Abstract
Spurred by the alleged relevance of the thia-Michael reaction in the bioactivity of various classes of cinnam(o)yl natural products and by the development of a quick NMR assay to study this reaction, we have carried out a systematic study of the “native” reactivity of these compounds with dodecanethiol and cysteamine as models, respectively, of simple thiols and reactive protein thiols that can benefit from iminium ion catalysis in Michael reactions. Cinnamoyl esters and amides, as well as cinnamyl ketones and oximes, did not show any reactivity with the two probe thiols, while cinnamaldehyde (1a) reacted with cysteamine to afford a mixture of a thiazoline derivative and compounds of multiple addition, and with aliphatic thiols to give a single bis-dithioacetal (6). Chalchones and their vinylogous C5-curcuminoid derivatives were the only cinnamoyl derivatives that gave a thia-Michael reaction. From a mechanistic standpoint, loss of conjugation in the adduct might underlie the lack of a native Michael reactivity. This property is restored by the presence of another conjugating group on the carbonyl, as in chalcones and C5-curcuminoids. A critical mechanistic revision of the chemical and biomedical literature on cinnamaldehyde and related compounds seems therefore required.
KEY WORDS: Cinnmaldeyde, Michael addition, Electrophiles, Conjugation, Cysteamine, Chalcones
Graphical abstract
1. Introduction
Cinnamon (Cinnamomum verum J. S. Presl. and C. aromaticum Nees.) is one of the oldest spices, being already mentioned in the early Chinese medical treatises and in the old Sanskrit texts1. The trade of cinnamon from India to the Mediterranean area is documented from the beginning of the Egyptian civilization1, and cinnamon quills and cinnamon oil are nowadays extensively used in flavoring, perfumery, beverages, and medicines. The main constituent of cinnamon oil (up to over 80%) is cinnamaldehyde (1a, Fig. 1), a pleiotropic bioactive agent of current interest as anti-diabetic and antifungal agent2. Cinnamaldehyde is a reactive compound, whose chemical “exuberance” is responsible not only for the many beneficial effects associated to cinnamon, but also for allergic reactions to cinnamon-containing perfumes, cosmetics, and sweets (cinnamon buns, cinnamon cereals and apple cakes)3. Severe allergic reactions of the skin and mucous membranes have also been observed in baking personnel and in workers processing cinnamon3, and because of this allergic potential, the acceptable daily intake (ADI) of 1a has been set at the rather low value of 1.25 mg/kg4. Cinnamaldehyde is also extensively used in detergents and household cleaner, and is, in an industrial perspective, a high production volume (HPV) material, with an estimated consumption of 1000 metric tons per year3.
Figure 1.

Chemical structures of cinnamaldehyde (1a), methylcinnamylketone (1b), methylcinnamate (1c), cinnamide (1d), chalcone (1e) and dicinnamylketone (1f), the oxime of cinnamaldehyde (2a) and its acetyl derivative (2b).
The allergic reactions to cinnamaldehyde have been related to its Michael reactivity and its ability to form stable adducts with proteins. A similar mechanism has been proposed to explain the capacity of 1a to activate TRPA1, the mustard oil receptor, by alkylation of the thiol-rich ankyrin moiety of this ion channel5. There is little doubt that cinnamaldehyde is capable to react with thiol groups, and this compound has, indeed, been extensively used in biochemical studies as a thiol-active agent, just like iodoacetamide or phenylarsine oxide6. However, the precise mechanism by which cinnamaldehyde traps thiols is unclear. The reaction of cinnamaldehyde with thiols has been considered the archetypal conjugated addition reaction that does not take place to any significant extent in the absence of secondary or primary amine7, establishing itself as a benchmark reaction to evaluate the performance of organocatalysts8. In accordance with this view, mutation studies with biological targets of 1a, including TRPA1, have highlighted the relevance of the formation of an imine with an arginine residue as a prelude to the Michael addition9. On the other hand, formation of hemithioacetals and not of Michael adducts was observed in the reaction of cinnamaldehyde and thiols in ionic liquids10, and spontaneous Michael reaction with thiols has also been reported, although the adducts could be characterized only after derivatization11. Confusion also exists in the Michael reactivity of cinnamoyl derivatives of general formula 1, that have been assumed, mainly in the biomedical literature, to behave as Michael acceptors without any clear demonstration of the actual occurrence of this reaction12. In the case of chalcones, formation of unstable Michael adducts, quickly reverting to the starting enones, has also been reported13.
Cinnamoyl derivatives might as well give Michael adducts under a judicious selection of catalysts, promoters, and pHs, but there is a surprising lack of information on the “native”, uncatalyzed reactivity of these compounds with thiols. Given the biomedical relevance of cinnamoyl derivatives in drug discovery and nutrition14, we have investigated the behavior of this class of compounds with two probe thiols, the odorless dodecanethiol as a model of a simple thiol, and cysteamine as a model of reactive thiol in a protein that can benefit from imine catalysis15.
2. Results and discussion
Cinnamaldehyde is an ambident electrophile, in principle capable to react with thiols both at the carbonyl and at the β-carbon. In practice, neither of these reactions is supposed to take place without Lewis acid catalysis (attack to the carbonyl and formation of the dithioacethal) or organocatalysis (pre-formation of an iminium ion followed by Michael addition)7, 8. In terms of frontier molecular orbitals, these maneuvers decrease the LUMO energy and remodulate its coefficients, increasing its value on the carbonyl (ipso) carbon (Lewis acid catalysis) or on the β-carbon (organocatalysis)7. In accordance with this view, reaction of cinnamaldehyde with cysteamine in DMSO (cysteamine assay, Fig. 2) gave as a major compound the thiazoline adduct 3 (Fig. 3), resulting from imine formation at the carbonyl group and Michael addition at the conjugated double bond. Several other compounds were also formed, resulting from the multidentate reactivity of cinnamaldehyde, and dilution with CDCl3 did not revert their formation. Compound 4a (Fig. 3) was the result of the intermolecular version of the process generating the thiazoline 3, with one molecule of cysteamine (or its disulfide oxidation product) trapping the carbonyl and the other one the olefinic double bond, while compounds 5a and 5b (Fig. 3) resulted from the reaction of two molecules of cinnamaldehyde with three molecule of cysteamine. The formation of these compounds shows that cinnamaldehyde acts as a multiple trap for “active” thiol groups, being capable also of reacting at its carbonyl site and with both sulfur and nitrogen nucleophilic sites.
Figure 2.
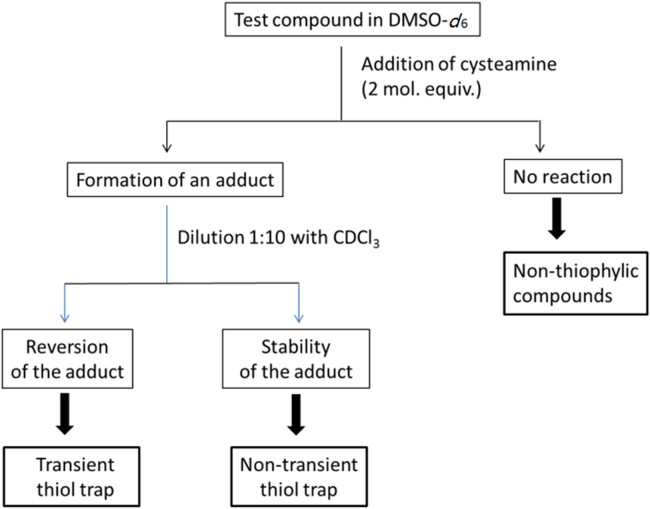
Classification of thiol-trapping compounds according to the cysteamine assay15.
Figure 3.
Chemical structures of compounds 3–5.
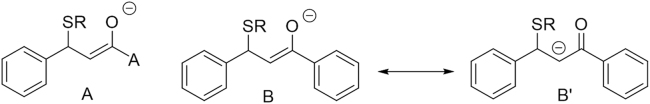
However, no reaction was observed with most of the other substrates investigated. Thus, both the oxime of cinnamaldehyde (2a, Fig. 1) and its acetyl derivative (2b, Fig. 1) were unreactive with cysteamine in DMSO, despite being claimed to be Michael acceptors16. No reaction was also observed with methylcinnamylketone (1b, Fig. 1), methylcinnamate (1c, Fig. 1) and cinnamide (1d, Fig. 1), in accordance with the chemical literature that lack clear evidence for these reactions, but in disagreement with the biomedical literature that assumes a Michael reactivity for these compounds. Within the compounds investigated, only chalcone (1e, Fig. 1) and its vinylogous analogue (dicinnamylketone, 1f, Fig. 1), a tetradeoxygenated analogue of the so-called C5-curcumin, gave a positive cysteamine assay, as evidenced by the disappearance of all the olefin proton resonances upon addition of cysteamine. In all cases where formation of a cysteamine adduct was observed, the reaction was irreversible. In comparative experiments where the reactivity of a 1:1:1 mixture of cinnamaldehyde (1a), chalcone (1e) and dicinnamylketone (1f) was treated with one equivalent of cysteamine, the most reactive compound was chalcone (1c). Taken together, these observations show that the presence of a β-phenyl group has a detrimental effect on the Michael reactivity of the electron-poor double bond, presumably because of the loss of conjugation associated to the formation of the Michael adduct (Fig. 4A). Michael reactivity is, however, restored by phenyl substitution on the carbonyl, either direct (chalcone, 1e) or vinylogous (dicinnamylketone, 1f), presumably because the intermediate enolate (Fig. 4B) can again benefit from conjugation, or otherwise get lost in the Michael adduct when the carbonyl substituent is non-conjugating (Fig. 4A).
Figure 4.
Effect of resonance on the structure of the Michael adducts of cinnam(o)yl derivatives. (A) Adduct from a generic substrate; (B) and (B') Adducts from chalcones.
We have previously observed that, with the exception of α,β-unsaturated aldehydes, most Michael acceptors that react with cysteamine in DMSO do not give any appreciable reaction in CDCl315. However, chalcone and dicinnamylketone reacted quickly with cysteamine also in CDCl3, and were even reactive with aliphatic thiols. Thiol adducts of chalcone have been reported to quickly revert to the starting enones during purification17, but no tendency to reversion was observed, and the adducts were stable under the conditions of the assay.
Spurred by the observation of an unusually high reactivity of chalcone with thiols, we also investigated the reaction of cinnamaldehyde with dodecanethiol. Much to our surprise, we observed formation of the bis-dithioacetal (6, (Fig. 5) contaminated by its corresponding thiosemiacetal when the two reagent were mixed in a 1:2 ratio in a variety of NMR solvents. The reaction has preparative value, and when carried out in CH2Cl2 with 4 equivalents of dodecanethiol, it afforded 6 in ca. 70% yield. No reaction occurred with dihydrocinnamaldehyde (7, Fig. 5), in accordance with the textbook notion that formation of dithioacetals from aldehyde requires the presence of strong Lewis acids to activate the carbonyl group18. An ethyl group is less encoumbered than an ethenyl, but the smooth reaction of cinnamaldehyde with thiols and the complete lack of reactivity of its dihydroderivative are difficult to explain on purely steric basis, and the observation is in sharp contrast to the notion that an α-unsaturation decreases the reactivity of a carbonyl toward nucleophilic addition. Since no reaction occurred when cinnamaldehyde was treated with alcohols, a possible explanation could be that conjugation to a phenyl reduces the hardness of the carbonyl to the point of making it possible for a weak and soft nucleophile like sulfur to attack it without any previous catalysis. On the other hand, no reaction occurred with simple amines like 2-phenylethylamine.
Figure 5.

Chemical structures of bis-dithioacetal (6) and dihydrocinnamaldehyde (7).
Taken together, the results of this comparative study show that cinnamyl derivatives can trap thiols in a Michael fashion only when loss of conjugation is compensated by the presence of another phenyl ring bound to the carbonyl, so that there is no overall loss of conjugation associated to the reaction. Cinnamaldehyde itself can trap thiols only after imine formation with cysteamine, or, with simple thiols, by attacking to the carbonyl. While our observations do not dismiss the potential for a mainstream Michael addition to take place with the other cinnam(o)yl derivatives under cellular conditions, they suggest that this would require a special chemical milieu or a special promotion/catalysis to take place.
3. Experimental
3.1. General experimental procedures
1H NMR (500 MHz) and 13C NMR (125 MHz) spectra were measured on a Varian INOVA spectrometer. Chemical shifts were referenced to the residual solvent signal (CDCl3: δH = 7.26, δC = 77.0; DMSO-d6: δH = 2.50). Low- and high-resolution ESI-MS spectra were obtained on an LTQ OrbitrapXL (Thermo Scientific) mass spectrometer. All compounds investigated are commercial (Aldrich) except the oxime 2a16, its acetyl derivative 2b16 and the cinnamide 1d19 that were prepared according to literature.
3.2. Cysteamine assay
In an NMR tube, an exact amount of substrate (ca. 5 mg) was dissolved in 500 μL dry DMSO-d6, and the 1H NMR spectrum was recorded. Two equivalents of cysteamine or dodecanethiol were then added, and the spectrum was immediately recorded, with acquisition finishing within 5 min from the addition. The reaction was typically monitored by observing the changes in the olefin region of the spectrum. To test the reversibility of the addition, the sample was diluted to 1:10 with CDCl3, and the spectrum was recorded again. Reversion was evaluated by comparing this spectrum with an original CDCl3 spectrum of the product under investigation.
3.3. Chemistry
3.3.1. 7-Phenyl-2,3,6,7-tetrahydro-1,4-thiazepine (3)
1H NMR (CDCl3): δ 7.30—7.20 (6H, m), 3.80 (1H, m), 2.92 (1H, m), 2.73 (1H, m), 2.53 (2H, m), 1.75 (2 H, m). ESI-MS: m/z 192 [M + H]+; HR-ESI-MS: m/z 192.0842; Calcd. for C11H14NS 192.0847.
3.3.2. E-2-((3-((2-Aminoethyl)thio)-3-phenylpropylidene)amino)ethane-1-thiol (4a)
1H NMR (CDCl3): δ 7.30—7.20 (5H, m), 6.50 (1H, t, J = 4.5 Hz), 3.80 (1H, m), 3.0 (2H, t, J = 6.5 Hz), 2.85—2.60 (6H, overlapped), 1.80 (2H, m). ESI-MS: m/z 269 [M + H]+; HR-ESI-MS: m/z 269.1141; Calcd. for C13H21N2S2 269.1146. The disulfide 4b was only detected by ESI-MS: m/z 349 [M + H]+.
3.3.3. Compounds 5a/5b
1H NMR (CDCl3): δ 8.10 (1H, d, J = 6.5 Hz), 7.55 (2H, d, J = 6.5 Hz), 7.38—7.20 (8H, m), 7.15 (1H, d, J = 15.5 Hz), 6.85 (1H, dd, J = 15.5, 6.5 Hz), 4.45 (1H, m), 3.70 (1H, m), 3.0 (4H, m), 2.65—2.55 (8H, overlapped), 1.75 (2H, m). ESI-MS: m/z 460 [M + H]+; HR-ESI-MS: m/z 460.1911; Calcd. for C24H34N3S3 460.1915.
3.3.4. Reaction of cinnamaldehyde with dodecanethiol
To a stirred solution of 1a (330 mg, 2 mmol) in CHCl3 (5 mL), dodecanethiol (1.610 g, 8 mmol, 4 mol equiv.) was added. After stirring overnight at room temperature, the reaction was worked up by washing with 2% NaOH to remove the excess dodecanethiol and then with brine. The residue was purified by gravity column chromatography on silica gel (petroleum ether) to afford 832 mg 6 (80%) as a colorless and odorless oil.
3.3.5. E-(3-Phenylprop-2-ene-1,1-diyl)bis(dodecylsulfane) (6)
IR υmax (KBr): 1645, 1620, 1480, 1290, 1110, 987, 896 cm—1. 1H NMR (CDCl3): 7.35 (5H, m), 6.54 (1H, d, J = 15.6 Hz), 6.18 (1H, dd, J = 15.6, 9.5 Hz), 4.45 (1H, d, J = 9.5 Hz), 2.60 (4H, m), 1.60 (4H, m), 1.52 (4H, m), 1.30 (32H, m), 0.85 (6H, t, J = 6.7 Hz). ESI-MS: m/z 519 [M + H]+; HR-ESI-MS: m/z 519.4049; Calcd. for C33H59S2 519.4058.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Orazio Taglialatela-Scafati, Email: scatagli@unina.it.
Giovanni Appendino, Email: giovanni.appendino@uniupo.it.
References
- 1.Gray E.W., Miller J.I. The spice trade of the Roman Empire 29 B.C.—A.D. 641. J Rom Stud. 1970;60:222–224. [Google Scholar]
- 2.Rao P.V., Gan S.H. Cinnamon: a multifaceted medicinal plant. Evid Based Complement Altern Med. 2014;2014:642942. doi: 10.1155/2014/642942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cocchiara J., Letizia C.S., Lalko J., Lapczynski A., Api A.M. Fragrance material review on cinnamaldehyde. Food Chem Toxicol. 2005;43:867–923. doi: 10.1016/j.fct.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 4.Council of Europe. Natural flavouring substances, their sources and added artificial flavouring substances. In: Matieres aromatisantes naturelles, leurs sources, et matieres aromatisantes artificielles ajoutees. Strasbourg, France: Council of Europe; 1974. p.145.
- 5.Kumar H., Kim I.S., More S.V., Kim B.W., Choi D.K. Natural product-derived pharmacological modulators of Nrf2/ARE pathway for chronic diseases. Nat Prod Rep. 2014;31:109–139. doi: 10.1039/c3np70065h. [DOI] [PubMed] [Google Scholar]
- 6.Kuipers D.P., Scripture J.P., Gunnink S.M., Salie M.J., Schotanus M.P., Ubels J.L. Differential regulation of GLUT1 activity in human corneal limbal epithelial cells and fibroblasts. Biochimie. 2013;95:258–263. doi: 10.1016/j.biochi.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marigo M., Schulte T., Franzén J., Jørgensen K.A. Asymmetric multicomponent domino reactions and highly enantioselective conjugated addition of thiols to α,β-unsaturated aldehydes. J Am Chem Soc. 2005;127:15710–15711. doi: 10.1021/ja055291w. [DOI] [PubMed] [Google Scholar]
- 8.Blanco V., Carlone A., Hänni K.D., Leigh D.A., Lewandowski B. A rotaxane-based switchable organocatalyst. Angew Chem Int Ed. 2012;51:5166–5169. doi: 10.1002/anie.201201364. [DOI] [PubMed] [Google Scholar]
- 9.Buey R.M., Calvo E., Barasoain I., Pineda O., Edler M.C., Matesanz R. Cyclostreptin binds covalently to microtubule pores and lumenal taxoid binding sites. Nat Chem Biol. 2007;3:117–125. doi: 10.1038/nchembio853. [DOI] [PubMed] [Google Scholar]
- 10.Yadav J.S., Reddy B.V., Kondaji G. Eco-friendly and highly chemoselective 1,3-oxathio- and 1,3-dithioacetalization of aldehydes using ionic liquids. Chem Lett. 2003;32:672–673. [Google Scholar]
- 11.Sirotanovic K.D., Bajlon-Pastor M.M. Addition of mercaptans to unsaturated aldehydes. II. Addition of ethylmercaptan, amylmercaptan, and benzylmercaptan to unsaturated aromatic aldehydes. Glas Hem Drustva Beogr. 1966;31:329–337. [Google Scholar]
- 12.Gersch M., Kreuzer J., Sieber S.A. Electrophilic natural products and their biological targets. Nat Prod Rep. 2012;29:659–682. doi: 10.1039/c2np20012k. [DOI] [PubMed] [Google Scholar]
- 13.Amslinger S., Al-Rifai N., Winter K., Wörmann K., Scholz R., Baumeister P. Reactivity assessment of chalcones by a kinetic thiol assay. Org Biomol Chem. 2013;11:549–554. doi: 10.1039/c2ob27163j. [DOI] [PubMed] [Google Scholar]
- 14.Zhu R., Liu H., Liu C., Wang L., Ma R., Chen B. Cinnamaldehyde in diabetes: a review of pharmacology, pharmacokinetics and safety. Pharamcol Res. 2017;122:78–89. doi: 10.1016/j.phrs.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 15.Avonto C., Taglialatela-Scafati O., Pollastro F., Minassi A., Di Marzo V., De Petrocellis L. An NMR spectroscopic method to identify and classify thiol-trapping agents: revival of Michael acceptors for drug discovery? Angew Chem Int Ed. 2011;50:467–471. doi: 10.1002/anie.201005959. [DOI] [PubMed] [Google Scholar]
- 16.DeFalco J., Steiger D., Gustafson A., Emerling D.E., Kelly M.G., Duncton M.A. Oxime derivatives related to AP18: agonists and antagonists of the TRPA1 receptor. Bioorg Med Chem Lett. 2010;20:276–279. doi: 10.1016/j.bmcl.2009.10.113. [DOI] [PubMed] [Google Scholar]
- 17.Allen C.F., Fournier J.O., Humphlett W.J. The thermal reversibility of the Michael reaction: IV. Thiol adducts. Can J Chem. 1964;42:2616–2620. [Google Scholar]
- 18.Smith MB, March J. In: March's advanced organic chemistry: reactions, mechanisms, and structure. 6th ed. Hoboken: Whiley; 2007. p. 1227–80.
- 19.Gowda R.R., Chakraborty D. FeIII-catalyzed synthesis of primary amides from aldehydes. Eur J Org Chem. 2011;2011:2226–2229. [Google Scholar]