Abstract
The purpose of this study is to evaluate the in vivo retention capabilities of poloxamer-based in situ hydrogels for vaginal application with nonoxinol-9 as the model drug. Two in situ hydrogel formulations, which contained 18% poloxamer 407 plus 1% poloxamer 188 (GEL1, relative hydrophobic) or 6% poloxamer 188 (GEL2, relative hydrophilic), were compared with respect to the rheological properties, in vitro hydrogel erosion and drug release. The vaginal retention capabilities of these hydrogel formulations were further determined in two small animal models, including drug quantitation of vaginal rinsing fluid in mice and isotope tracing with 99mTc in rats. The two formulations exhibited similar phase transition temperatures ranging from 27 to 32 °C. Increasing the content of poloxamer 188 resulted in higher rheological moduli under body temperature, but slightly accelerated hydrogel erosion and drug release. When compared in vivo, GEL1 was eliminated significantly slower in rat vagina than GEL2, while the vaginal retention of these two hydrogel formulations behaved similarly in mice. In conclusion, increases in the hydrophilic content of formulations led to faster hydrogel erosion, drug release and intravaginal elimination. Rats appear to be a better animal model than mice to evaluate the in situ hydrogel for vaginal application.
Abbreviations: AUC, area under curve; EO, hydrophilic ethylene oxide; F127, Poloxamer 407; F68, poloxamer 188; GEL1, 1% poloxamer 188 + 18% poloxamer 407; GEL2, 6% poloxamer 188 + 18% poloxamer 407; HLB, hydrophile--lipophile balance; ICR, Institute of Cancer Research; MRT, mean residence time; MW, molecular weight; N-9, Nonoxynol-9; PEO-PPO-PEO, poly(ethylene oxide)a-poly(propylene oxide)b-poly(ethylene oxide)a; PO, hydrophobic propylene oxide; RP-HPLC, reverse-phase high performance liquid chromatography; SVF, simulated vaginal fluid
KEY WORDS: Vaginal administration, Poloxamer, Thermosensitive hydrogel, Retention, Nonoxinol-9
Graphical abstract
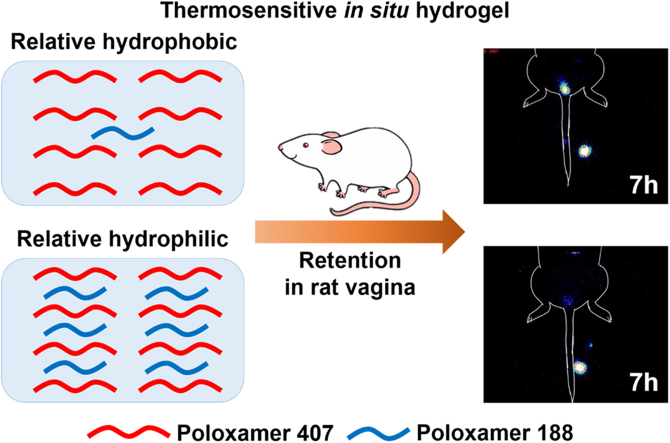
For the poloxamer-based thermosensitive hydrogel, higher content of poloxamer 188 in the formulation led to shortened intravaginal retention after topical administration to rats, which was attributed to the increased hydrophilicity of the hydrogel.
1. Introduction
The vagina is the primary organ for topical application of contraceptives. Dosage forms for vaginal administration include suppositories, films, effervescent tablets and hydrogels. Vagina-applied semisolid dosage forms are much more popular than solid dosage forms due to their convenient administration, flexible dose, rapid onset, and good lubrication1. However, rapid physiological clearance caused by mucous secretion and turnover impedes effective vaginal retention of such semisolid dosage forms. The clearance in the vaginal lumen is so powerful that the residence time of conventional formulations is usually too short for the bioactive agents to exert their therapeutic role, leading to insufficient dose and/or duration of action2. Abdominal motions also accelerate the removal of vaginal formulations. Accordingly, if semisolid formulations have sufficient viscosity or bioadhesive capability, their vaginal elimination will be efficiently slowed down3, 4.
Presently, thermosensitive in situ hydrogel formulations have attracted increasing interest in the field of mucosal administration5, 6, 7, 8. Applications of thermosensitive in situ hydrogels are based on their phase transition in response to the temperature increase from ambient to physiological temperature. For vaginal administration, such formulations are characterized by easy application because the low viscosity under room temperature would allow quickly spreading and even flowing into the fold regions of vaginal mucosa. In addition, the formed hydrogel in the vaginal lumen favors prolonged residence of the loaded drug in the vagina9. Preclinical studies carried out on mouse or rabbit models have proven the advantages of in situ hydrogel for vaginal use5, 10. Furthermore, when used for contraception, the hydrogel would form a protective layer on the surface of vaginal mucosa, which will inhibit sperm motility and simultaneously function as lubricant during sexual activity.
Among the thermosensitive polymers, poly(ethylene oxide)a-poly(propylene oxide)b-poly(ethylene oxide)a (PEO-PPO-PEO), also known as the generic name of poloxamer and the trade name Pluronic, has been studied most extensively for vaginal use. This is a category of triblock copolymers with the molecular weights (MW) ranging 1100–14,000 Da and the weight ratios of poly(ethylene oxide) block to poly(propylene oxide) block varying from 1:9 to 8:2.
At concentrations above a critical value, aqueous poloxamer solutions have inverse thermal sensitivity11. The gelation of aqueous poloxamer solutions is closely related to dehydration of the hydrophobic poly(propylene oxide) blocks, followed by formation of micelles12. In order to obtain a suitable gelation temperature, poloxamer-based in situ hydrogel is usually composed of two poloxamers of different grades, relatively hydrophobic poloxamer 407 (Pluronic F127) and relatively hydrophilic poloxamer 188 (Pluronic F68)13, 14, 15. The presence of poloxamer 188 usually leads to increased hydrophilicity and higher gelation temperature. However, the influence of poloxamer 188 contents on the in vivo performance of the hydrogel has rarely been addressed. Additionally, the appropriate small animal model for evaluating the intravaginal residence of poloxamer-based in situ hydrogels has not been assessed. The present report addresses these two questions.
In order to determine the relationship between the concentration of poloxamer 188 and intravaginal residence, two poloxamer-based thermosensitive in situ hydrogel formulations with similar gelation temperature were evaluated for in vivo use. Nonoxynol-9 (N-9), the most widely applied topical spermicide16, was used as the model drug. To facilitate comparison, the formulations should contain similar compositions except for differing poloxamer 188 concentrations designed to produce differing intravaginal residence times. According to our previous work17, two N-9-containing in situ hydrogel formulations, relatively hydrophobic GEL1 (18% poloxamer 407/1% poloxamer 188) and relatively hydrophilic GEL2 (18% poloxamer 407/6% poloxamer 188) were compared for rheological properties, in vitro hydrogel erosion, drug release, and vaginal residence in two animal models.
2. Experimental
2.1. Materials and animals
Poloxamer 407 (F127) and poloxamer 188 (F68) were kindly provided by BASF Co., Ltd. (Ludwigshafen, Germany). N-9 was bought from Guanghui Technology Co., Ltd. (Dalian, China). 99mTcO4Na was provided by GMS Pharmaceutical Co., Ltd. (Shanghai, China). Triton X-100 was bought from Solarbio Life Science Co., Ltd. (Beijing, China). Ultrapure water was produced by the Milli-Q system (Millipore, Schwalbach, Germany). All other chemicals used in this study were of analytical grade and commercially available.
Female adult ICR (Institute of Cancer Research) mice (28–30 g) and SD (Sprague–Dawley) rats (220–230 g) were provided by Super B.K. laboratory animal Co., Ltd. (Shanghai, China) and maintained at 22 ± 2 °C on a 12 h light--dark cycle with access to food and water ad libitum. All animal experiments were carried out in accordance with the guidelines published by the National Institutes of Health for the care and use of laboratory animals (NIH Publications No. 8023).
2.2. Preparation of in situ hydrogel
In situ hydrogel was prepared using the “cold method” as previously reported18 at the concentration of 4% N-9, 18% poloxamer 407 and 1% (for GEL1) or 6% (for GEL2) poloxamer 188. Briefly, the required amount of poloxamers and N-9 (if necessary) for each formulation was weighed and added to chilled sodium acetate solution (16 mmol/L, pH 4.5) under stirring in a flask. The obtained mixture was placed at 4 °C until the polymer was dissolved completely. All the samples were prepared on a weight basis and reported as weight percent (%, w/w).
2.3. Rheological measurements
Thermosensitive gelation processes of GEL1 and GEL2 were investigated upon temperature sweeping from 20 to 40 °C using a rotatory rheometer (Bohlin Gemini II, Malvern, UK) under the oscillation mode with a fixed frequency of 1 Hz and a steady shear strain of 0.02 at a heating rate of 1 °C/min.
To evaluate the effect of dilution by vaginal secretions on gelation capability of the in situ hydrogel, similar measurements were carried out on the mixtures of GEL1 or GEL2 with simulated vaginal fluid (SVF) at the volume ratio of 5:0.5 or 5:0.75. SVF was prepared according to previous literature19 with the following composition (g/L): NaCl, 3.51; KOH, 1.40; Ca(OH)2, 0.222; bovine serum albumin, 0.018; lactic acid, 2.00; acetic acid, 1.00; glycerol, 0.160; urea, 0.400; and glucose, 5.00. The pH of SVF was adjusted to about 4.2 using HCl.
2.4. Hydrogel erosion
A membraneless model was used to assess the in vitro erosion of the hydrogel as described previously18 with minor modifications. Briefly, a flat-bottomed vial with an effective dissolution area of 3.80 cm2 containing approximately 2 g hydrogel was immersed in a water bath of 37 °C for 10 min in order to allow the polymer solution to form hydrogel. The dissolution medium (10 mL SVF) pre-equilibrated at 37 °C was carefully layered over the upper surface of the solidified hydrogel. Then the vial was placed in a thermostatic shaker set at 37 °C and 100 rpm. At predetermined time points, the dissolution medium was poured out, the outer surface of the vial was wiped dry and the weight of the vial plus the hydrogel was recorded. Then, the hydrogel was further equilibrated at 37 °C for 10 min, followed by replenishment of 10 mL pre-heated dissolution medium. The above process was repeated until the hydrogel was completely dissolved.
2.5. Drug release
The release of N-9 from hydrogel was monitored using the cell method as described in Appendix XII E of British Pharmacopeia (2011). Briefly, 3.0 g hydrogel was placed in the extraction cell (effective release area of 15.90 cm2) covered with a piece of 3 μm microporous filter membrane, which was then placed in the vessel of a dissolution tester (RC806, Tianfa Technology Co., Ltd., Tianjin, China) containing 900 mL SVF stirring at 37 °C and 100 rpm.
At predetermined time points, an aliquot of 100 μL release medium was sampled for analysis using RP-HPLC (reverse-phase high performance liquid chromatography, Agilent 1100 series, USA) equipped with a Diamonsil® column (C18, 250 mm×4.6 mm, 5 μm, Dikma) under the following conditions: column temperature at 25 °C, mobile phase of methanol–water (88:12), flow rate at 1 mL/min, detection wavelength at 228 nm and injection volume of 50 μL. This method was validated beforehand with respect to the linear range, accuracy, precision and specificity.
2.6. In vivo residence
2.6.1. Intravaginal residence in mice
Female ICR mice were acclimated for at least one week before being used and allowed to free access to standard food and tap water. Three groups of mice (66 mice per group) were tested including (1) 4% w/v N-9 aqueous solution, (2) GEL1 and (3) GEL2. For each group, the mice were further divided into 11 sub-groups (6 mice per sub-group) to collect data at the following time points: 0, 20 and 40 min, then 1, 2, 3, 4, 5, 6, 7 and 8 h.
The formulations were administrated into mice vagina using a microliter syringe equipped with a blunt needle. After inserting the needle approximately 0.5 cm into the vagina, 10 μL of each formulation was discharged rapidly and the mouse was kept in an inverted position for about 10 s to assure gelation. To minimize systematic errors caused by possible residue of hydrogel in the syringe, the weight of the syringe before (Wa) and after (Wb) administration was recorded to calculate the accurate amount of hydrogel injected into the vagina (Wadm,g) according to the following equation:
| (1) |
At each determined time point, the vagina of six mice in each group was carefully rinsed by 50 μL pre-warmed normal saline for 10 times. The rinsing solution was collected for RP-HPLC analysis conducted under the same condition as described in Section 2.5. Before analysis, the collected rinsing solution from each mouse was diluted to 1 mL with water, spiked with the internal standard Triton X-100 and filtered by 0.22 μm membrane. The retention percentage of applied hydrogel was calculated based on the detected N-9 concentrations (Cdet,mg/mL) according to the following equation:
| (2) |
2.6.2. Intravaginal residence in rats
A total 12 female SD rats were divided into 3 groups (n = 4) and intravaginally treated with radiolabeled GEL1, GEL2 or solution, respectively. For GEL1 and GEL2, 3 mL of each hydrogel was vortically mixed with 100 μL 99mTcO4Na. For the solution, 3 mL aqueous medium with the same composition as GEL1 but containing no poloxamers was mixed with 100 μL 99mTcO4Na. Etherized rats were intravaginally administered with 0.2 mL hydrogel or solution using 1 mL disposable syringe equipped with a blunt gavage needle, which was inserted 1 cm into the orificium. After administration, the rats were allowed to move freely. Then scintigraphic imaging was taken with a γ-ray camera (BHP6602, Binsong Photon Technology Co., Ltd., Beijing, China) every hour until no radioactive signal was detectable in the vagina. To locate the vagina and calculate the retention percentage of radioactive isotopes, a 1.5 mL Eppendorf centrifuge tube containing 0.2 mL of the same formulation was placed near to the base of the rat tail as reference.
2.7. Statistics
The pharmacokinetic parameters of vaginal residence were calculated using DAS software (Ver. 2.0, Mathematical Pharmacology Professional Committee of China). Data were evaluated by Student׳s t-test, and differences were considered to be significant at a level of P < 0.05.
3. Results and discussion
3.1. Thermosensitivity and rheological property
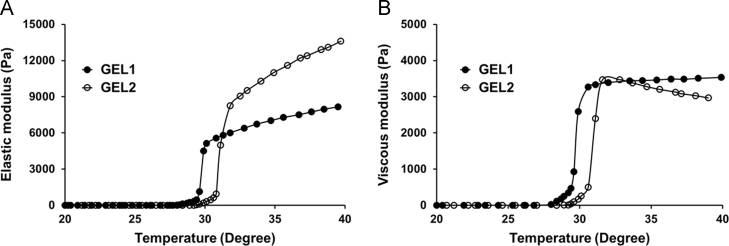
The elastic modulus Gʹ and viscous modulus Gʹʹ are both important rheological parameters for poloxamer-based in situ hydrogel. For GEL1, the elastic modulus Gʹ and viscous modulus Gʹʹ increased sharply in the temperature range of 27–30 °C (Fig. 1). For GEL2, a similar phase transition occurred in the temperature range of 28.5–31.5 °C (Fig. 1). The gelation temperature of GEL2 was slightly higher than that of GEL1, but this difference is of no practical importance.
Figure 1.
Temperature-dependent profiles of the elastic modulus Gʹ (A) and viscous modulus Gʹʹ (B) of the hydrogel formulations determined under the oscillation mode with a fixed frequency of 1 Hz and a steady shear strain of 0.02 at a heating rate of 1 °C/min. GEL1 is shown in solid circles, and GEL2 is shown in hollow circles.
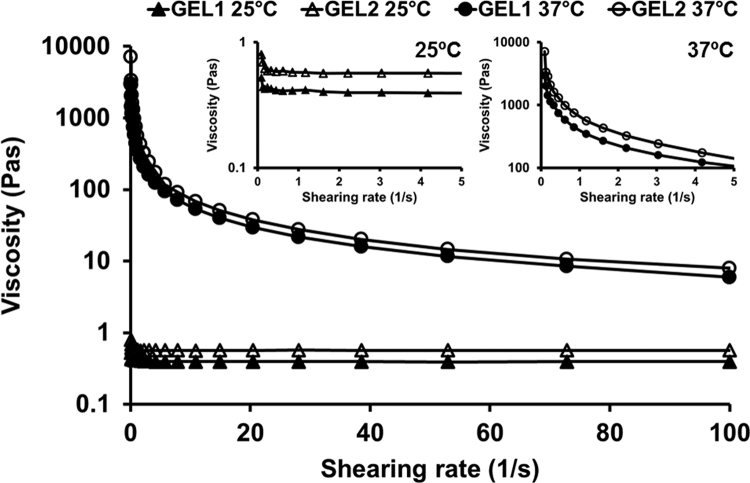
The concentration of poloxamer 188 in the formulations showed significant effect on the elastic modulus, but scarcely affected the viscous modulus. At 37 °C, the Gʹ value of GEL2 was about 4500 Pa higher than that of GEL1, while the Gʹʹ values of GEL1 and GEL2 were similar. The two formulations were both liquid at 25 °C and changed to pseudoplastic hydrogel at 37 °C. After phase transition, the viscosity of the formulations increased from less than 1 Pas to thousands of Pas at a shear rate of 0.1 s–1 (Fig. 2).
Figure 2.
The viscosity of GEL1 and GEL2 as a function of shearing rates under 25 °C (triangles) and 37 °C (circles). Enlarged curves are presented in order to facilitate comparing the viscosity of the two formulations under low shearing rates.
It was expected that higher content of poloxamer 188 in the formulation GEL2 resulted in higher gelation temperature, which is also consistent with previous literatures13, 14, 15. A typical example is a recently reported in situ gelling system based on the combination of poloxamer 407 and poloxamer 188 for ocular delivery, where poloxamer 188 was added in the formulation to raise the gelation temperature to the physiological range. This phenomenon could be explained by the thermosensitive gelation mechanism of poloxamer solutions. Based on 13C NMR data, gelation of aqueous poloxamer solutions was attributed to dehydration of hydrophobic poly(propylene oxide) blocks12. As a triblock copolymer, poloxamer 407 has a molar ratio of 0.28 (56/202) between hydrophobic propylene oxide (PO) and hydrophilic ethylene oxide (EO), while which is 0.17 (27/160) for poloxamer 188. The lowered PO/EO molar ratio in the in situ hydrogel caused by addition of poloxamer 188 led to increased hydrophilicity and gelation temperature.
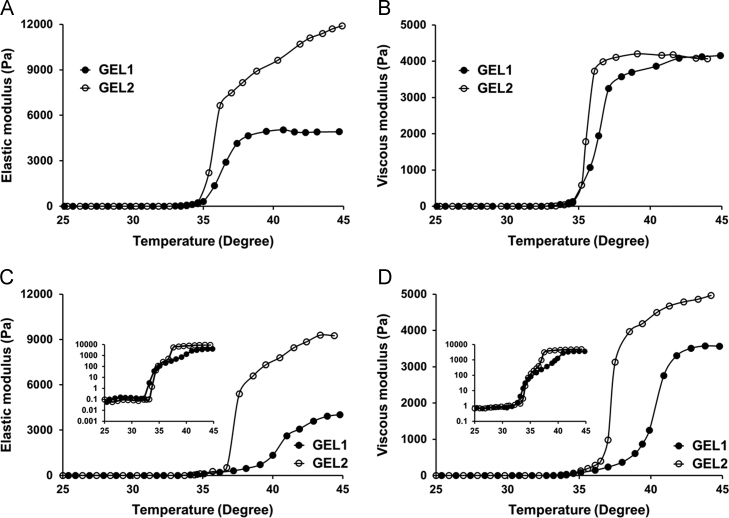
Because the volume of vaginal fluids usually varies from 0.5 to 0.75 mL20, 21, the formulations were mixed with SVF to simulate extreme dilution in the in vivo circumstance. As shown in Fig. 3A and B, when diluted with 0.5 mL SVF, both GEL1 and GEL2 remained semi-solid under body temperature. Again, the Gʹ value of GEL2 was about 5000 Pa higher than that of GEL1 while the Gʹʹ values remained similar. When diluted with 0.75 mL SVF, both GEL1 and GEL2 still began to form hydrogel below 37 °C, although the gelation process flattened out (Fig. 3C and D). The difference between the Gʹ values of these two formulations still exists. It is worth noting that at body temperature the Gʹʹ value of GEL1 became obviously lower than that of GEL2. It is known that the gelation capability of poloxamer solutions depends closely on the polymer concentration22, 23, 24. Our result indicated that both the two formulations had adequate anti-dilution capacity, assuring that they could form hydrogels after administration in the vagina even after dilution by vaginal fluid.
Figure 3.
Effect of dilution on the gelation process of the hydrogel formulations (5 mL) with 0.5 mL (A and B) or 0.75 mL (C and D) simulated vaginal fluid. The elastic modulus Gʹ (A and C) and viscous modulus Gʹʹ (B and D) changed as a function of temperature. GEL1 is shown in solid circles, and GEL2 is shown in hollow circles. In order to illustrate the gelation process clearly, the rheological curves with a logarithmic ordinate are inserted in (C) and (D).
3.2. Hydrogel erosion and drug release
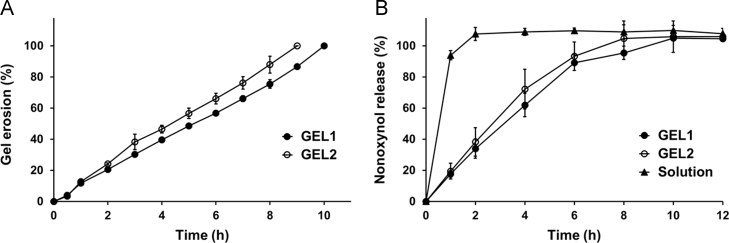
Poloxamer-based hydrogel undergoes erosion in aqueous medium at a rate associated with contact surface, stirring condition and hydrogel composition in the membraneless model. As shown in Fig. 4A, GEL1 dissolved completely in 10 h, slightly but significantly slower than GEL2. When the hydrogel was directly exposed to SVF, the erosion of both the two formulations precisely followed zero-order kinetics. The difference in the erosion rates may be attributed to the composition of the hydrogel, namely the different contents of poloxamer 188 in the formulations.
Figure 4.
Gel erosion (A) and N-9 release (B) of the thermosensitive in situ hydrogels in simulated vaginal fluid at 37 °C (n=4, mean±S.D.). GEL1 is shown in solid circles, GEL2 is shown in hollow circles, and N-9 solution is shown in solid triangles.
In the in vitro release study, the upper surface of the hydrogel was covered by a filter membrane with 3-μm micropores, through which poloxamer could diffuse into the aqueous medium. Under this condition, N-9 was totally released from both the formulations in 10 h (Fig. 4B), which was consistent with the in vitro erosion of the hydrogels. Furthermore, the release rate of N-9 from GEL1 was slightly but not significantly slower than that from GEL2. Slower erosion corresponding to slower drug release was in agreement with previous literature13. The release of N-9 from the two hydrogels during the initial 6 h both followed zero-order kinetics, indicating the erosion-controlled release mechanism.
It should be noted that addition of poloxamer 188 may exert two opposing influences. On one hand, increase in the total concentration of the polymers increased the strength of formed hydrogel and thus retarded the hydrogel erosion and drug release. On the other hand, the higher hydrophilicity of poloxamer 188 favored the hydrogel erosion in aqueous environment and thus accelerated the drug release. Our experimental data revealed that the balance between these two mechanisms ultimately resulted in a slight influence on the hydrogel erosion and drug release by the variations in poloxamer 188 concentration. This result is consistent with the report of Baloglu et al.25 that the rates of drug release were similar from various hydrogel formulations containing different concentrations and ratios of poloxamers 407 and 188.
3.3. In vivo residence
3.3.1. Vaginal residence in mice
The HPLC analysis method of N-9 in the vaginal rinsing fluid was validated with respect to the linear range, precise, accuracy, specificity and recovery. When vaginal rinsing was conducted immediately after vaginal administration, >90% of the applied dose could be detected with good repeatability.
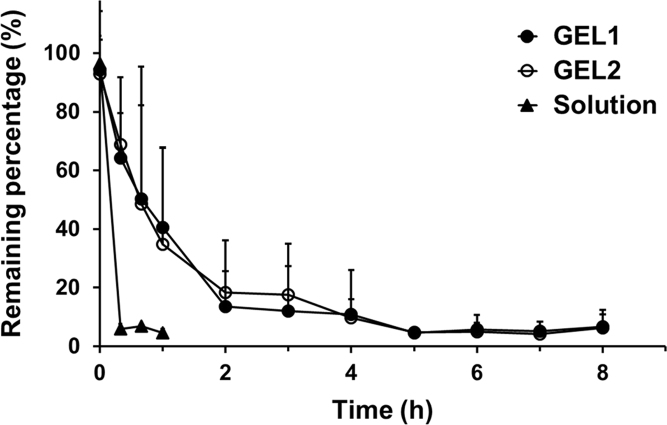
As shown in Fig. 5, elimination of N-9 solution was so fast that only less than 6% of the total dose remained in the mouse vagina 20 min after administration. In contrast, the elimination of both GEL1 and GEL2 was much slower. The N-9 loaded in the hydrogels was almost completely eliminated from the mouse vagina 5 h after administration.
Figure 5.
Time profiles of the remaining percentage of N-9 in ICR mice vagina after intravaginal application of GEL1 (solid circles), GEL2 (hollow circles) and solution (triangles). Data are mean±S.D., n = 6.
The elimination profiles of N-9 in mouse vagina were similar for GEL1 and GEL2. When analyzed using the statistical moment method, no difference was found between GEL1 and GEL2 with respect to the intravaginal pharmacokinetic parameters including the mean residence time (MRT) and the area under curve (AUC), while the AUCs of both hydrogel formulations were more than 6 times higher compared to that of solution (Table 1). The minor differences in hydrogel erosion and drug release in the mouse model suggests that this model does not discriminate between the in situ hydrogel formulations.
Table 1.
Statistical moment analysis on the profiles of vaginal retention percentage vs. time in mice.
| Formulation | Pharmacokinetic parametera |
Ratio of AUC(0–t)b |
|
|---|---|---|---|
| MRT(0–t) (h) | AUC(0–t) (%·h) | Intravaginal F (%) | |
| GEL1 | 2.29 ± 0.21 | 136.1 ± 43.8 | 648.1 |
| GEL2 | 2.23 ± 0.46 | 140.6 ± 10.3 | 669.5 |
| Solution | 0.22 ± 0.01 | 21.0 ± 1.6 | – |
Data are mean±S.D., n = 6.
The prolonged retention was evaluated by the ratio of AUC(0–t) between the hydrogel and the solution.
3.3.2. Vaginal residence in rats
Isotope 99mTc was used as a tracer to compare the vaginal residence of GEL1 and GEL2 in rats due to its capability of continuously in situ imaging and minimal radiological hazard due to the short half-life. To exclude possible deviation caused by isotope decay, a centrifuge tube containing a same volume of the same formulation was placed nearby the animal for normalization.
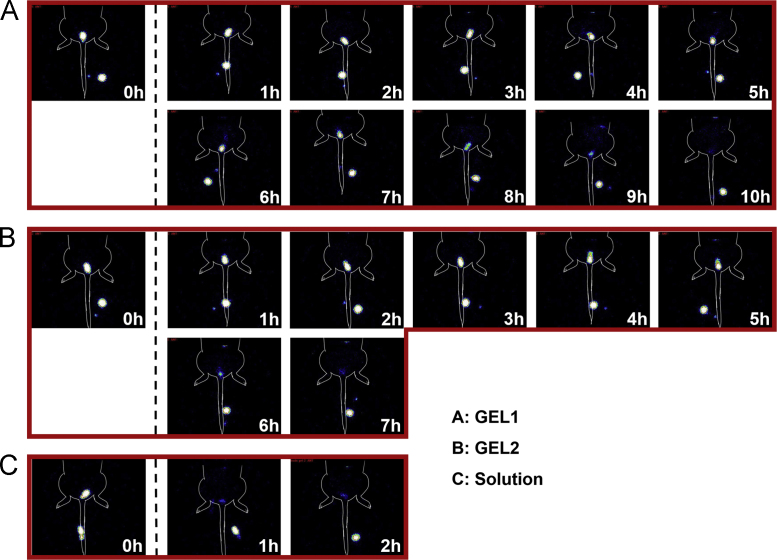
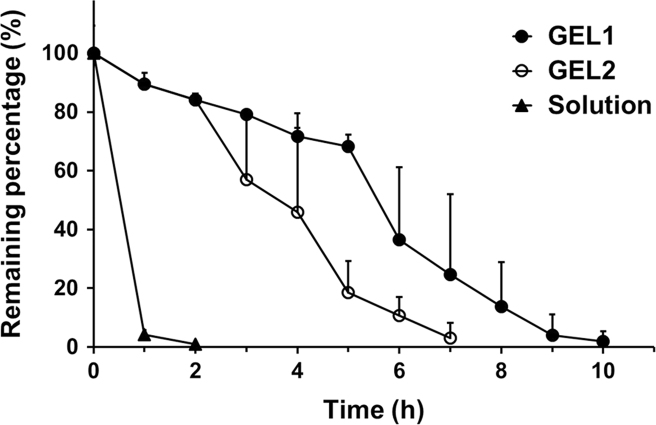
As shown in Figure 6, Figure 7, a significant difference in vaginal retention between GEL1 and GEL2 was found in rats. The elimination of GEL1 was slow in the first 5 h, accelerated during 5–9 h after administration and finally completed after about 10 h. By contrast, the elimination of GEL2 accelerated from 2 h after administration and completed in 7 h. The in vivo retention of the hydrogels was highly consistent with their in vitro erosion and release. Pharmacokinetic parameters given in Table 2 also showed a significant difference between the retention characteristics of GEL1 and GEL2 in rats. The AUC ratio between GEL2 and the solution was almost the same in rats with that in mice, but the AUC ratio between GEL1 and the solution was substantially higher.
Figure 6.
Scintigraphic imaging of time-dependent changes after administration of 99mTcO4Na-spiked GEL1 (A), GEL2 (B), and solution (C) in SD rat vagina.
Figure 7.
Time profiles of the remaining percentage of 99mTcO4Na in SD rat vagina after intravaginal application of GEL1 (solid circles), GEL2 (hollow circles) and solution (triangles). Data are mean±S.D., n = 4.
Table 2.
Statistical moment analysis on the profiles of vaginal retention percentage vs. time in rats.
| Formulation | Pharmacokinetic parametera |
Ratios of AUC(0–t)b |
|
|---|---|---|---|
| MRT(0–t) (h) | AUC(0–t) (%·h) | Intravaginal F (%) | |
| GEL1 | 3.47 ± 0.53c | 522.7 ± 69.7c | 957.3 |
| GEL2 | 2.01 ± 0.49 | 347.5 ± 70.4 | 636.4 |
| Solution | 0.33 ± 0.01 | 54.6 ± 3.0 | – |
Data are mean±S.D., n = 4.
The prolonged retention was evaluated by the ratio of AUC(0–t) between the hydrogel and the solution.
Significantly different compared with GEL2 (P < 0.05).
When rats were used as the animal model coupling with radioisotope imaging, the results seemed meaningful. Even in vitro, GEL1 and GEL2, which only differed by 5% poloxamer 188, were marginally different in erosion rate and drug release. It is not surprising that a such difference between GEL1 and GEL2 was not reflected in mice, considering the limited volume of the mouse vaginal lumen. In our previous report, addition of carrageenan in poloxamer hydrogel slowed down the erosion of hydrogel by almost four times, but only prolonged drug retention to a limited extent in mice10. By contrast, the rat vagina is relatively deeper in length and larger in volume, which is more similar to that of the primate and permits the in vivo discrimination of vaginal formulations.
Poloxamer is a family of amphiphilic copolymers. The hydrophile-lipophile balance (HLB) value is 22 for poloxamer 407 and 29 for poloxamer 188, respectively11, 26, 27. Adding poloxamer 188 to the poloxamer 407 aqueous solution resulted in enhanced hydrophilicity of the formed hydrogel. By comparison between the results of in vitro and in vivo evaluations on GEL1 and GEL2, it could be rationally concluded that relative hydrophilic formulation led to faster hydrogel erosion, drug release and intravaginal elimination. Our finding is also consistent with a recently published study on ocular administration, which revealed that, under a fixed total polymer concentration, higher content of hydrophilic poloxamer 188 in the formulation resulted in faster removal from the corneal surface after application28. These phenomena could be explained by Marchetti group׳s work29, in which the authors found that the presence of hydrophilic additives like polyethylene glycol accelerated drug release from poloxamer-based hydrogel even at low concentration, and attributed the accelerated release to the higher osmotic pressure between the hydrogel and the dissolution medium created by the increased hydrogel hydrophilicity.
Rheological assessment is usually employed to predict the in vivo behavior of in situ hydrogel30, 31, 32. However, there is still controversy over the importance of rheological parameters in the evaluation of hydrogel performance. Baloglu et al.25 found that significant difference in rheological moduli and viscosity led to similar rates of drug release from poloxamer-based hydrogels for vaginal application. In the present study, at body temperature, the elastic modulus Gʹ of GEL2 was always much higher than that of GEL1 (Figure 1, Figure 3), but the intravaginal elimination of GEL2 in rats was faster than that of GEL1. Obviously, the elastic modulus was not correlated with the intravaginal retention. Elastic modulus Gʹ, or “storage modulus”, describing the elastic deformation of the hydrogel33, was not related with the hydrogel erosion and elimination, which could be clearly seen in Figure 4, Figure 7. By contrast, viscous modulus Gʹʹ, reflecting the viscous nature of the hydrogel, might provide more useful information, since the Gʹʹ value of GEL1 before dilution was slightly higher at body temperature than that of GEL2 (Fig. 1B). Our results suggested that the viscous nature of the poloxamer-based hydrogel might be a more important characteristic correlating with the intravaginal retention.
Two extreme fates for in situ hydrogels after vaginal administration are possible. The worst one is complete mixing with vaginal fluid, while the best one is immediately forming gel without being diluted by vaginal fluid. The actual in vivo situation probably falls between these two scenarios and can only be identified from experimental data. In this study, the Gʹʹ value of GEL1 was gradually lowered than that of GEL2 after dilution (Fig. 3), but the intravaginal residence of GEL1 in rats was much longer than that of GEL2. Perhaps the in situ hydrogels formed immediately after administration without or with only slight dilution by vaginal fluid. This speculation seems reasonable since the thermal-induced gelation occurred rapidly as shown by the sharp phase transition (Fig. 1) and there was not much effective agitation in the vaginal lumen.
4. Conclusions
Consequently, besides gelation temperature, the content of poloxamer 188 also affected in vitro and in vivo performance of the poloxamer 407-based in situ hydrogel. When the concentration of poloxamer 407 was fixed in the formulation, more poloxamer 188 increased the hydrophilicity of the formed hydrogel, resulting in accelerated hydrogel erosion, drug release and intravaginal elimination. The viscous modulus, rather than elastic modulus, was shown to be related to the intravaginal retention of the hydrogel. Rats are the more appropriate animal model as compared with mice for the in vivo comparison of the poloxamer-based hydrogels for vaginal application, especially for the in situ hydrogel formulations with similar in vitro characteristics.
Acknowledgments
This study was supported by the National Key Technology R&D Program (2012BAI31B04), the Open Project Program of National Population and Family Planning Key Laboratory of Contraceptives Drugs and Devices (2016KF08), and the National Natural Science Foundation of China (81102385).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Schwartz J.L., Weiner D.H., Lai J.J., Frezieres R.G., Creinin M.D., Archer D.F. Contraceptive efficacy, safety, fit, and acceptability of a single-size diaphragm developed with end-user input. Obstet Gynecol. 2015;125:895–903. doi: 10.1097/AOG.0000000000000721. [DOI] [PubMed] [Google Scholar]
- 2.D׳Cruz O.J., Uckun F.M. Vaginal microbicides and their delivery platforms. Expert Opin Drug Deliv. 2014;11:723–740. doi: 10.1517/17425247.2014.888055. [DOI] [PubMed] [Google Scholar]
- 3.Kieweg S.L., Geonnotti A.R., Katz D.F. Gravity-induced coating flows of vaginal gel formulations: in vitro experimental analysis. J Pharm Sci. 2004;93:2941–2952. doi: 10.1002/jps.20194. [DOI] [PubMed] [Google Scholar]
- 4.Kieweg S.L., Katz D.F. Squeezing flows of vaginal gel formulations relevant to microbicide drug delivery. J Biomech Eng. 2006;128:540–553. doi: 10.1115/1.2206198. [DOI] [PubMed] [Google Scholar]
- 5.Li C., Han C., Zhu Y., Lu W., Li Q., Liu Y. In vivo evaluation of an in-situ hydrogel system for vaginal administration. Pharmazie. 2014;69:458–460. [PubMed] [Google Scholar]
- 6.Caramella C.M., Rossi S., Ferrari F., Bonferoni M.C., Sandri G. Mucoadhesive and thermogelling systems for vaginal drug delivery. Adv Drug Deliv Rev. 2015;92:39–52. doi: 10.1016/j.addr.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Cho H.J., Balakrishnan P., Park E.K., Song K.W., Hong S.S., Jang T.Y. Poloxamer/cyclodextrin/chitosan-based thermoreversible gel for intranasal delivery of fexofenadine hydrochloride. J Pharm Sci. 2011;100:681–691. doi: 10.1002/jps.22314. [DOI] [PubMed] [Google Scholar]
- 8.Bruschi M.L., Jones D.S., Panzeri H., Gremião M.P., de Freitas O., Lara E.H. Semisolid systems containing propolis for the treatment of periodontal disease: in vitro release kinetics, syringeability, rheological, textural, and mucoadhesive properties. J Pharm Sci. 2007;96:2074–2089. doi: 10.1002/jps.20843. [DOI] [PubMed] [Google Scholar]
- 9.Priya J.H., John R., Alex A., Anoop K.R. Smart polymers for the controlled delivery of drugs--a concise overview. Acta Pharm Sin B. 2014;4:120–127. doi: 10.1016/j.apsb.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y., Zhu Y.Y., Wei G., Lu W.Y. Effect of carrageenan on poloxamer-based in situ gel for vaginal use: improved in vitro and in vivo sustained-release properties. Eur J Pharm Sci. 2009;37:306–312. doi: 10.1016/j.ejps.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 11.Kabanov A.V., Batrakova E.V., Alakhov V.Y. Pluronic block copolymers as novel polymer therapeutics for drug and gene delivery. J Control Release. 2002;82:189–212. doi: 10.1016/s0168-3659(02)00009-3. [DOI] [PubMed] [Google Scholar]
- 12.Ur-Rehman T., Tavelin S., Gröbner G. Effect of DMSO on micellization, gelation and drug release profile of Poloxamer 407. Int J Pharm. 2010;394:92–98. doi: 10.1016/j.ijpharm.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Xuan J.J., Yan Y.D., Oh D.H., Choi Y.K., Yong C.S., Choi H.G. Development of thermo-sensitive injectable hydrogel with sustained release of doxorubicin: rheological characterization and in vivo evaluation in rats. Drug Deliv. 2011;18:305–311. doi: 10.3109/10717544.2010.544690. [DOI] [PubMed] [Google Scholar]
- 14.dos Santos A.C., Akkari A.C., Ferreira I.R., Maruyama C.R., Pascoli M., Guilherme V.A. Poloxamer-based binary hydrogels for delivering tramadol hydrochloride: sol-gel transition studies, dissolution--release kinetics, in vitro toxicity, and pharmacological evaluation. Int J Nanomed. 2015;10:2391–2401. doi: 10.2147/IJN.S72337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xuan J.J., Balakrishnan P., Oh D.H., Yeo W.H., Park S.M., Yong C.S. Rheological characterization and in vivo evaluation of thermosensitive poloxamer-based hydrogel for intramuscular injection of piroxicam. Int J Pharm. 2010;395:317–323. doi: 10.1016/j.ijpharm.2010.05.042. [DOI] [PubMed] [Google Scholar]
- 16.Baldwin M.K., Jensen J.T. Contraception during the perimenopause. Maturitas. 2013;76:235–242. doi: 10.1016/j.maturitas.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Yang F.J., Chen J.Y., Feng L.L., Wei G., Lu W.Y. Preparation and evaluation of nonoxynol-9-loaded thermoreversible gel for vaginal application. Chin Pharm J. 2014;49:2008–2013. [Google Scholar]
- 18.Li C., Li C., Liu Z., Li Q., Yan X., Liu Y. Enhancement in bioavailability of ketorolac tromethamine via intranasal in situ hydrogel based on poloxamer 407 and carrageenan. Int J Pharm. 2014;474:123–133. doi: 10.1016/j.ijpharm.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 19.Owen D.H., Katz D.F. A vaginal fluid simulant. Contracept. 1999;59:91–95. doi: 10.1016/s0010-7824(99)00010-4. [DOI] [PubMed] [Google Scholar]
- 20.Campaña-Seoane M., Peleteiro A., Laguna R., Otero-Espinar F.J. Bioadhesive emulsions for control release of progesterone resistant to vaginal fluids clearance. Int J Pharm. 2014;477:495–505. doi: 10.1016/j.ijpharm.2014.10.066. [DOI] [PubMed] [Google Scholar]
- 21.Aka-Any-Grah A., Bouchemal K., Koffi A., Agnely F., Zhang M., Djabourov M. Formulation of mucoadhesive vaginal hydrogels insensitive to dilution with vaginal fluids. Eur J Pharm Biopharm. 2010;76:296–303. doi: 10.1016/j.ejpb.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Dalla-Bona A.C., Stoisiek K., Oesterheld N., Schmehl T., Gessler T., Seeger W. Characterization of lung-delivered in-situ forming controlled release formulations. J Pharm Pharmacol. 2015;67:1349–1354. doi: 10.1111/jphp.12434. [DOI] [PubMed] [Google Scholar]
- 23.Oshiro A., da Silva D.C., de Mello J.C., de Moraes V.W., Cavalcanti L.P., Franco M.K. Pluronics f-127/l-81 binary hydrogels as drug-delivery systems: influence of physicochemical aspects on release kinetics and cytotoxicity. Langmuir. 2014;30:13689–13698. doi: 10.1021/la503021c. [DOI] [PubMed] [Google Scholar]
- 24.Xu X., Shen Y., Wang W., Sun C., Li C., Xiong Y. Preparation and in vitro characterization of thermosensitive and mucoadhesive hydrogels for nasal delivery of phenylephrine hydrochloride. Eur J Pharm Biopharm. 2014;88:998–1004. doi: 10.1016/j.ejpb.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Baloglu E., Karavana S.Y., Senyigit Z.A., Hilmioglu-Polat S., Metin D.Y., Zekioglu O. In-situ gel formulations of econazole nitrate: preparation and in-vitro and in-vivo evaluation. J Pharm Pharmacol. 2011;63:1274–1282. doi: 10.1111/j.2042-7158.2011.01315.x. [DOI] [PubMed] [Google Scholar]
- 26.Moghimi S.M., Hunter A.C. Poloxamers and poloxamines in nanoparticle engineering and experimental medicine. Trends Biotechnol. 2000;18:412–420. doi: 10.1016/s0167-7799(00)01485-2. [DOI] [PubMed] [Google Scholar]
- 27.Oh K.T., Bronich T.K., Kabanov A.V. Micellar formulations for drug delivery based on mixtures of hydrophobic and hydrophilic Pluronic® block copolymers. J Control Release. 2004;94:411–422. doi: 10.1016/j.jconrel.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 28.Al Khateb K., Ozhmukhametova E.K., Mussin M.N., Seilkhanov S.K., Rakhypbekov T.K., Lau W.M. In situ gelling systems based on Pluronic F127/Pluronic F68 formulations for ocular drug delivery. Int J Pharm. 2016;502:70–79. doi: 10.1016/j.ijpharm.2016.02.027. [DOI] [PubMed] [Google Scholar]
- 29.Ricci E.J., Lunardi L.O., Nanclares D.M., Marchetti J.M. Sustained release of lidocaine from poloxamer 407 gels. Int J Pharm. 2005;288:235–244. doi: 10.1016/j.ijpharm.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 30.Wei G., Xu H., Ding P.T., Li S.M., Zheng J.M. Thermosetting gels with modulated gelation temperature for ophthalmic use: the rheological and gamma scintigraphic studies. J Control Release. 2002;83:65–74. doi: 10.1016/s0168-3659(02)00175-x. [DOI] [PubMed] [Google Scholar]
- 31.Chang J.Y., Oh Y.K., Choi H.G., Kim Y.B., Kim C.K. Rheological evaluation of thermosensitive and mucoadhesive vaginal gels in physiological conditions. Int J Pharm. 2002;241:155–163. doi: 10.1016/s0378-5173(02)00232-6. [DOI] [PubMed] [Google Scholar]
- 32.Mayol L., Quaglia F., Borzacchiello A., Ambrosio L., La Rotonda M.I. A novel poloxamers/hyaluronic acid in situ forming hydrogel for drug delivery: rheological, mucoadhesive and in vitro release properties. Eur J Pharm Biopharm. 2008;70:199–206. doi: 10.1016/j.ejpb.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 33.Bonacucina G., Martelli S., Palmieri G.F. Rheological, mucoadhesive and release properties of Carbopol gels in hydrophilic cosolvents. Int J Pharm. 2004;282:115–130. doi: 10.1016/j.ijpharm.2004.06.012. [DOI] [PubMed] [Google Scholar]