Abstract
Traditional Chinese medicine (TCM) has played a pivotal role in maintaining the health of Chinese people and is now gaining increasing acceptance around the global scope. However, TCM is confronting more and more concerns with respect to its quality. The intrinsic “multicomponent and multitarget” feature of TCM necessitates the establishment of a unique quality and bioactivity evaluation system, which is different from that of the Western medicine. However, TCM is investigated essentially as “herbal medicine” or “natural product”, and the pharmacopoeia quality monographs are actually chemical-markers-based, which can ensure the consistency only in the assigned chemical markers, but, to some extent, have deviated from the basic TCM theory. A concept of “quality marker” (Q-marker), following the “property-effect-component” theory, is proposed. The establishment of Q-marker integrates multidisciplinary technologies like natural products chemistry, analytical chemistry, bionics, chemometrics, pharmacology, systems biology, and pharmacodynamics, etc. Q-marker-based fingerprint and multicomponent determination conduce to the construction of more scientific quality control system of TCM. This review delineates the background, definition, and properties of Q-marker, and the associated technologies applied for its establishment. Strategies and approaches for establishing Q-marker-based TCM quality control system are presented and highlighted with a few TCM examples.
KEY WORDS: Traditional Chinese medicine, Q-marker, Quality control, Property-effect-component
Graphical abstract
This review in detail delineates the new concept of quality marker (Q-marker), and the strategies for the establishment of Q-marker-based holistic quality control system of traditional Chinese medicine (TCM), as illustrated by several example cases.
1. Introduction
Traditional Chinese medicine (TCM) has been attracting more and more attention and receiving an increasing acceptance from a global scope due to its vital role in prevention and treatment of diseases. Despite TCM has a long history of clinical practice, a major barrier hindering its modernization and globalization is the lack of solid scientific evidence, that is, the data that clearly elucidate the chemical composition, reveal the definite mechanism of action, validate the effectiveness by “double-blind” clinical trials, and enable the practical quality control to ensure the safety, efficacy, and consistency, by means of modern biomedicine tools. World Health Organization (WHO), by releasing Beijing Declaration, encouraged the integration of evidence-based traditional medicines into national healthcare system, and promoted the regulation and standards that could ensure the appropriate, safe, and effective use of traditional medicines1. Chinese scientists have been endeavoring to uncover the TCM “black box”, and significant progress in the modernization and globalization of TCM has been made in the past two decades, such as the multidisciplinary research platform establishment, significant scientific achievements, booming TCM industry, clinical evaluation, and globalization and professionals training2. However, having a unique theory completely different from the Western medicine, TCM research currently is still in the era of “let a hundred flowers blossom and a hundred schools of thoughts contend”, without a standardized and commonly accepted research strategy that can be followed.
The “multicomponent and multitarget” property of TCM gives rise to the necessity for developing a new theory or evaluation system for the therapeutic basis research, and thus establishing a more scientific quality control system for TCM. On one hand, the development of analytical techniques, such as multidimensional chromatography and LC–MS, has enabled the characterization of 500 plus components from a single herb, simultaneous quantitation of 40 plus compounds from a TCM formula3, 4, 5, 6, and even qualitative identification or quantitative evaluation of an herb from different TCM formula preparations7, 8. Automatic annotation of plant metabolites by in silico database is also achievable9, 10. On the other hand, a number of new theories/strategies and analytical techniques, such as phytomics QC11, spectrum-effect relationship12, 13, metabolomics/chinmedomics14, 15, serum pharmacology/serum pharmacochemistry16, 17, biochromatography18, affinity ultrafiltration/LC–MS coupled with in silico molecular docking19, 20, and activity index (AI)21 or combination index (CI)22, etc., have been developed to screen the bioactive components responsible for the holistic efficacy and to investigate the synergetic effect of multiple components. Network pharmacology is a powerful tool that can elucidate the targets network and inspire drug discovery23. However, by these strategies, TCM is investigated essentially as “herbal medicine” or “natural product”, by which some important TCM factors like the nature/flavor and channel entry have been ignored.
Many factors, such as the species, cultivation/production area conditions (geo-herbalism), harvesting, processing, transportation/storage conditions, extraction/purification, ADME (absorption, distribution, metabolism, and excretion), and interaction of diverse components24, can affect the quality of TCM, which render the study of TCM quality being a systematic endeavor. The quality control system adopted in Chinese Pharmacopoeia in general is chemical marker oriented. The chemical composition and content of markers in TCM medicinal materials, extractives, products, and formulae, are measured by UV, TLC, HPLC (or UHPLC), GC, LC–MS, or GC–MS, for authenticity identification and quality evaluation. Still remarkable insufficiencies occur in the current pharmacopoeia quality monographs, and doubts centered at the relationship between the chemical markers and the holistic efficacy. On occasion, the markers are nonspecific, the detection of which fails to discriminate the official species from the unofficial ones and adulteration in TCM formulae. Thereby, a scientific TCM quality control system should embrace the drug nature and monitor the effect-associated, specific chemical markers by commonly accepted analytical techniques.
Recently, a Q-marker concept towards this endeavor was proposed24, 25, 26, by considering the factors that influence the quality of TCM, to settle the issues in quality standards and quality assurance. The parameters that can affect Q-markers and multidiscipline-based strategies for establishing Q-markers were presented. In contrast to the conventional chemical markers, Q-markers are specific, effect-related, and in alignment with the basic theory of TCM (nature/flavor and channel entry, and the formulation principle). Q-marker-based TCM quality control is thus considered more scientific and beneficial to accelerating the modernization and globalization of TCM. This review in detail depicts the background, definition, and properties of the new concept of Q-marker. Practical strategies for constructing the holistic quality control system of TCM are provided, based on the competent practice in establishing TCM quality standards for Chinese Pharmacopoeia, United States Pharmacopoeia, and European Pharmacopoeia. We expect the concept of Q-marker can arouse peer resonance, and we together make more contribution to the modernization and globalization of TCM.
2. Concept of Q-marker and its role in quality control of TCM
2.1. Background
Comprehensive researches on the quality of TCM and establishment of the quality standards are deemed as a complicated and systematic task due to its highly complex chemical composition. Despite collections of documents are available associated with the chemical analysis and bioactivity evaluation, the strategies and approaches developed by different research groups are diverse and even controversial. Whether the chemical markers used in pharmacopoeia are responsible for and directly related to the clinical effect of TCM (involving the raw drug materials, extractives, products, and formula preparations in diverse dosage forms) is often questionable. Given approximately 1/3 of the species collected in Chinese Pharmacopoeia (2015 edition) having 2–5 different plant sources, the authentication of the TCM materials hence becomes a vital factor in establishing the quality standards. Development of the practical analytical methods (qualitative and quantitative analyses) to capture the fingerprint and content information of multiple chemical markers (specific and controllable) is the basis for quality control of TCM. The concept of Q-marker was proposed by considering the complex factors from the basic theory of TCM, the formulation, preparation technology, dosage form, and usage of formula preparations, to standardize TCM quality research and quality standards elaboration, to enhance the quality consistency, controllability, and traceability. It would benefit the manufacturing process control and quality management of TCM products.
2.2. Definition of Q-marker
The Q-marker of TCM refers to the intrinsic or processing/preparation-resultant chemical substances closely associated with the functional properties that exist in the raw materials and products of TCM (involving the decoction pieces, decoctions, extractives, and Chinese patent medicines), which can be used as the indicators for quality control of TCM to embody the safety and effectiveness. However, the chemicals that are absorbed or newly generated after an in vivo process (such as the human in vivo metabolites, gut enzymes or microorganism transformed chemicals) in need of additional structural elucidation are out of this scope. From the definition, the basic properties of Q-marker can be outlined in four aspects: 1) they are the intrinsic chemical components in TCM materials and products, or processing/preparation-resultant; 2) they are functional properties-associated, with definite chemical structures; 3) they can be qualitatively characterized and quantitatively determined; and 4) for the formulae, the representative substances of the Monarch are firstly considered, and those from the Minister, Assistant, and Guide, should be considered as well, following the compatibility theory. The factors, involving 1) cell and tissue specificity, 2) organ specificity, 3) developmental specificity for biosynthesis, 4) the extrinsic factors of growing process (drug materials), and 5) the preparation factors (formula preparations), can affect Q-markers. In establishment of Q-markers, special attention should be paid to the components that ensure the authenticity (identity marker), differentiate the quality difference (superiority/inferiority marker), and identify the geo-authenticity (geo-authentic marker).
2.3. Approaches for establishing Q-markers
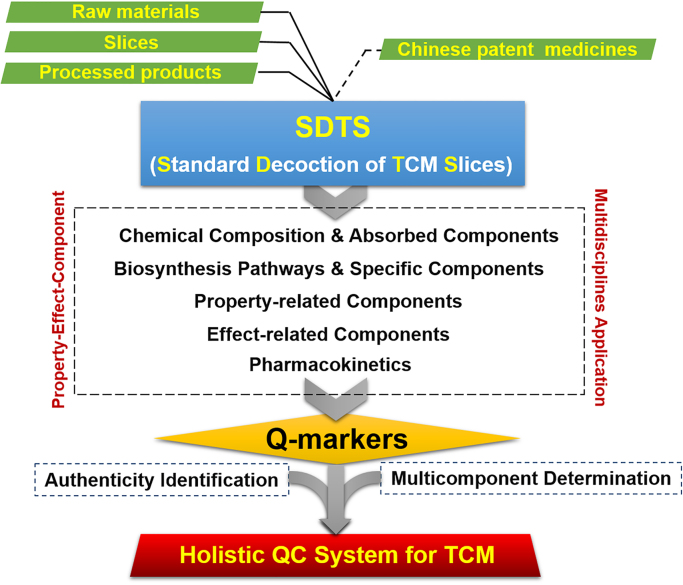
Standard decoction of TCM slices (SDTS) is the standard form for clinical use, and can be a core segment to trace the efficacy of TCM27. Therefore, the quality research using SDTS as a reference sample is conducted to establish the Q-markers, which can be traced to the raw drug materials and slices (as well as the processed products) and extended to the formula preparations (Chinese patent medicines). A strategy for establishing the Q-markers of SDTS has been proposed as follows24, 25, as shown in Fig. 1.
-
1)
Sample requirement. The samples should be representative and authenticated to a definite species based on the requirements of Chinese Pharmacopoeia for TCM raw materials and slices; conventional identification and DNA barcoding can be integrated28.
-
2)
Processing of the slices. The processing should be in accordance with the pharmacopoeia requirements or the approaches used in the geo-authentic location or major producing regions.
-
3)
Quality examination of the decoction. Before decocting, the content of markers, appearance, test items, and water content, should be examined.
-
4)
Sample amount. A recommended amount, 100 g; batch number, 10 above.
-
5)
Quantity of water. 6–8 folds of the slice amount in volume.
-
6)
Soaking time and decoction times. Recommended soaking time, 30 min; decoction times: twice (30 and 20 min, respectively).
-
7)
Concentration method. Recommended concentration under reduced pressure and at the temperature lower than 50 °C; final volume, 5 folds of the drug weight; preservation, at a low temperature avoiding light.
-
8)
Fingerprint of SDTS. HPLC, GC, LC–MS, and GC–MS, are recommended to count the peak number and identify the peaks. Those peaks that are specific and associated with the effect will be selected as the Q-markers. The fingerprint is determined by comparing with the reference drug or reference standards.
-
9)
Quantitative determination of markers. HPLC, GC, LC–MS, and GC–MS approaches are recommended. Single standard to determine multicomponents (SSDMC) is a practical multicomponent determination method29, 30.
Figure 1.
A general flowchart for Q-marker-based establishment of holistic quality control system of TCM.
A triarchic theory of “property-effect-component”, by integrating multidiscipline-based strategies (including natural products chemistry, analytical chemistry, bionics, computer aided design, pharmacology, system biology, and pharmacodynamics) is recommended to discover effect-associated markers following the TCM basic theory, which are regarded as the Q-markers.
-
1)
Comprehensive analysis of chemical composition and identification of the absorbed components. By combining phytochemical approaches (column chromatography, preparative HPLC, and NMR) and LC–MS (high-resolution MS is recommended), the chemical composition is globally profiled and characterized. The absorbed components and their metabolites are identified by LC–MS.
-
2)
Exploration of biosynthetic pathways and specificity. For the characterized components, further biosynthetic pathways and specificity (among the genus, species, organ, tissue, and growth and development stages) should be considered to discern which thereof are the specific components.
-
3)
Discovery of property-related components. Bionics techniques, such as the electronic nose (EN) and electronic tongue (ET), coupled with phytochemical isolation (total extract, fractions, and single compounds) are used to screen the components associated with five flavors of TCM (pungent, sweet, sour, bitter, and salty)31, 32, 33, 34, 35. Molecular docking (bitter-taste receptor: hTAS2R10, positive ligand: quinine; pungent-taste receptor: OR7D4, positive ligand: capsaicine; GPCR) can further give more evidence for the discovery of property-related components.
-
4)
Discovery of effect-associated components. The extract and single compounds are tested on multiple models (including animals, excised organs, cells, enzymes, etc.) related to the major effect. Network pharmacology analysis of representative compounds is performed to predict the multitarget action pathways. Metabolomics enables discovery of the metabolism pathways associated with the therapeutic effects.
-
5)
Pharmacokinetics study. The pharmacokinetics (PK) parameters (Cmax, tmax, AUC0–t, AUC 0–∞, Vd, t1/2, MRT0–t, and CL) of the extract (TCM raw materials and formulae), single TCM material, and representative compounds in animals are determined. Alternations of the parameters conduce to elucidate the reasonability of compound compatibility.
3. Classic cases for the establishment of quality marker components (Q-markers)
Consistent with the concept of Q-marker, the authors have practiced for more than twenty years in the establishment of quality marker components for quality control of TCM raw materials and TCM formula. Classic cases are illustrated here.
Establishment of the Q-markers that ensure the authenticity of Ginseng Radix et Rhizoma (Panax ginseng; Ren-Shen) is of difficulty, since the chemical difference among the congeneric species (such as Panax quinquefolius and Panax notoginseng) and different parts (the root/rhizome, stem/leaf, flower, berry, and seed) needs to be clarified. Nontargeted metabolomics and artificial nerve network (ANN) were employed to explore the identity markers for five different parts of P. ginseng36. Moreover, a “commercial-homophyletic” comparison-induced biomarkers verification strategy was proposed to find the robust markers that could embody the inherent chemical differences. Given the bioactivity and specificity37, 38, ginsenosides were mainly used to establish the quality markers. Consequently, the seed contains very few ginsenosides and can be easily discriminated from the other four parts. Eleven robust marker compounds, involving ginsenosides Re, Rg1, Rg2, Rc, Rf, F1, Ro, vina-R4, acetyl-Rh13/R19, floral-I/J, and a flavonoid, were diagnostic for the exact identification among the root/rhizome, stem/leaf, flower, and berry of P. ginseng. Particularly, root and leaf that are recorded in Chinese Pharmacopoeia (2015 edition) with differentiated nature/flavor and channel entry differ in ginsenosides Rf, F1, and Rd. The composition of ginsenosides in the roots of the congeneric P. ginseng, P. quinquefolius, and P. notoginseng, was also elucidated39. Seventeen saponins, including the identity markers and significantly different components, were deduced for their discrimination, which were also diagnostic for identifying them from the TCM formula preparations. In particular, a new identity marker, Rs1, can be used to discriminate P. ginseng from P. quinquefolius.
Corydalis Rhizoma (Corydalis yanhusuo; Yan-Hu-Suo) is a famous TCM herb that can promote qi circulation and relieve pains. Its Q-markers were established as a demonstrative study to construct the quality control approaches and quality standards associated with safety and efficacy40. Firstly, the LC–MS analysis of the chemical components of a standard extract of vinegar-processed Corydalis Rhizoma identified 28 alkaloids. Secondly, based on the biosynthesis, specificity, and the content, tertrahydropalmatine, corydaline, coptisine, palmatine, dehydrocorydaline, D-tetrahydrojatrorrhizine, and protopine, were the potential Q-markers. Thirdly, the pharmacodynamic experiments demonstrated the antalgic effects of the 60% ethanol extract and tetrahydropalmatine on the animal, excised organ, and cell models. Network pharmacology analysis revealed the pathways were associated with the hormonal regulation, central analgesia, spasmolysis, inflammation, and immune-regulation. Accordingly, tetrahydropalmatine, palmatine, D-glaucine, and biflorine, are the major therapeutic basis, and regarded as the Q-markers. Subsequent drug property screening experiments by molecular docking (bitter receptor: hTAS2R10 and GPCR) testified that tetrahydropalmatine and protopine can be the substances associated with the bitter and pungent taste properties. Eleven prototype alkaloids and six metabolites were detected in the plasma of rats orally administrated with a Corydalis Rhizoma extract, and seven prototype alkaloids were found in the brain tissues. Tetrahydropalmatine, corydaline, and biflorine that could penetrate into blood–brain barrier (BBB), exert the antalgic effect. Based on all aforementioned evidence, seven alkaloids (corydaline, tetrahydropalmatine, coptisine, protopine, palmatine, dehydrocorydaline, and D-tetrahydrojatrorrhizine) are finally selected as the Q-markers of Corydalis Rhizoma.
Whether the Q-markers established based on the drug material can be extended to the TCM formula, the Q-markers of Yuanhu Zhitong Dropping Pill (YZDP) were investigated41. YZDP is prepared from Corydalis Rhizoma (vinegar-processed) and Angelicae Dahuricae Radix. By applying the similar research strategy, 51 components (including 28 alkaloids and 23 coumarins) were characterized from YZDP, and 26 prototype components and 14 metabolites were identified in the rat plasma. Fifteen prototype components thereof could pass through the BBB. Protoberberine alkaloids are the potential substances for the bitter taste, while tetrahydropalmatine, biflorine, and imperatorin, are the possible pungent taste related substances. The targets of YZDP on dysmenorrhea are the pitocin receptors, M receptors, H1 receptors, and synthesis of prostaglandin. The multitarget mechanism of action of YZDP is related to the hormonal regulation, central analgesia, spasmolysis of smooth muscle, antiinflammation, and immunoregulation, which is analogous to that discovered for Corydalis Rhizoma. The signaling pathways of glyceryl phosphalide metabolism, amino acids metabolism, and sphingolipid metabolism, were disturbed, by which dysmenorrhea could be effectively relieved. Formulation can enhance the absorption of corydaline, tetrahydropalmatine, and biflorine, and delay the absorption and prolong the residence time of imperatorin and isoimperatorin. Ultimately, five components (corydaline, tetrahydropalmatine, biflorine, imperatorin, and isoimperatorin), are established as the Q-markers for YZDP, of which the former two alkaloids were included in the Q-markers of Corydalis Rhizoma. The selection criteria for establishing the Q-markers of TCM formulae highlight the Monarch herb, but the Minister, Assistant, and Guide herbs are also taken into consideration.
4. Construction of the holistic quality control system of TCM based on Q-markers
When the “quality consistency, controllability, traceability, and manufacturing-related” Q-markers are defined, in the next, a holistic quality control system of TCM can be established by monitoring these markers with practical analytical approaches. Core issues in constructing TCM quality control system are the authenticity identification, quality assessment, and foreign substances control (pesticide residue, heavy metal, and aflatoxins, etc.). Q-markers, as the transferable substances, play a vital role in the process of authenticity identification and quality assessment for TCM (including the raw materials, extractives, products, and compound formula preparations), and these two aspects are therefore highlighted.
4.1. Authenticity identification
A primary goal of performing quality control is to ensure the authenticity of a drug, that is, to identify the correct species from the counterfeits and surrogates. Diverse identification methods based on the experience, morphology, microscopy, physiochemical properties, TLC, fingerprint, characteristic chromatogram, and DNA sequencing, have been employed in the identification item of Chinese Pharmacopoeia (2015 edition)1, 42. Amongst them, TLC and fingerprint are capable to capture the overall chemical profiles43, 44, 45, and characteristic chromatogram gives the composition information of characteristic components that are of the authentication significance and also the substance basis of Q-markers. Despite approximately 1/3 of TCMs collected in Chinese Pharmacopoeia are from multiple plant sources, discovery and definition of the species thereof is a premise for elaborating a feasible and scientific quality standard to ensure the efficacy and consistency46. Application of Q-marker could help the selection of high-quality species that possesses the best clinical effect. As a typical example, Ganoderma (Ling-Zhi) is from the dried fruiting bodies of Canoderma lucidum (Leyss. Ex Fr.) Karst and C. sinense Zhao, Xu et Zhang as recorded in Chinese Pharmacopoeia. However, it was found that, different from C. lucidum, C. sinense contains extremely rare triterpenic acids47, a significant group of bioactive constituents closely related to the therapeutic effects48, 49, 50. C. lucidum is finally selected as the unique authentic species in establishing the quality standard monograph of Ganoderma lucidum Fruiting Body for Herbal Medicine Compendium of United States Pharmacopoeia (https://hmc.usp.org/monographs/ganoderma-lucidum-fruiting-body-1-0).
4.2. Quality assessment
Multicomponent determination is commonly accepted as the golden standard for assessing the quality of TCM51, 52, 53. Content determination of the established Q-markers, by routine HPLC, GC, and LC–MS, is an important factor for establishing a scientific TCM quality standard. The quantitative markers can involve a series of bioactive analogs or different subtype of components depending on the knowledge of the therapeutic basis. Simultaneous quantitation of multimarkers, on one hand, is beneficial to a holistic quality evaluation and to discriminate adulterants in extractives, products, and compound formula preparations, and on the other hand, puts forward a strict requirement on reference substances. The use of more reference standards can greatly increase the testing cost. The introduction of “single standard to determine multicomponents” (SSDMC) method meets the necessity of multimarker monitoring, but only utilizes a single reference standard that is easily accessible29, 30, 54. SSDMC has been employed to establish the TCM quality standards for United States Pharmacopoeia and European Pharmacopoeia, which is also expected to be widely adopted by Chinese Pharmacopoeia in the future. Currently the reported SSDMC methods are based on HPLC–UV analysis, which in general can simultaneously determine less than 10 quantitative markers. To achieve the comprehensive quality evaluation for compound formula preparations that contain more than ten herbal medicines like Niuhuang Shangqing Pill5, the coverage of SSDMC should be further expanded, in which chromatographic separation and unbiased detection are the two major issues. Multidimensional liquid chromatography (MDLC) is a powerful tool in separating and quantifying the multicomponents of TCM compound formula preparations8. Charged aerosol detector (CAD), reported as a new universal detector55, 56, may be utilized in SSDMC to determine different subcategories of herbal metabolites (Q-markers). Whether the hybridization of MDLC and CAD detection suits the establishment of SSDMC approaches is in need of strict experimental validation29. Moreover, multicomponent determination coupled with chemometrics can visualize the authenticity identification and quality assessment, discovery of Q-markers, and even identification of the medicinal part57, 58, 59.
5. Successful cases of formulating scientific quality standards based on Q-markers
Ginseng is ranked among the most popular natural products worldwide60. Three Panax species, P. ginseng (Asian ginseng), P. quinquefolius (American ginseng), and P. notoginseng (Sanchi ginseng), are largely consumed as the healthcare products, dietary supplements, and herbal medicines37. Based on the lucubration of the chemical difference of these congeneric ginseng drugs39, 61, 62, we have successfully elaborated or improved the quality standard monographs of P. ginseng, P. quinquefolius, P. notoginseng, and red ginseng (the processed product of P. ginseng) for Herbal Medicines Compendium of United States Pharmacopoeia, which are here used as a successful case to establish Q-marker-based quality standards. Different growing environment, ages, and processing, can affect the quality, resulting in the differentiated strengths of Ginseng drugs in the markets. The chemical similarity (ginsenosides) renders it particularly important to monitor the Q-markers that enable identification of the authentic species from its counterfeits and surrogates when establishing the quality standard monographs.
Firstly, a sample library of Ginseng is established, which contains multiple batches of samples for each species (generally>10)57. They have clear information of purchasing channels and places of production, and have been strictly authenticated. For P. ginseng, the samples between wild and cultivated, and from different parts, should be discriminated. The samples of red ginseng should be collected from GMP-qualified manufacturers. P. notoginseng samples include the taproot (Zhugen), rhizome (Jiankou), branching root (Jintiao), and fibrous root (Xugen). Secondly, LC–MS fingerprint coupled with multivariate statistical analysis is used to capture the global chemical information and discover the differential components. High-resolution mass spectrometry, including QTOF and LTQ-Orbitrap, coupled with UHPLC or offline comprehensive 2D-LC facilitates the high-throughput or profound qualitative analysis of ginsenosides from different Panax species and different parts3, 36, 39, 63, 64. Thirdly, a high-performance TLC approach that capacitates the species discrimination is established focusing on the examination of ginsenoside Q-markers. High-sensitivity and high-resolution TLC chromatograms are obtained by means of an automatic sampling applicator and temperature-humidity controllable automatic developing instrument on an HP-TLC silica gel plate. The TLC chromatograms of P. ginseng, P. quinquefolius, P. notoginseng, and red ginseng, show distinct spots corresponding to the ginsenoside markers that include m-Rb1, m-Rc (or m-Rd), Rc, and Rf. Moreover, an HPLC–UV fingerprint method is developed to establish the characteristic chromatograms. The presence/absence and relative intensity of nine ginsenosides (noto-R1, Rg1, Re, Rf, Rb1, Ro, Rc, Rb2, and Rd) are diagnostic for the identification and discrimination of P. ginseng, P. quinquefolius, P. notoginseng, and red ginseng. Fourth, SSDMC is used for quality evaluation. For red ginseng, ginsenoside Rg1 is selected as the single reference standard to determine the contents of eight ginsenosides (Rg1, Re, Rf, Rb1, Ro, Rc, Rb2, and Rd). In the case of quality assessment of P. notoginseng, a five-minute, UHPLC-based SSDMC method is developed that enables the simultaneous determination of five major saponins (noto-R1, Rg1, Re, Rb1, and Rd). On the other hand, UHPLC/QTOF-MS-based metabolomics is employed to establish the characteristic components of Xueshuantong and Xuesaitong, two similar Notoginseng total saponins products64.
New attempts have been made aiming at improving the analysis efficiency and the identification coverage when facing the quality control of TCM compound formula preparations (also known as Chinese patent medicines, CPMs). A new strategy, namely “monomethod-heterotrait matrix”, is proposed, which can identify all the compositional drugs from a single CPM or identify a TCM from different CPMs, and quantify the Q-markers (from the monarch drug) from different CPMs. As the classic examples, selective ion monitoring (SIM) coupled with UHPLC separation is able to identify Notoginseng Radix et Rhizoma (San-Qi), Carthumi Flos (Hong-Hua), and Chuanxiong Rhizoma (Chuang-Xiong) from Shuxiong tablet65, and, on the other hand, can identify Carthumi Flos from eleven different CPMs using quinochalcone C-glycosides as the Q-markers7. The contents of five major Notoginseng saponins (representative of the monarch drug P. notoginseng) in eight different CPMs are determined by a multiheart cutting 2D-LC approach8. These cases testify the feasibility of “monomethod-heterotrait matrix” strategy in the qualitative and quantitative analyses of CPMs with much improved efficiency and potency.
6. Conclusions
The modernization and globalization of TCM have become an overwhelming tendency supported by the impressive progress in TCM standardization technologies. The concept of Q-markers is a reasonable system that can guide the quality investigations following the basic TCM theory. Application of multidisciplinary techniques benefits the discovery of Q-markers. Q-markers-based quality standard will be regarded as a more scientific quality control approach for TCM drug materials, extractives, products, and compound formula preparations. It is also a general conception that Q-marker-based quality control for Chinese medicine production from the raw materials to patent medicinal products would be more beneficial for the transitivity and traceability in TCM production process.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Dean Guo, Email: daguo@simm.ac.cn.
Changxiao Liu, Email: liuchangxiao@163.com.
References
- 1.Guo D.A., Wu W.Y., Ye M., Liu X., Cordell G.A. A holistic approach to the quality control of traditional Chinese medicine. Science. 2015;347:S23–S31. [Google Scholar]
- 2.Zhang B.L., Zhang J.H. Twenty years׳ review and prospect of modernization research on traditional Chinese medicine. China J Chin Mater Med. 2015;40:3331–3334. [PubMed] [Google Scholar]
- 3.Qiu S., Yang W.Z., Shi X.J., Yao C.L., Yang M., Liu X. A green protocol for efficient discovery of novel natural compounds: characterization of new ginsenosides from the stems and leaves of Panax ginseng as a case study. Anal Chim Acta. 2015;893:65–76. doi: 10.1016/j.aca.2015.08.048. [DOI] [PubMed] [Google Scholar]
- 4.Qiao X., Lin X.H., Ji S., Zhang Z.X., Bo T., Guo D.A. Global profiling and novel structure discovery using multiple neutral loss/precursor ion scanning combined with substructure recognition and statistical analysis (MNPSS): characterization of terpene-conjugated curcuminoids in Curcuma longa as a case study. Anal Chem. 2016;88:703–710. doi: 10.1021/acs.analchem.5b02729. [DOI] [PubMed] [Google Scholar]
- 5.Liang J., Wu W.Y., Sun G.X., Wang D.D., Hou J.J., Yang W.Z. A dynamic multiple reaction monitoring method for the multiple components quantification of complex traditional Chinese medicine preparations: niuhuang Shangqing pill as an example. J Chromatogr A. 2013;1294:58–69. doi: 10.1016/j.chroma.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 6.Wang Q., Song W., Qiao X., Ji S., Kuang Y., Zhang Z.X. Simultaneous quantification of 50 bioactive compounds of the traditional Chinese medicine formula Gegen-Qinlian decoction using ultra-high performance liquid chromatography coupled with tandem mass spectrometry. J Chromatogr A. 2016;1454:15–25. doi: 10.1016/j.chroma.2016.05.056. [DOI] [PubMed] [Google Scholar]
- 7.Si W., Yang W.Z., Guo D.A., Wu J., Zhang J.X., Qiu S. Selective ion monitoring of quinochalcone C-glycoside markers for the simultaneous identification of Carthamus tinctorius L. in eleven Chinese patent medicines by UHPLC/QTOF MS. J Pharm Biomed Anal. 2016;117:510–521. doi: 10.1016/j.jpba.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 8.Yao C.L., Yang W.Z., Wu W.Y., Da J., Hou J.J., Zhang J.X. Simultaneous quantitation of five Panax notoginseng saponins by multi heart-cutting two-dimensional liquid chromatography: method development and application to the quality control of eight notoginseng containing Chinese patent medicines. J Chromatogr A. 2015;1402:71–81. doi: 10.1016/j.chroma.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Ridder L., van der Hooft J.J., Verhoeven S., de Vos R.C., Bino R.J., Vervoort J. Automatic chemical structure annotation of an LC–MSn based metabolic profile from green tea. Anal Chem. 2013;85:6033–6040. doi: 10.1021/ac400861a. [DOI] [PubMed] [Google Scholar]
- 10.Qiu F., Fine D.D., Wherritt D.J., Lei Z.T., Sumner L.W. PlantMAT: a metabolomics tool for predicting the specialized metabolic potential of a system and for large-scale metabolite identifications. Anal Chem. 2016;88:11373–11383. doi: 10.1021/acs.analchem.6b00906. [DOI] [PubMed] [Google Scholar]
- 11.Tilton R., Paiva A.A., Guan J.Q., Marathe R., Jiang Z.L., van Eyndhoven W. A comprehensive platform for quality control of botanical drugs (PhytomicsQC): a case study of Huangqin Tang (HQT) and PHY906. Chin Med. 2010;5:30. doi: 10.1186/1749-8546-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu G.L., Xie M., Yang X.Y., Song Y., Yan C., Yang Y. Spectrum-effect relationships as a systematic approach to traditional Chinese medicine research: current status and future perspectives. Molecules. 2014;19:17897–17925. doi: 10.3390/molecules191117897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qi J., Yu B.Y. A new methodology for the quality evaluation of traditional Chinese medicine-integrated spectrum-effect fingerprint research. Chin J Nat Med. 2010;8:171–176. [Google Scholar]
- 14.Wang P.C., Wang Q.H., Yang B.Y., Zhao S., Kuang H.X. The progress of metabolomics study in traditional Chinese medicine research. Am J Chin Med. 2015;43:1281–1310. doi: 10.1142/S0192415X15500731. [DOI] [PubMed] [Google Scholar]
- 15.Wang X.J., Zhang A.H., Sun H., Han Y., Yan G.L. Discovery and development of innovative drug from traditional medicine by integrated chinmedomics strategies in the post-genomic era. Trends Anal Chem. 2016;76:86–94. [Google Scholar]
- 16.Yan G.L., Sun H., Zhang A.H., Han Y., Wang P., Wu X.H. Progress of serum pharmacochemistry of traditional Chinese medicine and further development of its theory and method. China J Chin Mater Med. 2015;40:3406–3412. [PubMed] [Google Scholar]
- 17.Wang Y.L., Li G.Q., Zhou Y., Yin D.K., Tao C.L., Han L. The difference between blood-associated and water-associated herbs of Danggui-Shaoyao San in theory of TCM, based on serum pharmacochemistry. Biomed Chromatogr. 2016;30:579–587. doi: 10.1002/bmc.3586. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y., Kong L., Hu L.H., Lei X.Y., Yang L., Chou G.X. Biological fingerprinting analysis of the traditional Chinese prescription Longdan Xiegan Decoction by on/off-line comprehensive two-dimensional biochromatography. J Chromatogr B. 2007;860:185–194. doi: 10.1016/j.jchromb.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 19.Song H.P., Chen J., Hong J.Y., Hao H.P., Qi L.W., Lu J. A strategy for screening of high-quality enzyme inhibitors from herbal medicines based on ultrafiltration LC–MS and in silico molecular docking. Chem Commun. 2015;51:1494–1497. doi: 10.1039/c4cc08728c. [DOI] [PubMed] [Google Scholar]
- 20.Wang F., Xiong Z.Y., Li P., Yang H., Gao W., Li H.J. From chemical consistency to effective consistency in precise quality discrimination of Sophora flower-bud and Sophora flower: discovering efficacy-associated markers by fingerprint-activity relationship modeling. J Pharm Biomed Anal. 2017;132:7–16. doi: 10.1016/j.jpba.2016.09.042. [DOI] [PubMed] [Google Scholar]
- 21.Wang S.F., Wang H.Q., Liu Y.N., Wang Y., Fan X.H., Cheng Y.Y. Rapid discovery and identification of anti-inflammatory constituents from traditional Chinese medicine formula by activity index, LC–MS, and NMR. Sci Rep. 2016;6:31000. doi: 10.1038/srep31000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long F., Yang H., Xu Y.M., Hao H.P., Li P. A strategy for the identification of combinatorial bioactive compounds contributing to the holistic effect of herbal medicines. Sci Rep. 2015;5:12361. doi: 10.1038/srep12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hao D.C., Xiao P.G. Network pharmacology: a Rosetta stone for traditional Chinese medicine. Drug Dev Res. 2014;75:299–312. doi: 10.1002/ddr.21214. [DOI] [PubMed] [Google Scholar]
- 24.Liu C.X., Chen S.L., Xiao X.H., Zhang T.J., Hou W.B., Liao M.L. A new concept on quality marker of Chinese materia medica: quality control for Chinese medicinal products. Chin Tradit Herbal Drugs. 2016;47:1443–1457. [Google Scholar]
- 25.Liu C.X., Cheng Y.Y., Guo D.A., Zhang T.J., Li Y.Z., Hou W.B. A new concept on quality marker for quality assessment and process control of Chinese medicines. Chin Herbal Med. 2017;9:3–13. [Google Scholar]
- 26.Guo D.A. Quality marker concept inspires the quality research of traditional Chinese medicines. Chin Herbal Med. 2017;9:1–2. [Google Scholar]
- 27.Chen S.L., Liu A., Li Q., Toru S., Zhu G.W., Sun Y. Research strategies in standard decoction of medicinal slices. China J Chin Mater Med. 2016;41:1367–1375. doi: 10.4268/cjcmm20160801. [DOI] [PubMed] [Google Scholar]
- 28.Chen S.L., Pang X.H., Yao H., Han J.P., Luo K. Identification system and perspective for DNA barcoding traditional Chinese materia medica. World Sci Technol/Mod Tradit Chin Med Mater Med. 2011;13:747–754. [Google Scholar]
- 29.Hou J.J., Wu W.Y., Da J., Yao S., Long H.L., Yang Z. Ruggedness and robustness of conversion factors in method of simultaneous determination of multi-components with single reference standard. J Chromatogr A. 2011;1218:5618–5627. doi: 10.1016/j.chroma.2011.06.058. [DOI] [PubMed] [Google Scholar]
- 30.Hou J.J., Wu W.Y., Liang J., Yang Z., Long H.L., Cai L.Y. A single, multi-faceted, enhanced strategy to quantify the chromatographically diverse constituents in the roots of Euphorbia kansui. J Pharm Biomed Anal. 2014;88:321–330. doi: 10.1016/j.jpba.2013.08.049. [DOI] [PubMed] [Google Scholar]
- 31.Sun Y.P., Zhang T.J., Cao H., Xu J., Gong S.X., Chen C.Q. Expression of pungent-taste herbs and their applications in clinical compatibility. Chin Tradit Herbal Drugs. 2015;46:785–790. [Google Scholar]
- 32.Zhang J.Y., Cao H., Gong S.X., Xu J., Han Y.Q., Zhang T.J. Expression of sweet-taste of Chinese materia medica and its application in clinical compatibility. Chin Tradit Herbal Drugs. 2016;47:533–539. [Google Scholar]
- 33.Cao H., Zhang J.Y., Gong S.X., Xu J., Zhang T.J., Liu C.X. Expression of sour-taste properties of Chinese materia medica and their applications in clinical compatibility. Chin Tradit Herbal Drugs. 2015;46:3617–3622. [Google Scholar]
- 34.Zhang J.Y., Cao H., Xu J., Han Y.Q., Gong S.X., Zhang T.J. Expression of bitter taste of Chinese materia medica and its application in clinical compatibility. Chin Tradit Herbal Drugs. 2016;47:187–193. [Google Scholar]
- 35.Zhang J.Y., Cao H., Gong S.X., Xu J., Han Y.Q., Zhang T.J. Expression of salt-taste herbs and their applications in clinical compatibility. Chin Tradit Herbal Drugs. 2016;47:2797–2802. [Google Scholar]
- 36.Qiu S., Yang W.Z., Yao C.L., Qiu Z.D., Shi X.J., Zhang J.X. Nontargeted metabolomic analysis and “commercial-homophyletic” comparison-induced biomarkers verification for the systematic chemical differentiation of five different parts of Panax ginseng. J Chromatogr A. 2016;1453:78–87. doi: 10.1016/j.chroma.2016.05.051. [DOI] [PubMed] [Google Scholar]
- 37.Yang W.Z., Hu Y., Wu W.Y., Ye M., Guo D.A. Saponins in the genus Panax L. (Araliaceae): a systematic review of their chemical diversity. Phytochemistry. 2014;106:7–24. doi: 10.1016/j.phytochem.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 38.Park J.D., Rhee D.K., Lee Y.H. Biological activities and chemistry of saponins from Panax ginseng C. A. Meyer. Phytochem Rev. 2005;4:159–175. [Google Scholar]
- 39.Yang W.Z., Qiao X., Li K., Fan J.R., Bo T., Guo D.A. Identification and differentiation of Panax ginseng, Panax quinquefolium, and Panax notoginseng by monitoring multiple diagnostic chemical markers. Acta Pharm Sin B. 2016;6:568–575. doi: 10.1016/j.apsb.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang T.J., Xu J., Han Y.Q., Zhang H.B., Gong S.X., Liu C.X. Quality markers research on Chinese materia medica: quality evaluation and quality standards of Corydalis Rhizoma. Chin Tradit Herbal Drugs. 2016;47:1458–1467. [Google Scholar]
- 41.Zhang T.J., Xu J., Shen X.P., Han Y.Q., Hu J.F., Zhang H.B. Relation of “property-response-component” and action mechanism of Yuanhu Zhitong Dropping pills based on quality marker (Q-marker) Chin Tradit Herbal Drugs. 2016;47:2199–2211. [Google Scholar]
- 42.Liang Y.Z., Xie P.S., Chan K. Quality control of herbal medicines. J Chromatogr B. 2004;812:53–70. doi: 10.1016/j.jchromb.2004.08.041. [DOI] [PubMed] [Google Scholar]
- 43.Zhong X.K., Li D.C., Jiang J.G. Identification and quality control of Chinese medicine based on the fingerprint techniques. Curr Med Chem. 2009;16:3064–3075. doi: 10.2174/092986709788803051. [DOI] [PubMed] [Google Scholar]
- 44.Tian R.T., Xie P.S., Liu H.P. Evaluation of traditional Chinese herbal medicine: Chaihu (Bupleuri Radix) by both high-performance liquid chromatographic and high-performance thin-layer chromatographic fingerprint and chemometric analysis. J Chromatogr A. 2009;1216:2150–2155. doi: 10.1016/j.chroma.2008.10.127. [DOI] [PubMed] [Google Scholar]
- 45.Xie P.S., Chen S.B., Liang Y.Z., Wang X.H., Tian R.T., Upton R. Chromatographic fingerprint analysis–a rational approach for quality assessment of traditional Chinese herbal medicine. J Chromatogr A. 2006;1112:171–180. doi: 10.1016/j.chroma.2005.12.091. [DOI] [PubMed] [Google Scholar]
- 46.Wu W.Y., Guo D.A. Strategies for elaboration of comprehensive quality standard system on traditional Chinese medicine. China J Chin Mater Med. 2014;39:351–356. [PubMed] [Google Scholar]
- 47.Da J., Wu W.Y., Hou J.J., Long H.L., Yao S., Yang Z. Comparison of two officinal Chinese pharmacopoeia species of Ganoderma based on chemical research with multiple technologies and chemometrics analysis. J Chromatogr A. 2012;1222:59–70. doi: 10.1016/j.chroma.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 48.Gill B.S., Navgeet, Kumar S. Ganoderic acid targeting multiple receptors in cancer: in silico and in vitro study. Tumor Biol. 2016;37:14271–14290. doi: 10.1007/s13277-016-5291-8. [DOI] [PubMed] [Google Scholar]
- 49.Shi L., Ren A., Mu D.S., Zhao M.W. Current progress in the study on biosynthesis and regulation of ganoderic acids. Appl Microbiol Biotechnol. 2010;88:1243–1251. doi: 10.1007/s00253-010-2871-1. [DOI] [PubMed] [Google Scholar]
- 50.Sanodiya B.S., Thakur G.S., Baghel R.K., Prasad G.B., Bisen P.S. Ganoderma lucidum: a potent pharmacological macrofungus. Curr Pharm Biotechnol. 2009;10:717–742. doi: 10.2174/138920109789978757. [DOI] [PubMed] [Google Scholar]
- 51.Qi L.W., Yu Q.T., Li P., Li S.L., Wang Y.X., Sheng L.H. Quality evaluation of Radix Astragali through a simultaneous determination of six major active isoflavonoids and four main saponins by high-performance liquid chromatography coupled with diode array and evaporative light scattering detectors. J Chromatogr A. 2006;1134:162–169. doi: 10.1016/j.chroma.2006.08.085. [DOI] [PubMed] [Google Scholar]
- 52.Yuan J.P., Wang J.H., Liu X., Kuang H.C., Zhao S.Y. Simultaneous determination of free ergosterol and ergosteryl esters in Cordyceps sinensis by HPLC. Food Chem. 2007;105:1755–1759. [Google Scholar]
- 53.He D.X., Chen B., Tian Q.Q., Yao S.Z. Simultaneous determination of five anthraquinones in medicinal plants and pharmaceutical preparations by HPLC with fluorescence detection. J Pharm Biomed Anal. 2009;49:1123–1127. doi: 10.1016/j.jpba.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 54.Da J., Cheng C.R., Yao S., Long H.L., Wang Y.H., Khan I.A. A reproducible analytical system based on the multi-component analysis of triterpene acids in Ganoderma lucidum. Phytochemistry. 2015;114:146–154. doi: 10.1016/j.phytochem.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 55.Poplawska M., Blazewicz A., Bukowinska K., Fijalek Z. Application of high-performance liquid chromatography with charged aerosol detection for universal quantitation of undeclared phosphodiesterase-5 inhibitors in herbal dietary supplements. J Pharm Biomed Anal. 2013;84:232–243. doi: 10.1016/j.jpba.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 56.Viinamäki J., Ojanperä I. Photodiode array to charged aerosol detector response ratio enables comprehensive quantitative monitoring of basic drugs in blood by ultra-high performance liquid chromatography. Anal Chim Acta. 2015;865:1–7. doi: 10.1016/j.aca.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 57.Liang Y.Z., Xie P.S., Chau F. Chromatographic fingerprinting and related chemometric techniques for quality control of traditional Chinese medicines. J Sep Sci. 2010;33:410–421. doi: 10.1002/jssc.200900653. [DOI] [PubMed] [Google Scholar]
- 58.Wu W.Y., Guo D.A. Several thoughts and suggestions on international quality standard system construction of traditional Chinese medicine. World Sci Technol/Mod Tradit Chin Med Mater Med. 2014;16:496–501. [Google Scholar]
- 59.Wang J.R., Yau L.F., Gao W.N., Liu Y., Yick P.W., Liu L. Quantitative comparison and metabolite profiling of saponins in different parts of the root of Panax notoginseng. J Agric Food Chem. 2014;62:9024–9034. doi: 10.1021/jf502214x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qi L.W., Wang C.Z., Yuan C.S. Isolation and analysis of ginseng: advances and challenges. Nat Prod Rep. 2011;28:467–495. doi: 10.1039/c0np00057d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang W.Z., Ye M., Qiao X., Liu C.F., Miao W.J., Bo T. A strategy for efficient discovery of new natural compounds by integrating orthogonal column chromatography and liquid chromatography/mass spectrometry analysis: its application in Panax ginseng, Panax quinquefolium and Panax notoginseng to characterize 437 potential new ginsenosides. Anal Chim Acta. 2012;739:56–66. doi: 10.1016/j.aca.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 62.Shi X.J., Yang W.Z., Qiu S., Yao C.L., Shen Y., Pan H.Q. An in-source multiple collision-neutral loss filtering based nontargeted metabolomics approach for the comprehensive analysis of malonyl-ginsenosides from Panax ginseng, P. quinquefolius, and P. notoginseng. Anal Chim Acta. 2017;952:59–70. doi: 10.1016/j.aca.2016.11.032. [DOI] [PubMed] [Google Scholar]
- 63.Yang W.Z., Zhang J.X., Yao C.L., Qiu S., Chen M., Pan H.Q. Method development and application of offline two-dimensional liquid chromatography/quadrupole time-of-flight mass spectrometry–fast data directed analysis for comprehensive characterization of the saponins from Xueshuantong injection. J Pharm Biomed Anal. 2016;128:322–332. doi: 10.1016/j.jpba.2016.05.035. [DOI] [PubMed] [Google Scholar]
- 64.Yao C.L., Yang W.Z., Zhang J.X., Qiu S., Chen M., Shi X.J. UHPLC–Q-TOF-MS-based metabolomics approach to compare the saponin compositions of Xueshuantong injection and Xuesaitong injection. J Sep Sci. 2017;40:834–841. doi: 10.1002/jssc.201601122. [DOI] [PubMed] [Google Scholar]
- 65.Yao C.L., Yang W.Z., Si W., Pan H.Q., Qiu S., Wu J. A strategy for establishment of practical identification methods for Chinese patent medicine from systematic multi-component characterization to selective ion monitoring of chemical markers: shuxiong tablet as a case study. RSC Adv. 2016;6:65055–65066. [Google Scholar]