Abstract
Elevated cyclooxygenase-2 (COX-2) and the associated inflammation within the brain contribute to glioblastoma development. However, medical use of COX inhibitors in glioblastoma treatment has been limited due to their well-documented vascular toxicity and inconsistent outcomes from recent human studies. Prostaglandin E2 (PGE2) has emerged as a principal mediator for COX-2 cascade-driven gliomagenesis. Are PGE2 terminal synthases and receptors feasible therapeutic targets for glioblastoma?
Keywords: EP receptors, Glioblastoma, Gliomagenesis, mPGES-1, Neuroinflammation, PGE2
Cyclooxygenase Cascade and Glioblastoma
Glioblastoma multiforme (GBM) is the highest-grade astrocytoma in the World Health Organization (WHO) grading system and represents the most common primary malignant brain tumor in adults, yet it lacks satisfactory treatment [1]. A better understanding of the molecular biology of GBM is desired to develop novel therapeutics with improved outcomes (Box 1). As the first cancer type that has been systematically studied by The Cancer Genome Atlas (TCGA) consortium, GBM can be categorized in four transcriptomic groups – proneural, neural, classical and mesenchymal, each of which is associated with complex genetic and/or epigenetic alterations that lead to signaling and clinical specificity [2]. In the meantime, studies on human GBM patients and animal xenograft models have uncovered some common molecular events that are correlated with and might be involved in the initiation and progression of malignant gliomas. For instance, the induction of COX-2 has been widely reported in most human glioblastomas and proposed as a major factor promoting tumor development. However, the prospect of COX-2 as a therapeutic target for GBM has been dampened by recent inconsistent outcomes from a number of population studies and the early termination of several clinical trials [3]. These differing results are not unexpected, given that five prostanoid products of COX-2 activate a total of nine G protein-coupled receptors (GPCRs) to regulate a myriad of pro- and anti-inflammatory consequences [4]. Adverse effects from the broad impact of COX-2 inhibition also brought tremendous attention during the past decade, leading to the withdrawal of two legendary COX-2-targeted drugs – rofecoxib (Vioxx®) and valdecoxib (Bextra®) from the USA market. The Jekyll and Hyde nature of the COX-2 cascade suggests that targeting its downstream prostanoid synthases or receptors might provide more specificity [4, 5].
BOX 1. Challenges for Current GBM Treatment.
With an annual incidence rate of over five per 100,000 populations and nearly 17,000 new diagnoses each year in the USA, GBM is typically associated with poor quality of life and dismal prognosis. The overall two-year survival rate is less than 10% and five-year survive rate is below 5% in unselected patients. Surgical removal in combination with conventional chemotherapy and radiotherapy represent the mainstay of treatment for GBM. However, even with these standard therapies, the prognosis still remains poor with a median overall survival under 15 months [1]. There are several complicating factors that make GBM particularly difficulty to treat: i). GBM cells – especially those in recurrent GBMs – are extremely resistant to chemotherapy and radiotherapy; ii). The highly infiltrative nature of glioblastoma makes thorough surgical removal impossible; iii). The brain is vulnerable to damage caused by therapies and has limited capacity for self-repair; iv). Most anti-tumor drugs including many immunotherapeutic agents cannot reach the tumor sites due to their poor brain penetration; v). Traditional treatment does not distinguish the tremendous molecular heterogeneity among different GBM subtypes [2]. Future efforts to seek novel therapeutics for this lethal brain cancer must hinge on a better understanding of the biology underlying these contributory factors.
Prostaglandin E Synthases
The tumor-promoting effects of COX-2 induction are largely mediated by its prostaglandin product PGE2 [6]. As the terminal enzyme that synthesizes PGE2 from intermediate PGH2, prostaglandin E synthase (PGES) has three isoforms – membrane-associated PGES-1 (mPGES-1 or PTGES), mPGES-2 (or PTGES2) and cytosolic PGES (cPGES or PTGES3) [7](Figure 1). Among these three isozymes, mPGES-1 is functionally coupled to COX-2 and, similarly to COX-2, is rapidly induced to synthesize PGE2 from COX-2-derived PGH2 in response to various detrimental stimuli [8]. Expression of mPGES-1 was found to be higher in recurred grade II gliomas that required a second surgical removal than in gliomas that were operated only once [9], suggesting a positive correlation between mPGES-1 level and the glioma grade. Also, mPGES-1 is elevated in human GBM cell lines as compared with human primary astrocytes. Genetic ablation or pharmacological inhibition of mPGES-1 can block the PGE2 release from GBM cells and impede their growth. This anti-proliferative effect can be recapitulated by protein kinase A (PKA) inhibitor H89 and reversed by exogenous PGE2 [10], indicating that PGE2 mediates GBM cell activities – at least in part – via a cAMP/PKA pathway. In addition, conditioned glioma cell culture medium was shown to enhance the expression of both COX-2 and mPGES-1 in microglia by releasing some yet-to-be-identified factor [11], suggesting another mechanism whereby gliomas increase local PGE2 levels engaging active microglia [12].
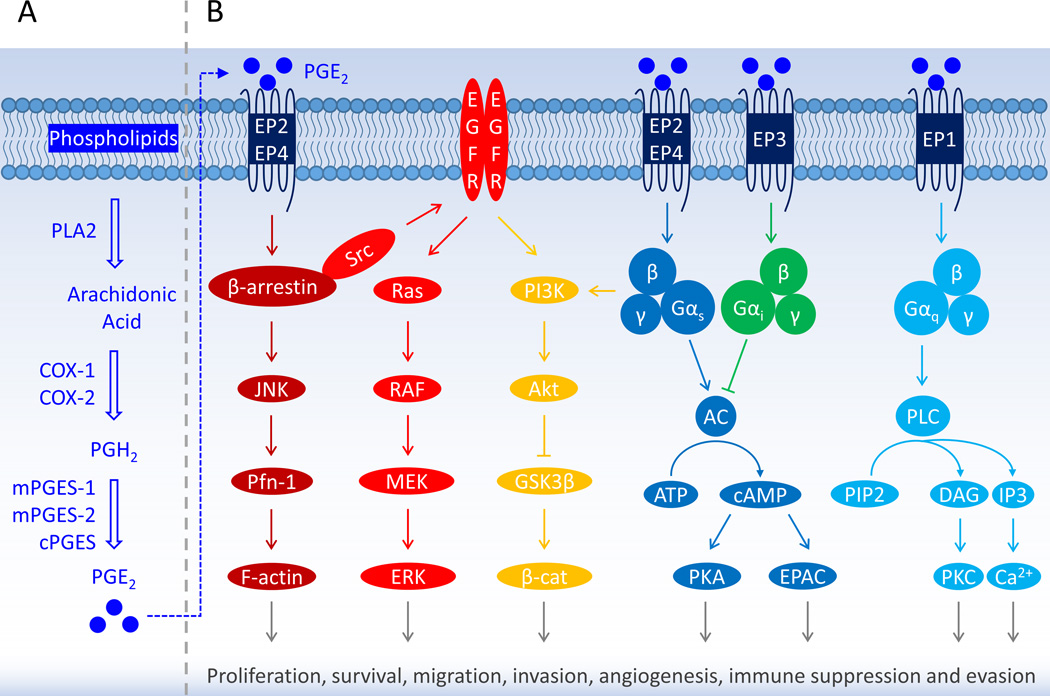
Figure 1. PGE2 Signaling through EP Receptors Promotes Gliomagenesis.
(A) Biosynthesis of prostaglandin E2 (PGE2). With various stimuli, arachidonic acid is liberated from membrane phospholipids by phospholipase A2 (PLA2), then converted into prostaglandin H2 (PGH2) – an intermediate molecule – by cyclooxygenase (COX). Unsteady PGH2 is further catalyzed by tissue-specific prostanoid synthases to five prostanoid products comprised of prostaglandins PGE2, PGD2 and PGF2α, thromboxane A2 (TXA2), and prostacyclin PGI2. The COX is the checkpoint enzyme in the synthesis of prostanoids and in mammals has two isoforms – COX-1 and COX-2. The COX-1 is constitutively expressed in most normal tissues to synthesize homeostatic prostanoids that are essential for maintaining many physiological functions; whereas the COX-2 is present at low levels under normal conditions but is rapidly and robustly induced to mediate various pathological processes that are usually associated with severe inflammatory reactions in response to tissue injuries and other detrimental stimuli [7]. (B) Prostanoids through a suite of G protein-coupled receptors (GPCRs) regulate a diversity of physiological and pathological events, e.g., inflammatory responses by prostaglandins, particularly PGE2; vasodilation by PGI2; vasoconstriction by TXA2. Two receptors (DP1 and DP2) for PGD2 have been identified up to date and four for PGE2 (EP1, EP2, EP3 and EP4), and each of the other three prostanoids acts on a single receptor – FP for PGF2α, IP for PGI2, and TP for TXA2. Multiple molecular signaling pathways have been proposed to mediate the effects of PGE2 on the regulation of glioblastoma cell survival, proliferation, migration, invasion, angiogenesis, immunosuppression, etc. Only the major pathways are indicated here by different colors. Abbreviations: AC, adenylyl cyclase; ATP, adenosine triphosphate; β-cat, β-catenin; cAMP, cyclic adenosine monophosphate; COX, cyclooxygenase; cPGES, cytosolic prostaglandin E synthase; DAG, diacylglycerol; EGFR, epidermal growth factor receptor; EPAC, exchange protein directly activated by cAMP; EPs, prostaglandin E2 receptors; ERK, extracellular signal-regulated kinase; F-actin, filamentous actin; GSK3β, glycogen synthase kinase 3β; IP3, inositol 1,4,5-trisphosphate; JNK, c-Jun N-terminal kinase; MEK, mitogen-activated protein kinase kinase; mPGES, membrane-associated PGES; Pfn-1, profilin-1; PGE2, prostaglandin E2; PGH2, prostaglandin H2; PI3K, phosphoinositide 3-kinase; PIP2, phosphatidylinositol 4,5-bisphosphate; PKA, protein kinase A; PKC, protein kinase C; PLA2, phospholipase A2; PLC, phospholipase C; RAF, rapidly accelerated fibrosarcoma kinase.
However, it was reported that a high level of mPGES-1 might also be associated with increased apoptosis in glioblastoma [13]. Ablation of mPGES-1 expression can increase the apoptotic sensitivity of human primary culture GBM cells and their proliferation in xenograft mice. Interestingly, intracellular PGE2 injection – but not the direct addition of PGE2 into the culture medium – induced Bax-dependent apoptosis in these GBM cells. These findings together suggest that the mPGES-1-mediated apoptotic effect is independent of cytoplasmic membrane-bound PGE2 receptors [13], and highlight the importance of balance between extracellular and cytosolic PGE2 in the regulation of apoptosis. These observations – if common in other GBM cells – also raise the concern that targeting mPGES-1 might curtail its beneficial effect on controlling GBM cell apoptosis.
Prostaglandin E2 Receptors
PGE2 mediates various physiological and pathological events via acting on four GPCRs – EP1–EP4. EP1 receptor is Gαq-coupled to mediate the mobilization of cytosolic Ca2+ and activation of protein kinase C (PKC); EP2 and EP4 receptors are coupled to Gαs that activates adenylyl cyclase, resulting in the synthesis of cAMP from ATP; EP3 receptor is mainly coupled to Gαi and its activation downregulates the cytosolic cAMP levels. Among these four PGE2 receptors, EP1, EP2 and EP4 have widely been reported to facilitate tumor formation and growth; EP3 may be indirectly involved in tumorigenesis as its expression is often decreased in various tumor tissues (Figure 1) [4–6, 10].
EP1 selective antagonist SC51089 and non-selective EP antagonist AH6809 were reported to decrease the in vitro proliferation of COX-2-positive human GBM cells, and to slow the growth of tumor xenografts with these cells in mice. These results recapitulated the anti-proliferative effect of COX-2 inhibition by NS398 in a similar model [14], and suggest that EP1 receptor might be involved in COX-2/PGE2-mediated GBM proliferation. In addition, PGE2 can increase the migration of human GBM cell lines in vitro, which can be largely blocked by selective inhibition on EP2 or EP4 receptor [15]. EP4 receptor expression in human GBM cells and low-grade glioma cells is regulated by COX-2 activity and appears to positively correlate with cell growth [16]. L-161982 – a selective antagonist of EP4 receptor, but not sulprostone – a selective EP3 agonist, was found to decrease GBM cells growth through activating type II PKA, suggesting the involvement of the PGE2/EP4 signaling in mediating the proliferative effects [10].
Both PGE2 and selective EP2 agonist – butaprost have recently been reported to enhance GBM cell survival and proliferation after radiation through trans-activating the epithelial growth factor receptor (EGFR) and β-catenin (Figure 1). Thus, EP2 receptor activation by PGE2 that is induced after radiotherapy might help to sustain GBM cell growth and survival, and hence contributes to the radiation-resistance of the tumors [17]. In addition, COX-2-derived PGE2 can induce Id1, a transcriptional regulator important for the tumor cell self-renewal and radiation resistance, via EP4 receptor-dependent activation of mitogen-activated protein kinase (MAPK) and early growth response protein 1 (EGR-1) [18], suggesting that EP4 receptor activation by PGE2 plays a critical role in GBM cell proliferation and resistance to radiation therapy as well. It appears that EP2 and EP4 receptors work synergistically to promote GBM activities as they share much of downstream cAMP signaling pathways (Figure 1), although they might be differentially expressed in various GBM cells [18].
Concluding Remarks
A better understanding of GBM biology will facilitate developing effective therapeutics for the most aggressive glioma. Identification of genetic and epigenetic alterations in GBMs to reveal the tumor heterogeneity will help develop specific therapies for different glioblastoma subtypes. Future efforts should also focus on the inflammatory nature of tumors to seek common molecular mechanisms that cause the genesis and progression of glioblastomas and might lead to new therapies that could benefit the majority of GBM patients (Box 1). Recent frustrations from efforts aimed at repurposing COX inhibitors in treating malignant gliomas suggest that COX-2 inhibition alone is unlikely to be a viable strategy to suppress glioblastomas due to its broad effects, although short-term exposure might provide some beneficial effects. The downstream PGE2 terminal synthases and EP receptors might provide novel targets to develop next-generation therapeutics for GBM with more specificity than the generic blockade of COX-2. However, it will be critical to validate their candidacy as molecular targets in proof-of-concept studies using selective small-molecule inhibitors.
Acknowledgments
We thank Q. Richard Lu and Atsuo T. Sasaki for their critical comments on the manuscript. J.J. is supported by the National Institutes of Health (NIH)/National Institute of Neurological Disorders and Stroke (NINDS) grant R00NS082379, NARSAD Young Investigator Grant 20940 from the Brain & Behavior Research Foundation, and the University of Cincinnati (UC) Gardner Neuroscience Institute/Neurobiology Research Center Pilot Research Program. J.Q. is supported by the China Scholarship Council (No. 201506780008). Z.S. is supported by the National Natural Science Foundation of China (No. 31271444 and No. 81201726), the Guangdong Natural Science Funds for Distinguished Young Scholar (No. 2014A030306001), the Guangdong Special Support Program for Young Talent (No. 2015TQ01R350), and the Science and Technology Program of Guangdong (No. 2016A050502027).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310:1842–1850. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- 2.Brennan CW, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155:462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qiu J, et al. Cyclooxygenase-2 in glioblastoma multiforme. Drug Discov Today. 2016 doi: 10.1016/j.drudis.2016.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang J, Dingledine R. Prostaglandin receptor EP2 in the crosshairs of anti-inflammation, anti-cancer, and neuroprotection. Trends Pharmacol Sci. 2013;34:413–423. doi: 10.1016/j.tips.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang J, Dingledine R. Role of prostaglandin receptor EP2 in the regulations of cancer cell proliferation, invasion, and inflammation. J Pharmacol Exp Ther. 2013;344:360–367. doi: 10.1124/jpet.112.200444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dey A, et al. Anti-Inflammatory Small Molecules To Treat Seizures and Epilepsy: From Bench to Bedside. Trends Pharmacol Sci. 2016;37:463–484. doi: 10.1016/j.tips.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samuelsson B, et al. Membrane prostaglandin E synthase-1: a novel therapeutic target. Pharmacol Rev. 2007;59:207–224. doi: 10.1124/pr.59.3.1. [DOI] [PubMed] [Google Scholar]
- 9.Mattila S, et al. The terminal prostaglandin synthases mPGES-1, mPGES-2, and cPGES are all overexpressed in human gliomas. Neuropathology. 2009;29:156–165. doi: 10.1111/j.1440-1789.2008.00963.x. [DOI] [PubMed] [Google Scholar]
- 10.Payner T, et al. Microsomal prostaglandin E synthase-1 regulates human glioma cell growth via prostaglandin E(2)-dependent activation of type II protein kinase A. Mol Cancer Ther. 2006;5:1817–1826. doi: 10.1158/1535-7163.MCT-05-0548. [DOI] [PubMed] [Google Scholar]
- 11.Nakano Y, et al. Induction of prostaglandin E2 synthesis and microsomal prostaglandin E synthase-1 expression in murine microglia by glioma-derived soluble factors. Laboratory investigation. J Neurosurg. 2008;108:311–319. doi: 10.3171/JNS/2008/108/2/0311. [DOI] [PubMed] [Google Scholar]
- 12.Li W, Graeber MB. The molecular profile of microglia under the influence of glioma. Neuro Oncol. 2012;14:958–978. doi: 10.1093/neuonc/nos116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lalier L, et al. Increase in PGE2 biosynthesis induces a Bax dependent apoptosis correlated to patients' survival in glioblastoma multiforme. Oncogene. 2007;26:4999–5009. doi: 10.1038/sj.onc.1210303. [DOI] [PubMed] [Google Scholar]
- 14.Matsuo M, et al. Inhibition of human glioma cell growth by a PHS-2 inhibitor, NS398, and a prostaglandin E receptor subtype EP1-selective antagonist, SC51089. J Neurooncol. 2004;66:285–292. doi: 10.1023/b:neon.0000014537.15902.73. [DOI] [PubMed] [Google Scholar]
- 15.Gomes RN, Colquhoun A. Cbio-11 effect of Prostaglandin E2 on Cell Migration in U251 Mg and U87 Mg Human Glioma Cells. Neuro-Oncology. 2015;17:v57. [Google Scholar]
- 16.Kambe A, et al. The cyclooxygenase inhibitor sulindac sulfide inhibits EP4 expression and suppresses the growth of glioblastoma cells. Cancer Prev Res (Phila) 2009;2:1088–1099. doi: 10.1158/1940-6207.CAPR-09-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brocard E, et al. Radiation-induced PGE2 sustains human glioma cells growth and survival through EGF signaling. Oncotarget. 2015;6:6840–6849. doi: 10.18632/oncotarget.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cook PJ, et al. Cox-2-derived PGE2 induces Id1-dependent radiation resistance and self-renewal in experimental glioblastoma. Neuro Oncol. 2016;18:1379–1389. doi: 10.1093/neuonc/now049. [DOI] [PMC free article] [PubMed] [Google Scholar]