20%, respectively. There was no statistically significant difference in tumor control between different dose levels in both the lower and higher risk groups. On multivariate analysis, non-Caucasian race (P =0.0003), positive surgical margin (P=0.009); extracapsular extension (ECE, P=0.01) and treatment package time (TPT) ≥85 days (P=0.002) were independent correlates of worse LRC while, age ≥ 57 years (P< 0.0001); positive surgical margins (P=0.01); ECE (P=0.026) and TPT ≥85 days (P=0.003) were independently associated with worse OS.
Conclusions
This long-term report of PORT delivered at 1.8 Gy/day to total doses of 57.6 Gy to 68.4 Gy, without chemotherapy for HNSCC demonstrated that increasing dose did not significantly improve tumor control. On multivariate analysis, the only significant treatment variable was TPT. The results also confirm that positive surgical margins and/or nodal ECE remain the most significant predictive pathological factors.
INTRODUCTION
Gilbert Fletcher introduced the concept of post-operative radiotherapy (PORT) for head and neck squamous cell carcinoma (HNSCC) in the 1950s to address observed high rates of postoperative recurrences.[1] Subsequent reports confirmed that PORT can improve disease control, and probably survival, but there were no uniform or prospectively validated guidelines for fractional or total radiation dose selection.[2–6] Fletcher recommended a radiation dose of 60Gy in the 2nd edition of his Textbook of Radiotherapy.[7] He posited in this era antedating segmental imaging that an incremental dose of 10Gy was required to overcome relative hypoxia in the operative bed, whereas non-operated, non-tumor bearing volumes could be reliably controlled with just 50Gy. The University of Florida recommended that the dose be increased to 65Gy at 1.7–1.8Gy/fraction, and that higher-risk areas, including positive margins receive a definitive intent dose of 70Gy.[8] Thus, despite recognizing a benefit to PORT, questions regarding the ideal fractional dose, total dose, and the need for additional dose if clinical and pathologic features suggested a greater risk of recurrence remained unanswered. To address these question, Investigators at The University of XXXXXXXXXX Cancer Center evaluated the dose-response relationship for HNC PORT with this prospective, randomized study between 1983 and 1991. The question regarding fractional dose (i.e. 1.8 Gy vs. 2 Gy) was deemed less important, so these scientists focused on total dose which varied dependent on a risk stratification formula based on the then current understanding of clinical, surgical, and pathologic factors.
A preliminary report of the first 240 of 302 patients was published in 1993 with a median follow-up of 22 months.[9] The authors recommended a dose of 57.6Gy to “intermediate-risk” volumes, and 63Gy for “high-risk” volumes delivered at 1.8Gy/fraction, 5-days/week. More complete long-term follow up data was collected, but not reported. They recognized that treatment time factors were important, and, in fact, addressed this in their next trial randomizing patients to standard or accelerated fractionated PORT.
We analyzed the 20+ year follow up on the original data set for the complete cohort, and also performed further un-planned exploratory statistical analyses in the light of current understanding of risk-factors to better appreciate the time-dose-response relationships for PORT in HNSCC. Our specific aims include:
Long-term and final reporting of this prospective, risk-adaptive trial
Confirmation of tumor risk factors affecting outcomes after PORT without chemotherapy
Identification of time-dose dependent outcomes in PORT without chemotherapy
Identification of treatment package time (TPT, from surgery to completion of PORT) thresholds for PORT in HNSSC
Methods
The details of the study design, inclusion criteria, risk stratification, and radiotherapy techniques were described in details in the first report.[9] In brief, eligible patients had HNSCC in primary tumors of the oral cavity, oropharynx, hypopharynx, larynx, or neck nodes of unknown primary that required PORT. Patients had their primary tumor and disease in the neck assessed separately, and each was assigned to either a lower or higher dose risk groups using a separate empiric point system for each (Figure 1). The dose to be treated to sites in the lower risk group was randomized between dose levels A (57.6 Gy/32 fractions/6 weeks) and B (63 Gy in 35 fractions over 7 weeks), while the dose to be planned for sites in the higher risk group was randomized to dose level B or C (68.4 Gy/38 fractions/7.5 weeks). Note that dose level A was initially 52.2–54Gy, but after the first interim analysis showed several early recurrences, the minimal dose was changed to 57.6Gy. Tobacco data was not collected.
Figure 1.
Risk scores and study design.
Risk scoring criteria for primary site and neck. Patients’ primary sites and involved necks were independently assigned to higher or lower risk categories based on the cumulative point score representing lower versus higher risk of recurrence then randomized to different dose levels.
Statistical analysis
The Kaplan-Meier product limit test was used to calculate, from the date of surgery, rates of 5- and 10-year overall survival (OS), local control (LC), regional control (RC), locoregional control (LRC), freedom from distant metastasis (FDM), cancer–specific survival (CSS, death from primary cancer coded as an event and censoring all others). Comparison of disease control by risk category and dose level was done based on intention to treat assignment.
Competing risk of failure and death were performed with Weibull parametric fitting using cause of failure and cause of death, respectively as a competing risk variable for uncensored data. Univariate Cox proportional hazards assessments were performed to determine the correlation of patient, disease, and treatment-related variables with disease control and survival endpoints. For continuous variables that demonstrated a significant association with an outcome in initial univariate analysis, a subsequent recursive partitioning analysis (RPA) was implemented to identify appropriate cutoff point, and then continuous variables were converted to binary levels based on the resultant cutoff to be included in the final model. Eventually, all the following variables were examined: age (<57 vs ≥ 57 years), sex (male or female), race (Caucasian vs others), T stage (T1-T2 Vs T3-T4), N stage (N0 vs. N+), margin status (positive versus others “negative, converted and close”), number of positive nodes (0–1 vs ≥2), number of nodal groups (0–1 vs ≥2), extracapsular extension (positive vs others), nerve invasion (positive vs negative), nodal direct invasion (positive vs negative), biologically effective dose (BED), as a linear quadratic (LQ)-based formula with an overall time factor included for both primary and neck RT[10], and treatment package time TPT (<85 vs ≥85 days). Age and TPT binary levels were RPA-driven. The number of nodal groups were recorded but not the named neck levels. Multivariate survival analyses were done with Cox regression and included all variables with p < 0.30. Survival distributions between various risk groups were compared with log-rank test. Data were analyzed using JMP 11.2 Pro statistical software (SAS Institute, Cary, NC), and statistical significance was determined using a specified non–Bonferroni-corrected α of 0.05.
Results
Patients
From 1983 to 1991, 301 patients were enrolled. Of those, 26 were excluded because of interval disease recurrence after surgery and prior to radiation start, 8 were excluded because of refusal to start radiation, and 3 because of major protocol violation leaving 264 patients included in the current analysis. Median age was 61 years (range 34–86) and 195 (74%) were men. Table 1 summarizes patients and disease characteristics.
Table 1.
Patients and disease characteristics
| Characteristic | N. (%) |
|---|---|
|
| |
| Age | |
| Median (range) | 61 (34–86) |
|
| |
| Sex | |
| Male | 197 (75) |
| Female | 67 (25) |
|
| |
| Ethnicity | |
| Caucasian | 205 (78) |
| African American | 30 (11) |
| Hispanic | 28 (11) |
| Others | 1 (<1) |
|
| |
| Primary site | |
| Oral cavity | 74 (28) |
| Oropharynx | 49 (19) |
| Hypopharynx | 47 (18) |
| Larynx | 92 (35) |
| Unknown | 2 (<1) |
|
| |
| T stage | |
| T0 | 2 (<1) |
| T1 | 4 (1) |
| T2 | 50 (19) |
| T3 | 143 (55) |
| T4 | 65 (25) |
|
| |
| N stage | |
| N0 | 107 (41) |
| N1 | 51 (19) |
| N2 | 67 (25) |
| N3 | 39 (15) |
|
| |
| Surgical margins | |
| Negative | 124 (47) |
| Close | 67 (26) |
| +ve -> −ve | 44 (18) |
| Positive | 24 (9) |
| N/A | 2 (<1) |
|
| |
| Extracapsular extension | |
| No nodal disease | 61 (28) |
| Negative | 75 (24) |
| Positive | 128 (48) |
|
| |
| Nerve invasion | |
| Absent | 195 (74) |
| Present | 67 (26) |
| N/A | 2 (<1) |
Overall oncologic and survival data
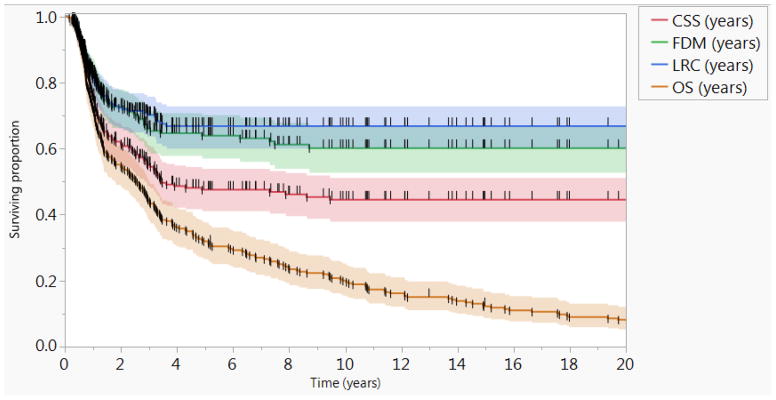
At the time of study final analysis, 7 patients were alive approximately 25 years following study closure, 4 patients were lost to follow-up, and 253 patients were deceased. The actuarial 5- and 10-year OS rates were 32% and 20%, respectively; and the actuarial 5- and 10-year CSS rates were 48% and 46%, respectively. Seventy-five patients (28%) had documented local and/or regional failure; 64 (85%) of those recurrences occurred in the first two years of follow up. The actuarial 5-year LRC rate was 67%, and there were no events beyond. There were 83 (31%) total distant recurrences with the 5- and 10-year FDM rates of 64% and 60%, respectively. For patients with LRC (n=189), the 5- and 10-year FDM rates of 67% and 63%, respectively. The actuarial 5- and 10-year RFS rates were 47% and 44%, respectively. A second primary cancer was documented in 70 patients (27%). Figure 2 shows the Kaplan-Meier curves of oncologic and survival outcomes for all patients.
Figure 2.
Survival and oncologic outcomes.
Kaplan-Meier curves calculated for all patients (n=264) showing locoregional control (LRC), freedom from distant metastasis (FDM), cancer specific survival (CSS), and Overall survival (OS). Short vertical lines represent censored data and shaded colors represent 95% confidence intervals
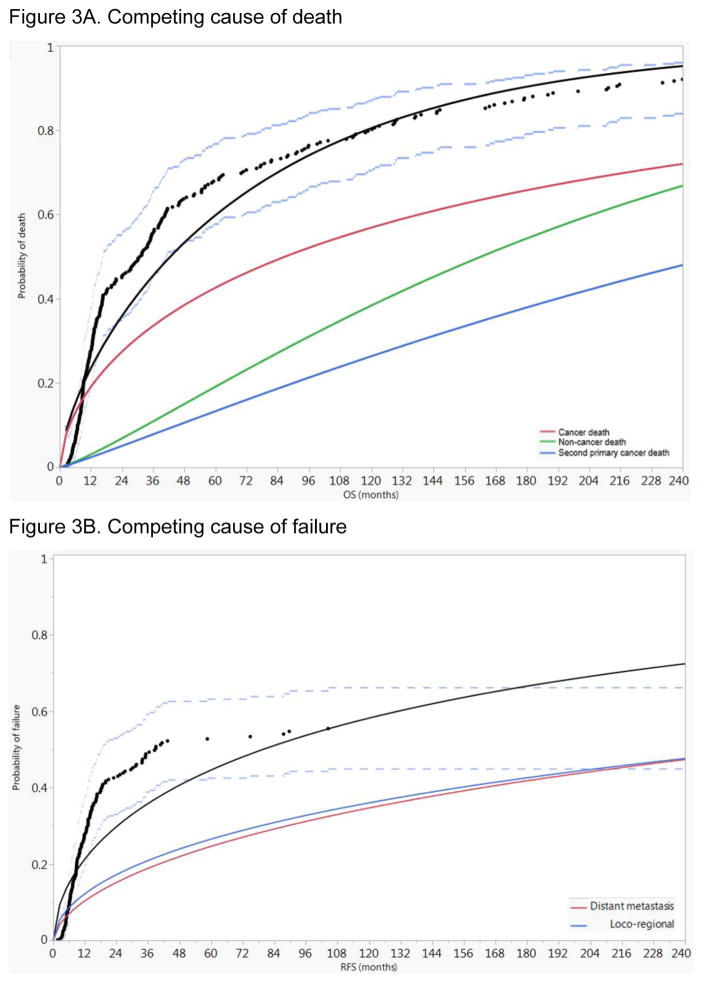
Assessment of competing causes of death for all patients revealed the predominance of cancer mortality throughout the entire follow up duration, followed by non-cancer-related death and death from second primary cancer (Figure 3A). Additionally, assessment of competing causes of first failure for all patients revealed the slight numeric predominance of loco-regional failure throughout the entire follow up duration, followed by distant metastasis (Figure 3B).
Figure 3.
Competing risk analysis for all patients A) competing risk of death cancer mortality predominates throughout the entire follow up duration, followed by non-cancer-related death and death from second primary cancer, B) competing risk of failure where loco-regional failure slightly exceeds the risk of distant metastasis. Black dots represent aggregated data points, black line represents fitted line of the aggregated data points, and dashed blue lines represent 95% confidence intervals.
Disease control by risk group and dose level
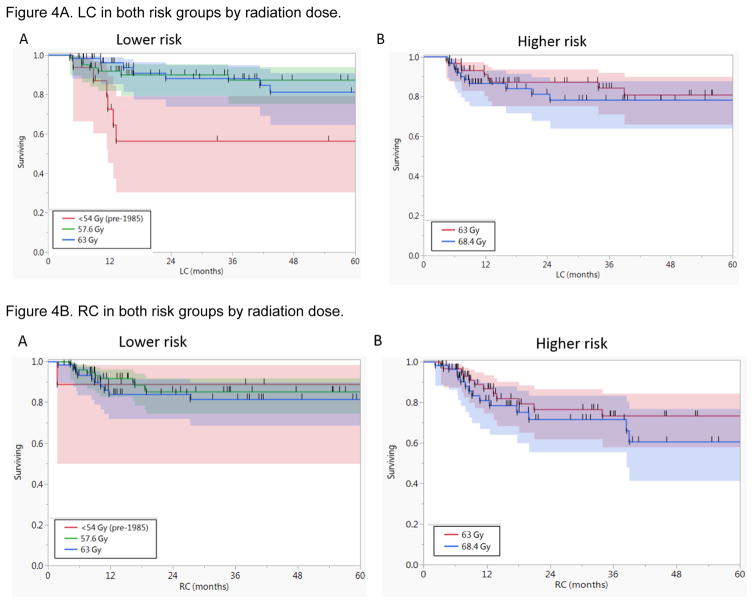
Forty-two patients (16%) developed local recurrence; 19 of 135 (14%) in the lower risk and 23 of 129 (18%) in the higher risk group of primary site recurrence based on the study designed risk scoring system for primary recurrence. Except for patients who were treated prior to 1985 with lower dose level A <54 Gy (n=17), there were no statistically significant differences in the 5-year LC rate between different dose levels in both the lower and higher risk groups (Supplementary Table 1, Figure 4A).
Figure 4.
Kaplan-Meier curves for A) local control (LC) in both risk groups by radiation dose, and B) regional control (RC). It shows there were no statistically significant differences in LC or RC rates between different dose levels in both the lower and higher risk groups except for LC of patients who were treated prior to 1985 with lower dose level A <54 Gy (n=17).
Neck recurrences occurred in 47 patients (18%); 21 of 148 (14%) in the lower risk, and 26 of 116 (22%) in the higher risk group of neck recurrence based on the study designed risk scoring system for neck recurrence. There were no statistically significant differences in the 5-year regional control (RC) rates between different dosing levels in both the lower and higher risk groups (Figure 4B). Additionally, the significant dose response demonstrated in the initial report for patients with ECE, was no longer noted in the current analysis. There was no significant dose response above 57.6 Gy for patients with ECE (n=15) with 5-year RC of 71% compared to 67% for ≥63 Gy (n=112, P=0.9)
Late toxicity
Sever late complication (i.e. grade 3 and 4 per RTOG late toxicity scoring system) were recorded in 30 patients (11%). There was no grade 5 toxicity. The most frequently encountered late toxicity was pharyngeal stricture and dysphagia in 17 patients, osteoradionecrosis of the mandible in 8 patients, sever neck fibrosis in 3 patients, and pharyngeocutaneous fistula in 2 patients. There was no dose-response relationship for late toxicities when analyzed by the maximum dose delivered, as there was no statistically significant difference in the distribution of late toxicity events between the three groups (P=0.9). The frequency was 4/43 (9.3%) for maximum dose ≤ 57.6 Gy; 15/128 (11.7%) for 63 Gy; and 11/93 (11.8%) to 68.4 Gy.
Outcome correlates
Locoregional control
Univariate analysis revealed that all of the following variables were significantly associated with improved LRC: Caucasian race, non-positive surgical margin, no extracapsular extension, negative nodal staging, neck RT higher BED, and package time < 85 days (P<0.05 for all). On multivariate analysis; Caucasian race (HR 0.4, 95% CI 0.2–0.6, P = 0.0003), negative surgical margins “including negative, converted, and close margins” (HR 0.4, 95% CI 0.2–0.8, P= 0.009), no ECE (HR 0.4, 95% CI 0.2–0.8, P=0.01) and TPT <85 days (HR 0.5, 95% CI 0.3–0.8, P=0.002) were independent correlates of better LRC.
Distant control
Univariate analysis revealed that all of the following variables were significantly associated with improved distant control: N0 stage, non-positive margin, lower number of positive nodes, lower number of nodal groups, no extracapsular extension, primary RT BED, and neck RT BED (P<0.05 for all). However, in multivariate analysis, lower number of nodal groups (i.e. 0–1 vs ≥ 2) was the only co-variable that remained significant (HR 0.4, 95% CI 0.2–0.6, P=0.0003).
Cancer specific survival
Univariate analysis revealed that all of the following variables were significantly associated with improved cancer specific survival; Caucasian race, non-positive surgical margins, lower number of positive nodes, lower number of nodal groups, no extracapsular extension, neck RT BED, primary RT BED, and package time < 85 days (P<0.05 for all). On multivariate analysis; Caucasian race (HR 0.5, 95% CI 0.4–0.8, P=0.001), non-positive surgical margins (HR 0.4, 95% CI 0.3–0.8, P=0.004) and package time < 85 days (HR 0.7, 95% CI 0.5–0.9, P=0.03) were independently associated with improved cancer specific survival.
Overall survival
Univariate analysis revealed that all of the following variables were significantly associated with improved survival: age < 57 years, N0 stage, negative surgical margins, lower number of positive nodes (0–1 vs ≥ 2), smaller number of positive nodal groups (0–1 vs ≥ 2), higher neck RT BED, and lower package time (< 85 days) (P <0.05). On multivariate analysis, age < 57 years (P <0.0001), negative surgical margins (P=0.011), negative ECE (P = 0.027) and package time < 85 days (P=0.003) were independently associated with improved survival. Table 2 shows the details of the cox regression analyses. Supplementary Figure S1 depicts the KM survival curves for all patients by margin status, ECE, and TPT.
Table 2.
Univariate and multivariate analyses of OS
| Characteristics | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Age | ≥ 57 years | 1 | 1 | ||
| < 57 years | 0.6 (0.4–0.7) | 0.0001* | 0.5 (0.4–0.7) | < 0.0001* | |
| Sex | Male | 1 | - | ||
| Female | 0.96 (0.7–1.3) | 0.790 | - | ||
| Race | Caucasian | 1 | - | ||
| Others | 1.1 (0.8–1.5) | 0.495 | - | ||
| Site | Oropharynx | 1 | 1 | ||
| Oral Cavity | 1.1 (0.8–1.6) | 1.1 (0.8–1.6) | |||
| Larynx | 0.97 (0.7–1.4) | 0.9(0.6–1.4) | |||
| Hypopharynx | 1.5 (1–2.3) | 0.069 | 1.1(0.8–1.8) | 0.410 | |
| T stage | T1-T2 | 1 | - | ||
| T3-T4 | 1.0 (0.8–1.4) | 0.798 | - | ||
| N stage | N1-N3 | 1 | 1 | ||
| N0 | 0.7 (0.6–0.96) | 0.024* | 0.91 (0.7–1.2) | 0.463 | |
| ECE | Positive | 1 | 1 | ||
| Negative | 0.6 (0.5–0.8) | 0.0002* | 0.9 (0.6–1.2) | 0.026* | |
| Margins status | Positive | 1 | 1 | ||
| Others | 0.5 (0.4–0.8) | 0.007* | 0.5 (0.3–0.85) | 0.011* | |
| Nerve invasion | Negative | 1 | 1 | ||
| Others | 1.2 (0.9–1.6) | 0.213 | 1.2 (0.8–1.6) | 0.385 | |
| Number of +ve nodes | ≥2 | 1 | 1 | ||
| 0–1 | 0.5 (0.4–0.7) | < 0.0001* | 0.7 (1.3–3.6) | 0.098 | |
| Number of nodal groups | ≥2 | 1 | 1 | ||
| 0–1 | 0.5 (0.4–0.7) | < 0.0001* | 0.8 (0.5–1.3) | 0.391 | |
| Direct invasion | Yes | 1 | 1 | ||
| No | 0.8 (0.6–1.1) | 0.0842 | 1.0 (0.7–1.6) | 0.655 | |
| primary RT BED | continuous | 0.0911 | 0.757 | ||
| Neck RT BED | continuous | < 0.0001* | 0.097 | ||
| Package Time | ≥85 days | 1 | 1 | ||
| < 85 days | 0.6 (0.5–0.8) | 0.0003* | 0.7 (0.5–0.9) | 0.003* | |
TPT
As the overall treatment time is inherently longer in dose C (38 fx), compared with dose B (35 fx), and dose A (32 fx), we further analyzed the impact of the identified TPT per each dose group. Because primary sites and involved necks were independently assigned to higher or lower risk categories based on a cumulative point score, patients included in each dose group were based on the maximum dose assignment per patient. The distribution of TPT as well as its components (time from surgery to RT start and RT total time) for each dose group is presented as box plots (Supplementary Figure S2). The figure demonstrates that time from surgery to RT start was more variable in each dose group compared with RT total time that showed relatively tighter distribution. In univariate survival analysis, TPT as a continuous variable remained significant in dose A group (P=.004) but it didn’t reach statistical significance in the other two dose groups. However, when Kaplan-Meier curves were plotted by the identified TPT cutoff binary variable of 85 days for each dose group (Supplementary Figure S3), it showed a separation of curves in the three dose groups in favor of TPT < 85 days that reached statistical significance in dose A group for both LRC and, notably, OS endpoints. Kaplan-Meier curves also separated in dose groups B and C in favor of TPT < 85 days but it didn’t reach statistical significance.
Discussion
This was the first prospective multi-disciplinary risk-based personalized trial to evaluate the effect of radiation dose on the outcomes of PORT for HNSCC. Herein we present the final report with long-term follow up of the complete cohort that also allows for the reevaluation of the data within the current contextual understanding of risk factors for recurrence and death. The most important finding is radiation dose, within the ranges used (1.8 Gy/day to total doses between 57.6 Gy – 68.4 Gy and no concurrent chemotherapy), simply did not significantly affect LC, LRC, or OS. However, our secondary analysis is consistent with other reports that the overall treatment package time (TPT), not radiation dose drives LRC, CSS, and OS.
With respect to tumor factors, we confirm the two now generally accepted “high-risk” factors of margin status and ECE were the only independent tumor-related risk factors predicting LRC and OS. Since only the number of nodal groups was recorded but not the named neck level, we could not make a conclusion whether low neck disease portends poorer outcomes. Additionally, when evaluating ECE only in patients with positive neck dissection, a numerical trend rather than a statistical significance was shown for the positive ECE sub-cohort (Supplementary Figure S4). Initial positive margins, either mucosal or deep, that are converted to negative, and also <5mm but negative (“close”) margins (groups 2, 3, and 4 in the original publication) had similar LRC and OS as those with initial negative margins.[9] Additionally, it was seen that the presence of a single involved LN did not worsen outcomes, but any multi-level nodal disease of any number did observably increase the risk for DM. This was likely though more driven by ECE. Other than margin status and ECE, none of the pathologic risk factors evaluated – the factors we now consider as “intermediate risk factors” –and current indications for single modality PORT- were detectably associated with elevated risk for local or regional recurrence in the context of PORT. This does not mean that those risk factors do not predict for postoperative recurrence, but that they were adequately addressed by the PORT given, regardless of dose within the range evaluated.
The fact that dose escalation in this study was not successful has been attributed to tumor cell regeneration during the additional time required to deliver doses >57.6Gy at <2Gy/fraction because repopulation offset any putative benefit of higher radiation dose during the additional time required for delivery.[11] However, to evaluate this concern, we performed a BED analysis with correction for time [10] to interrogate the effect of the received dose as an outcome driver in the current dataset. In multivariate analysis, BED was not an independent driver of any of the tested outcomes.
When this study was initiated the common belief was that patients might not be able to tolerate PORT at 2 Gy per fraction, so 1.8Gy was chosen. However, it is now well recognized that dose per fraction also affects overall treatment time, and prolongation of time may be lead to decreased tumor control. Subsequent prospective studies for the definitive radiation treatment of HNSCC confirmed that strategies used to address time factor challenges including hyper- and accelerated fractionation improved at least local control[12–14], adding to the milieu suggesting time and fractionation, rather than dose per se, are drivers of radiotherapy response. These findings and the recognized tolerance of PORT led to an empiric increase in fractional dose for PORT from 1.8 to 2Gy that is now accepted as the standard.[15, 16] However, to further improve the therapeutic index, risk stratification based on biological rather than conventional clinical and pathological factors may be needed.
The concept of “treatment package time” was introduced to define the interval from surgery to the completion of PORT.[17] We evaluated the TPT in this dataset as a continuous variable and we were able to identify the cutoff point of 85 days using RPA analysis that showed it was an independent predictor of LRC, CSS, and OS for the entire dataset. However, after stratification by each dose group, TPT showed higher impact in the lowest dose level (i.e. dose A). This confirms that TPT independently affects outcomes for PORT delivered at 1.8 Gy/day without chemotherapy especially when lower total doses (i.e. <60 Gy) are prescribed. The principal driver of variation in TPT in our study after control for risk assignment was the interval between surgery and start of radiation (Supplementary Figure S2). Thus, while tempting to attribute outcomes to TPT, the observation may just be a statistical correlate, and increasing time between surgery and radiation may reflect other hidden factors selecting for patients with worse outcomes. Subsequent studies though do suggest the importance of minimizing TPT. The original investigators incorporated treatment time into their next prospective trial randomizing patients to PORT with standard or accelerated fractionation.[11] That study showed accelerated fractionation led to better LRC, but only for patients with delay >6 weeks to start RT. Outcomes were best if RT was completed within less than 11 weeks from surgery. In a retrospective cohort from the University of Pennsylvania treatment time ≤100 days led to better LRC and OS. [18] Finally, a prospective Italian phase III multicenter trial also showed that accelerated PORT led to better LRC for patients with delay >6.9 weeks of starting RT.[19]
Other strategies to address the risk of repopulation as a function of time include concurrent and “sandwich” chemotherapy. RTOG 9501 and EORTC 22931 used concurrent chemotherapy that has the potential for greater cell kill to compensate for interval subclinical clonogen proliferation.[15, 16] Patients enrolled on RTOG 9501 were allowed to recover from surgery without active treatment for up to 8 weeks before RT began. RTOG 0024 gave weekly chemotherapy starting in the second week post-operatively to suppress clonogen repopulation during the interval in which the patient was recovering from surgery, and before post-operative chemo-radiation started. The RTOG 0024 regimen was well tolerated, and had better risk-adjusted LRC, DFS, and OS compared with concurrent therapy alone in RTOG9501.[20]
There are three main limitations of this study. The high-risk group did not get tested at 57.6Gy. It is conceivable 63Gy might not have an advantage over 57.6Gy for even the high-risk group, and that no dose-response relationship above 57.6Gy exists for PORT. Secondly, patients were classified “low” versus “high” risk independently for the primary site and neck. Thus, the same patient may have been both low risk in the neck and high risk at the primary site, or vice versa, and assigned different doses to those sites. Separating out the differences in control within those different volumes after treatment in the 2D era using opposed laterals and electrons, and without segmental imaging is impossible. Finally, our concepts of dose today, with 3 and 4D radiation target definition, and sophisticated computerized treatment planning were not the same as 30 years ago, where the delivered doses were likely less precise.
Now that 2Gy/fraction is used, the empiric doses used for PORT are 56Gy for intermediate- and 60Gy for high-risk areas. RTOG trials [15, 20, 21] allowed for additional boosting to as high as 66Gy, but it is optional and empiric, and the potential benefit not validated by prospective trials. Non-tumor bearing, non-operated, contiguous or adjacent areas felt to be at risk, albeit “lower,” are given 50–54Gy. In the context of integrated planning the common doses used are 60/57/54Gy for CTV’s 1, 2 and 3, respectively, and in 30 fractions.
This and other trials did not address radiation dosing in the context of concurrent chemotherapy. The radiation doses used with concurrent chemotherapy, e.g., cisplatin, are the same as those used without chemotherapy.[15, 16] The reason the radiation doses were kept the same as with RT-alone in initial chemo-RT trials evaluating the addition of chemotherapy is that RT was considered the gold standard treatment, so its full robustness was maintained, while chemotherapy dose intensity was reduced when necessary. Current and future related studies are evaluating the relative importance of specifics and degrees of margin status and ECE and chemotherapy and RT dose reduction in the setting of HPV-driven HNSCC (E3311), and whether the addition of concurrent cetuximab adds to PORT for intermediate risk cancers (RTOG 0920).
In conclusion, this long-term report of a radiation dose seeking trial of PORT for HNSCC demonstrated that increasing dose did not significantly improve tumor control. The likely explanation is that in fractionated radiation increasing the total time likely offsets any benefit of increased dose, and adds to toxicity. The question of dose (and time) remain unanswered as while the standard of care for patients with “high-risk” features is currently concurrent chemoradiation, the 2 seminal trials used differing doses (60 vs. 66 Gy in 6 or 6 ½ weeks).
Supplementary Material
Summary.
We present the final report of a phase III trial to assess dose-response for postoperative RT in head and neck cancer. Primary sites and involved necks were independently assigned to higher or lower risk categories based on point score system then randomized to receive different dose levels. Increasing dose did not significantly improve tumor control, treatment package time was the only significant treatment variable, and positive surgical margins and ECE were the most significant pathological factors.
Acknowledgments
We thank Pamela Allen and Mary-Jane Oswald for their assistance with data collection. We are thankful for the late K. Kian Ang, MD for his innumerable contributions for the execution of this trial and the presentation of the final results in the current format.
Footnotes
Conflict of interest statement: The authors declare no conflicts of interest.
Funding sources and financial disclosures: Drs., Mohamed and Fuller receive(d) funding support from the National Institutes of Health (NIH)/National Institute for Dental and Craniofacial Research (1R01DE025248-01/R56DE025248-01). Dr. Fuller received/receives grant and/or salary support from: the NIH/National Cancer Institute (NCI) Head and Neck Specialized Programs of Research Excellence (SPORE) Developmental Research Program Award (P50CA097007-10) and Paul Calabresi Clinical Oncology Program Award (K12 CA088084-06); a National Science Foundation (NSF), Division of Mathematical Sciences, Joint NIH/NSF Initiative on Quantitative Approaches to Biomedical Big Data (QuBBD) Grant (NSF 1557679); a General Electric Healthcare/MD Anderson Center for Advanced Biomedical Imaging In-Kind Award; an Elekta AB/MD Anderson Department of Radiation Oncology Seed Grant; the Center for Radiation Oncology Research (CROR) at MD Anderson Cancer Center; and the MD Anderson Institutional Research Grant (IRG) Program. Dr. Fuller has received speaker travel funding from Elekta AB. Supported in part by the National Institutes of Health (NIH)/National Cancer Institute (NCI) Cancer Center Support (Core) Grant CA016672 to The University of Texas MD Anderson Cancer Center. Dr. Rosenthal receives grants from: NIH/NCI (5R0CA148707-03) and (5R01CA160880-03).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maccomb WS, Fletcher GH. Planned combination of surgery and radiation in treatment of advanced primary head and neck cancers. The American journal of roentgenology, radium therapy, and nuclear medicine. 1957;77:397–414. [PubMed] [Google Scholar]
- 2.Feldman M, Fletcher GH. Analysis of the parameters relating to failures above the clavicles in patients treated by postoperative irradiation for squamous cell carcinomas of the oral cavity or oropharynx. International journal of radiation oncology, biology, physics. 1982;8:27–30. doi: 10.1016/0360-3016(82)90380-7. [DOI] [PubMed] [Google Scholar]
- 3.Barkley HT, Fletcher GH, Jesse RH, Lindberg RD. Papers of the First Joint Meeting of the American Radium Society, James Ewing Society, and the Society of Head and Neck Surgeons Management of cervical lymph node metastases in squamous cell carcinoma of the tonsillar fossa, base of tongue, supraglottic larynx, and hypopharynx. The American Journal of Surgery. 1972;124:462–7. doi: 10.1016/0002-9610(72)90067-0. [DOI] [PubMed] [Google Scholar]
- 4.Vikram B, Strong EW, Shah JP, Spiro R. Failure at the primary site following multimodality treatment in advanced head and neck cancer. Head & neck surgery. 1984;6:720–3. doi: 10.1002/hed.2890060303. [DOI] [PubMed] [Google Scholar]
- 5.Bartelink H, Breur K, Hart G, Annyas B, van Slooten E, Snow G. The value of postoperative radiotherapy as an adjuvant to radical neck dissection. Cancer. 1983;52:1008–13. doi: 10.1002/1097-0142(19830915)52:6<1008::aid-cncr2820520613>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 6.Cachin Y, Eschwege F. Combination of radiotherapy and surgery in the treatment of head and neck cancers. Cancer Treatment Reviews. 2:177–91. doi: 10.1016/s0305-7372(75)80002-8. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher GH. Textbook of radiotherapy. 2. Philadelphia: Lea & Febiger; 1973. [Google Scholar]
- 8.Marcus RB, Jr, Million RR, Cassissi NJ. Postoperative irradiation for squamous cell carcinomas of the head and neck: analysis of time-dose factors related to control above the clavicles. International journal of radiation oncology, biology, physics. 1979;5:1943–9. doi: 10.1016/0360-3016(79)90943-x. [DOI] [PubMed] [Google Scholar]
- 9.XXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXX
- 10.Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989;62:679–94. doi: 10.1259/0007-1285-62-740-679. [DOI] [PubMed] [Google Scholar]
- 11.Ang KK, Trotti A, Brown BW, Garden AS, Foote RL, Morrison WH, et al. Randomized trial addressing risk features and time factors of surgery plus radiotherapy in advanced head-and-neck cancer. International journal of radiation oncology, biology, physics. 2001;51:571–8. doi: 10.1016/s0360-3016(01)01690-x. [DOI] [PubMed] [Google Scholar]
- 12.Awwad HK, Lotayef M, Shouman T, Begg AC, Wilson G, Bentzen SM, et al. Accelerated hyperfractionation (AHF) compared to conventional fractionation (CF) in the postoperative radiotherapy of locally advanced head and neck cancer: influence of proliferation. British journal of cancer. 2002;86:517–23. doi: 10.1038/sj.bjc.6600119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Overgaard J, Mohanti BK, Begum N, Ali R, Agarwal JP, Kuddu M, et al. Five versus six fractions of radiotherapy per week for squamous-cell carcinoma of the head and neck (IAEA-ACC study): a randomised, multicentre trial. The Lancet Oncology. 2010;11:553–60. doi: 10.1016/S1470-2045(10)70072-3. [DOI] [PubMed] [Google Scholar]
- 14.Parsons JT, Mendenhall WM, Stringer SP, Cassisi NJ, Million RR. Twice-a-day radiotherapy for squamous cell carcinoma of the head and neck: the University of Florida experience. Head & neck. 1993;15:87–96. doi: 10.1002/hed.2880150202. [DOI] [PubMed] [Google Scholar]
- 15.Cooper JS, Zhang Q, Pajak TF, Forastiere AA, Jacobs J, Saxman SB, et al. Long-term follow-up of the RTOG 9501/intergroup phase III trial: postoperative concurrent radiation therapy and chemotherapy in high-risk squamous cell carcinoma of the head and neck. International journal of radiation oncology, biology, physics. 2012;84:1198–205. doi: 10.1016/j.ijrobp.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefèbvre J-L, Greiner RH, et al. Postoperative Irradiation with or without Concomitant Chemotherapy for Locally Advanced Head and Neck Cancer. New England Journal of Medicine. 2004;350:1945–52. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 17.XXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXX
- 18.XXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXX
- 19.Sanguineti G, Richetti A, Bignardi M, Corvo R, Gabriele P, Sormani MP, et al. Accelerated versus conventional fractionated postoperative radiotherapy for advanced head and neck cancer: results of a multicenter Phase III study. International journal of radiation oncology, biology, physics. 2005;61:762–71. doi: 10.1016/j.ijrobp.2004.07.682. [DOI] [PubMed] [Google Scholar]
- 20.XXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXX
- 21.Harari PM, Harris J, Kies MS, Myers JN, Jordan RC, Gillison ML, et al. Postoperative Chemoradiotherapy and Cetuximab for High-Risk Squamous Cell Carcinoma of the Head and Neck: Radiation Therapy Oncology Group RTOG-0234. J Clin Oncol. 2014 doi: 10.1200/JCO.2013.53.9163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.