Abstract
Refined-JQ (JQ-R) is a mixture of refined extracts from Coptis chinensis (Ranunculaceae), Astragalus membranaceus (Leguminosae) and Lonicera japonica (Caprifoliaceae), the three major herbs of JinQi-JiangTang tablet, a traditional Chinese medicine (TCM) formula. The mechanisms by which JQ-R regulates glucose metabolism and improves insulin sensitivity were studied in type 2 diabetic KKAy mice and insulin-resistant L6 myotubes. To investigate the mechanisms by which JQ-R improves insulin sensitivity, a model of insulin-resistant cells induced with palmitic acid (PA) was established in L6 myotubes. Glucose uptake and expression of factors involved in insulin signaling, stress, and inflammatory pathways were detected by immunoblotting. JQ-R showed beneficial effects on glucose homeostasis and insulin resistance in a euglycemic clamp experiment and decreased fasting insulin levels in diabetic KKAy mice. JQ-R also improved the plasma lipid profiles. JQ-R directly increased the activity of superoxide dismutase (SOD) and decreased malondialdehyde (MDA) as well as inducible nitric oxide synthase (iNOS) levels in insulin-resistant L6 cells, and elevated the insulin-stimulated glucose uptake with upregulated phosphorylation of AKT. The phosphorylation levels of nuclear factor kappa B (NF-κB p65), inhibitor of NF-κB (IκB α), c-Jun N-terminal kinase (JNK1/2) and extracellular-signal-regulated kinases (ERK1/2) were also changed after JQ-R treatment compared with the control group. Together these findings suggest that JQ-R improved glucose and lipid metabolism in diabetic KKAy mice. JQ-R directly enhanced insulin-stimulated glucose uptake in insulin-resistant myotubes with improved insulin signalling and inflammatory response and oxidative stress. JQ-R could be a candidate to achieve improved glucose metabolism and insulin sensitivity in type 2 diabetes mellitus.
KEY WORDS: TCM, Type 2 diabetes mellitus, Insulin signalling, Inflammation, Oxidative stress
Graphical abstract
A mixture of refined extracts from three major herbs of JinQi-JiangTang tablets (JQ-R) improved glucose metabolism in diabetic KKAy mice and L6 myotubes by stimulating glucose uptake via the insulin-dependent PI3K-AKT pathway.
1. Introduction
Insulin resistance is the major pathogenic factor during the development of type 2 diabetes mellitus. It will induce glucose dysmetabolism in multiple tissues, which is the main cause of hyperglycemia and diabetes. Skeletal muscle contributes the most (70%) to insulin-stimulated glucose uptake1. Thus, insulin resistance in skeletal muscle is most likely a major determinant of type 2 diabetes and is characterized by impaired insulin-stimulated glucose uptake2.
Many studies have demonstrated that palmitic acid exposure in myotubes was a causal factor leading to insulin resistance with increased inflammatory response and oxidative stress3, 4. The excessive stress not only directly affected cells by oxidizing biomolecules but also by activating various pathways, such as c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase 1/2 (ERK1/2), and nuclear factor kappa B (NF-κB)5. The activation and interactions between these kinases and insulin signaling pathways leads to defective glucose uptake and contributes to the development of insulin resistance. Therefore, it is important to aim at multi-tissues, multi-pathways and ameliorate diabetic symptoms through multi-targeted therapeutic agents.
JinQi-JiangTang tablet, manufactured by Tianjin Zhongxin Pharmaceutical Group Co., Ltd. (Tianjin, China), is composed of three traditional herbal medicines, Coptis chinensis (Ranunculaceae), Astragalus membranaceus (Leguminosae) and Lonicera japonica (Caprifoliaceae). Refined-JQ (JQ-R) contains the total alkaloids of C. chinensis, the saponins of A. membranaceus, and the polyhydric alcohols of L. japonica. It has been proven beneficial for diabetes pre-clinically and clinically, and has been used for the therapy of mild or moderate non-insulin-dependent diabetes6. These components were claimed to possess anti-diabetic and anti-inflammatory activities. Our previous studies clearly showed that oral administration of JQ-R significantly decreased fasting blood glucose levels and improved insulin resistance in pre-diabetic high-fat diet (HFD)-induced obesity in C57 mice7. Further analysis revealed that JQ-R improved HFD-induced insulin resistance partially via activating the AMPK signaling pathway in both liver and muscle. However, as the detailed mechanism by which JQ-R ameliorates the systemic symptoms of diabetes remains unclear, we hypothesize that JQ-R can improve insulin resistance in different stages of diabetes. The present investigation was performed to evaluate the anti-diabetic effects of JQ-R in KKAy mice, a spontaneous type 2 diabetes animal model characterized by hyperglycemia and insulin resistance. We also investigated the effect and mechanisms of JQ-R on insulin signaling and inflammatory pathways in rat L6 skeletal muscle myotubes.
2. Methods
2.1. Preparation and quantitative analysis of JQ-R
The preparation of the above mentioned components of the three herbs comprising JQ-R was conducted according to our previously described methods8. It was shown that alkaloids/saponins/polyhydric alcohols at the weight ratio of 15/10/5 achieved an optimal anti-diabetic effect in hyperglycemic and insulin-resistant mice7. This ratio was used in this study.
2.2. Animals and experiment design
Male KKAy mice and C57BL/6J mice (10 weeks old) were purchased from the Experimental Animal Center, Chinese Academy of Medical Sciences and Peking Union Medical College (Beijing, China). The KKAy mice were fed ad libitum with a high-caloric diet containing 15% protein, 17.2% fat and 50% carbohydrate. The C57BL/6J mice were fed standard commercial diet (Research Diets, Inc., Beijing, China). All the mice were kept in an air-conditioned room with a 12 h light/dark cycle with free access to water and food. All animal experiments were carried out in strict accordance with the Standards for Laboratory Animals (GB14925-2001) and the Guideline on the Humane Treatment of Laboratory Animals (MOST 2006a) established by the People׳s Republic of China, and all animal procedures were approved by Beijing Administration Office of Laboratory Animal (approval number SCXK-Beijing-2009-0004).
All KKAy mice were examined for their diabetic indexes after a two-month high-caloric diet, including fasting blood glucose, triglycerides and total cholesterol, body weight and the ability to lower plasma glucose at 40 min after injection of 0.4 IU/kg insulin. The following criteria for diabetes were selected for this study: (1) fasting blood glucose, triglycerides and total cholesterol greater than 250, 200 and 60 mg/dL, respectively; (2) initial body weight in the range of 45–50 g; and (3) plasma glucose decreased by 20% or less at 40 min after insulin injection. Forty-eight diabetic KKAy mice were chosen and divided into four groups (12 mice each, data for grouping are shown in Table 1): vehicle-treated (Con), metformin-treated at a 100 mg/kg B.W. (Met), and JQ-R-treated at 100 and 200 mg/kg B.W. (JQ-R100 and JQ-R200). Additionally, age- and gender-matched C57BL/6J mice (n=12) were given vehicle solvent as normal control (Nor). All groups were treated by oral gavage every day for 10 weeks. Body weight, food and water intake were recorded at appropriate time points. Insulin tolerance tests (ITT) and oral glucose tolerance (OGTT) were performed after treatment for 4 and 5 weeks. Fasting blood glucose, lipids and insulin levels were monitored after treatment for 6 weeks. Blood hemoglobin A1c, superoxide dismutase (SOD), malondialdehyde (MDA) and C-reaction protein (CRP) were determined after 8 weeks of treatment. Whole-body insulin sensitivity was measured using the hyperinsulinemic-euglycemic clamp at the end of treatment (6 mice/group).
Table 1.
Data for animal groups. Animals were grouped primarily according to the percent blood glucose decrease at 40 min in an insulin tolerance test; fasting blood glucose, TG, TC levels and body weight are shown.
| Group | Fasting blood glucose (mg/dL) | BG decrease at 40 min in ITT (%) | Triglyceride (mg/dL) | Cholesterol (mg/dL) | Body weight (g) |
|---|---|---|---|---|---|
| Nor | 102.5±6.5*** | 37.0±11.4*** | 90.3±12.3*** | 59.8±8.6*** | 28.7±1.9*** |
| Con | 376.4±78.8 | 3.8±13.9 | 493.0±241.8 | 90.1±22.8 | 47.1±2.3 |
| Met | 381.8±68.5 | 3.8±12.1 | 517.0±265.8 | 100.2±28.9 | 48.0±3.2 |
| JQ-R 100 (mg/kg) | 381.3±72.1 | 3.9±12.0 | 511.5±209.6 | 97.8±27.8 | 48.1±2.6 |
| JQ-R 200 (mg/kg) | 375.3±51.7 | 3.9±10.2 | 519.0±303.2 | 97.4±27.1 | 47.6±2.9 |
Data were expressed as mean±SD, n=12. ***P<0.001 compared with Con group.
2.3. Insulin tolerance test (ITT) and oral glucose tolerance test (OGTT)
For ITT, 0.4 IU/kg of insulin (Humulin, Lilly, USA) was injected intraperitoneally after 4 h of fasting, and blood was sampled before and at 40 and 90 min after insulin injection. For OGTT, animals were orally administered with dextrose (2 g/kg) after 4 h of fasting, and blood was collected for measurement of blood glucose before and at 30, 60, and 120 min after glucose loading9.
2.4. Hyperinsulinemic-euglycemic clamp
On the test day mice were fasted for 6 h and then anesthetized with pentobarbital sodium (50 mg/kg body weight, intraperitoneally) and placed on a heating pad at 37 °C. The right jugular vein was catheterized (Micro-renathane, 0.025×0.012") for the infusion of glucose and insulin. After the operation the animal rested for 30 min to lessen the stress. Following the adaptation period, the whole clamp was conducted and lasted for about 120 min. Human insulin was infused by syringe infusion pump (KD Scientific, Inc., USA) at the rate of 8 mIU/kg/min. Blood glucose was monitored every 10 min. When the blood glucose levels fell below 6.0 mmol/L, the infusion of glucose was driven to maintain blood glucose levels at 5.5±0.5 mmol/L. The glucose infusion rate (GIR) to maintain euglycemia in the steady state represents whole-body insulin sensitivity.
2.5. Cell culture and treatment
Rat L6 skeletal muscle cells were seeded in DMEM supplemented with 10% FBS and 1% antibiotic/antimycotic solution (10,000 U/mL penicillin G, 10 mg/mL streptomycin, 25 μg/mL amphotericin B) in 5% CO2 at 37 °C. Cells were differentiated to myotubes in medium supplemented with 2% FBS and all experiments were performed in differentiated myotubes 5–6 days after seeding. For this study insulin resistance in L6 myotubes was induced by incubation with bovine serum albumin (BSA)-conjugated palmitic acid (PA, 0.5 mmol/L) for 16 h. A stock concentration of palmitate was dissolved in 50% ethanol and conjugated with 10% free fatty acid (FFA)-free BSA. Vehicle control cells (Vehicle) were treated with vehicle containing BSA but lacking the fatty acid. Stock solutions of metformin (Met) and JQ-R were dissolved in dimethylsulfoxide (DMSO). PA control cultures (PA) received the same concentration of DMSO as in the drug-treated cultures. All treated L6 myotubes were exposed to a final concentration of Met (1 mmol/L) or JQ-R (2.5 and 5 mg/mL) with concurrent PA incubation.
2.6. Inflammatory signaling pathway detection
For the protein expression in inflammatory signalling pathway studies, L6 myotubes were plated in 6-well plates and well differentiated in medium supplemented with 2% FBS. After 5–6 days, differentiation from myogenic cell-type to myotube was confirmed by light microscopy. Insulin resistance in L6 myotubes was induced as described above. Vehicle control (Vehicle) cells were treated with vehicle containing BSA but lacking PA. For treatment, medium containing PA and co-incubated with either vehicle (DMSO) or the tested agents at the indicated concentrations (2.5 or 5 mg/mL JQ-R and 1 mmol/L Met) were added to each well and incubated for 16 h. Cell-lysate samples were collected and prepared for immunoblot analysis. Samples were homogenized in RIPA lysis buffer (C1053, Applygen Inc., China) supplemented with protease inhibitor cocktail (P1265, Applygen Inc., China). Protein content was assayed according to the method of bicinchoninic acid using BSA as standard. Protein samples (20 μg) were resolved electrophoretically on a 10% sodium dodecyl polyacrylamide gel and transferred to PVDF membranes. The samples were incubated with antibodies to NF-κB, IκB α, JNK, ERK, p38 MAPK as well as β-actin primary antibodies (Cell Signaling Technology, USA) followed by secondary antibody. The signal was visualized by using an enhanced chemiluminescence detection system (ChemiScope2850, CLiNX science Instruments, China). Protein band densities were analyzed using Gel-Pro-Analyzer 3.1 software.
2.7. Glucose uptake and AKT (Ser473) phosphorylation assay
For assay of glucose uptake and AKT (Ser473) phosphorylation for insulin stimulation, L6 myotubes were plated at 104/well in 96-well plates or 2×105/well in 6 well plates and incubated with 0.5 mmol/L PA for 16 h and treated with either vehicle (DMSO) or the test substances at the indicated concentrations. Before the glucose uptake experiments, all culture medium was removed from each well and changed to free-serum medium for 2 h, then replaced with 100 μL of culture medium in the absence or presence of fluorescent 2-NBDG or 2-NBDG together with the tested agents at the indicated concentrations. Plates were incubated at 37 °C with 5% CO2 for 30 min before incubation with 100 nmol/L of insulin for 10 min. The 2-NBDG uptake reaction was stopped by removing the incubation medium and washing the cells twice with cold phosphate-buffered saline (PBS). The fluorescent intensity of the cell lysate was detected on a Bioteck Synergy 2 microplate reader (Excitation at 488 nm, Emission at 520 nm)10. For AKT phosphorylation, L6 myotubes were treated with either vehicle (DMSO) or the tested agents at the indicated concentrations and co-incubated with 0.5 mmol/L palmitate for 16 h, then serum starved for 2 h and incubated with 100 nmol/L of insulin for 10 min11. The pelleted protein was resolved by SDS-PAGE, and total AKT, AKT (Ser473) phosphorylation and β-actin were detected by immunoblotting with primary antibody. Protein band densities were analyzed using Gel-Pro-Analyzer 3.1 software.
2.8. Phenotypic evaluation of mice and biochemical analysis of cell lysates
Fasting blood glucose, triglycerides, total cholesterol and low-density lipoprotein cholesterol levels were determined by enzymatic/colorimetric methods with commercial kits (BIOSINO bio-technology & science, Inc., China). Fasting blood insulin (FINS) was measured by ELISA (American Laboratories Product Co., USA, and R&D Systems, Inc., USA, respectively), following manufacturer׳s instructions. FFAs were measured by commercial kits (Sekisui Medical, Tokyo, Japan). The activities of SOD, inducible nitric oxide synthase (iNOS), CRP and the content of MDA were determined by enzymatic/colorimetric methods with commercial kits (Nanjing Jiancheng bioengineering institute, Inc., China).
2.9. Statistical analysis
Statistical analyses were conducted using Graphpad Prism 6.0 software. All results are presented as the mean±standard deviation (SD). Statistical analyses were performed between C57BL/6J mice (Nor) and vehicle-treated KKAy mice (Con) and between Con group and drug-treated mice (Met, JQ-R100, and JQ-R200). Similarly to the in vivo tests, the in vitro statistical analyses were performed between Vehicle and PA-induced insulin-resistant cells (PA) and between PA culture and drug-treated cultures (P+Met, P+JQ-R2.5, and P+JQ-R5). Differences were determined using one-way ANOVA with post hoc tests to compare to Con or the PA group. A P value <0.05 was considered statistically significant.
3. Results
3.1. JQ-R improves glucose homeostasis in diabetic KKAy mice
To evaluate the effects of JQ-R on glucose homeostasis, we performed OGTT in KKAy mice after 5 weeks of treatment. After glucose loading in OGTT, the peak blood glucose level was significantly increased at 30 min in the Con group, and the AUC value was increased by 3-fold compared with Nor group (P<0.001, Fig. 1B). Compared to the Con group, the peak blood glucose levels and the AUC values in the JQ-R group (100 and 200 mg/kg) were decreased markedly (P<0.05, P<0.01, Fig. 1A and B), indicating improved glucose metabolism. We also monitored the effect of JQ-R on blood glucose control after long-term treatment. As shown in Fig. 1C, the decrease in fasting blood glucose in the JQ-R group is nearly 40% greater as compared to the Con group after a 6 weeks of treatment (P <0.01 and P < 0.001, respectively). We also observed a significant (16–21%) decrease in HbA1c levels (P<0.05 and P<0.01) in the JQ-R group compared with the Con group after 8 weeks of treatment.
Figure 1.
JQ-R improves glucose metabolism in diabetic KKAy mice. (A) Blood glucose level during the OGTT; (B) area under the curve (AUC) of the blood glucose in OGTT; (C) fasting blood glucose; (D) HbA1c levels. Each value represents the mean±SD, n=12 mice. *P<0.05, **P<0.01 and ***P<0.001 versus Con group.
3.2. JQ-R improves insulin resistance in diabetic KKAy mice
As shown in Fig. 2A and B, JQ-R at a dosage of 200 mg/kg ameliorated impaired insulin tolerance with reducing the AUC in ITT after 4-week treatment. As expected, we also observed a significant decreased blood insulin levels compared with the Con group as a result of JQ-R treatment for 6 weeks of treatment (P<0.01, Fig. 2C). To measure systemic insulin sensitivity, hyperinsulinemic-euglycemic clamp studies were performed at the end of 10 weeks of treatment. GIR values in the JQ-R group were markedly higher (P<0.01, P<0.001, 12.8±2.0 mg/kg/min in JQ-R100 and 15.2±1.5 mg/kg/min in JQ-R200) as compared to 6.6±1.9 mg/kg/min in Con group and as shown in Fig. 2D, indicating that whole-body glucose homeostasis and insulin sensitivity were substantially improved after JQ-R treatment.
Figure 2.
JQ-R improves systemic insulin sensitivity in diabetic KKAy mice. (A) Blood glucose level during the ITT; (B) area under the curve (AUC) of blood glucose in ITT; (C) basal insulin levels; (D) glucose infusion rates (GIR) in clamp. Each value represents the mean±SD, n=12 mice. *P<0.05, **P<0.01 and ***P<0.001 versus Con group.
3.3. JQ-R adjusts the lipid profile in diabetic KKAy mice
The diabetic KKAy mice also developed dyslipidemia with evidently higher plasma triglyceride, free fatty acid, total cholesterol and low-density lipoprotein cholesterol levels than those of the Nor group, as shown in Fig. 3. Interestingly, JQ-R can alleviate the dyslipidemia, producing a decrease of nearly 50% both in blood total cholesterol (P<0.05) and FFA levels (P<0.001) compared with Con group after 6 weeks of treatment. Compared with the Con group, JQ-R intervention at a dosage of 200 mg/kg also decreased blood triglyceride (P<0.05) and low-density lipoprotein cholesterol levels (P<0.01), demonstrating improved blood-lipid profiles after JQ-R treatment in type 2 diabetic KKAy mice.
Figure 3.
JQ-R improves the lipid profile in diabetic KKAy mice. (A) Blood triglyceride level; (B) blood free fatty acid level; (C) blood total cholesterol level; (D) blood low-density lipoprotein cholesterol level. Each value represents the mean±SD, n=12 mice. *P<0.05, **P<0.01 and ***P<0.001 versus Con group.
3.4. JQ-R ameliorates systemic oxidative stress and inflammation status in diabetic KKAy mice
Since some fractions of JQ-R have been reported to display anti-oxidative and anti-inflammatory activities16, 17, it seems possible that JQ-R can improve oxidative stress or inflammation status. To assess this, we evaluated the effects of JQ-R treatment on blood inflammatory cytokines, including CRP, as well as other biomarkers of oxidative stress such as SOD and MDA levels after 8 weeks of treatment. As shown in Table 2, JQ-R can improve the biomarkers of systemic oxidative stress notably, with a more than 2-fold increase in blood SOD activity (P<0.01 and P<0.001), and a reduction of 12.6% and 14.7% in blood content of MDA levels (P<0.05 and P<0.01) compared with the Con group. Compared with Con group, JQ-R intervention at a dosage of 200 mg/kg also decreased the blood concentration of CRP (P<0.05).
Table 2.
Effect of JQ-R on blood SOD, MDA and CRP levels after 8 weeks of treatment in diabetic KKAy mice.
| Group | Dosage (mg/kg) | SOD (U/mL) | MDA (nmol/mL) | CRP (mg/L) |
|---|---|---|---|---|
| Nor | – | 68.6±17.3 | 7.8±1.1** | 1.8±1.5* |
| Con | – | 71.3±24.1 | 9.5±0.9 | 4.4±2.8 |
| Met | 100 | 223.8±80.7*** | 8.5±0.8* | 1.5±3.0 |
| JQ-R | 100 | 157.5±52.2** | 8.3±0.7* | 2.7±2.0 |
| 200 | 166.5±32.8*** | 8.1±0.6** | 1.7±1.6* |
Data were expressed as mean±SD, n=12. *P<0.05. **P<0.01. ***P<0.001 compared with Con group.
Not applicable.
3.5. JQ-R protects against augmented oxidative stress and inflammatory pathways in PA-induced insulin resistant L6 myotubes
Given that diabetic hyperglycemia, dyslipidemia, and systemic oxidative stress and chronic inflammation are key components of insulin resistance and many metabolic diseases, it is possible that down-regulation of oxidative stress or inflammation could play a protection role in insulin resistance either in tissues or cellular levels. As is well known, skeletal muscle insulin resistance is a common defect in type 2 diabetes. Here we used an insulin-resistant L6 myocytes model to assess the effects of JQ-R on these events.
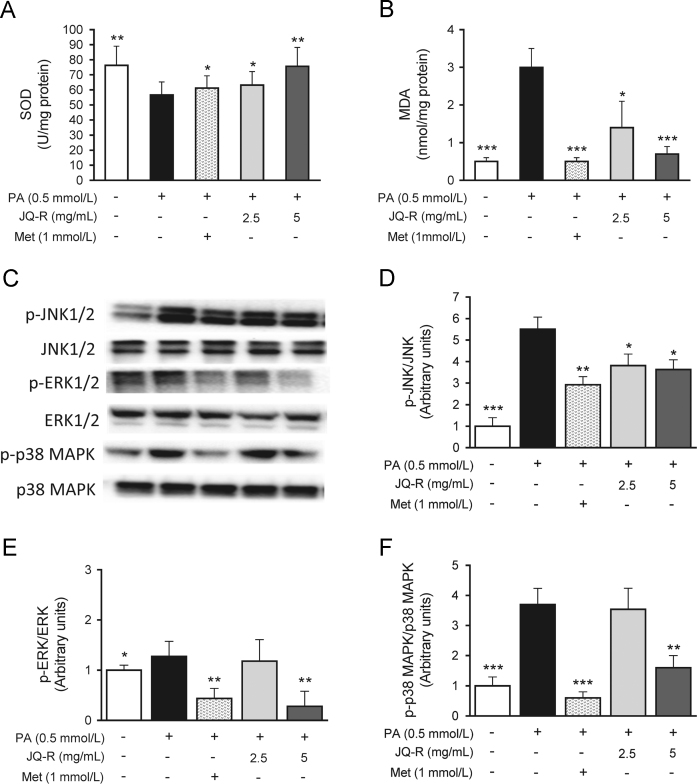
To confirm the protective effects of JQ-R against oxidative stress and inflammation, we monitored levels of SOD, MDA and activity of iNOS in the lysates of L6 myotubes. As seen in Figs. 4A, B and 5A, the level of SOD was reduced by 26% (P<0.01) in the PA control group and MDA content and the activity of iNOS were significant elevated 6- and 4-fold, respectively (P<0.001 and P<0.001), compared with the Vehicle group, indicating that palmitate caused increased oxidative stress and inflammation in skeletal muscle cells. JQ-R at different concentrations (2.5 and 5 mg/mL) restricted the production of MDA (P<0.05 and P<0.01) and reduced the activity of iNOS (P<0.01) by more than 50%. JQ-R at 5 mg/mL showed a marked elevation in the SOD level to that of Vehicle (P<0.01).
Figure 4.
Effect of JQ-R on biomarkers of oxidative stress and cellular stress-kinases protein expression in palmitate-induced insulin-resistant L6 myotubes. (A) Activity of SOD; (B) content of MDA; (C)–(F) bands are representative images for p-JNK1/2, p-ERK1/2 and p-MAPK p38 and total JNK1/2, ERK1/2 and MAPK p38 from three independent experiments. The scanned bar graph shows the fold induction over Vehicle L6 cells for each respective image. β-Actin was used as a loading protein. Vehicle: fully differentiated L6 cells, PA: fully differentiated L6 cells incubated with palmitic acid for 16 h. Data are presented as the mean±SD, n=3. *P<0.05, **P<0.01 and ***P<0.001 versus PA control group.
Figure 5.
Effect of JQ-R on NF-κB-signaling protein expression in palmitate-induced insulin-resistant L6 myotubes. (A) Activity of iNOS; (B)–(D) bands are representative images for p-IκB α and p-NF-κB p65 and total IκB α and NF-κB p65 from three independent experiments. The scanned bar graph shows the fold induction over Vehicle L6 cells for each respective image. β-Actin was used as a loading protein. Vehicle: fully differentiated L6 cells, PA: fully differentiated L6 cells incubated with palmitic acid for 16 h. Data are presented as the mean±SD, n=3. *P<0.05, **P<0.01 and ***P<0.001 versus PA control group.
It is well known that oxidative stress activates a variety of stress-sensitive intracellular signaling pathways. The activation of stress-activated protein kinase JNK, extracellular signal-regulated kinase 1/2 (ERK1/2) and p38 has been also implicated in the modulation of insulin resistance. Therefore, the expression of the regulatory factors in these pathways was investigated. As shown in Fig. 4C–F, exposure to palmitate for 16 h strongly stimulated phosphorylation of JNK, ERK1/2 and p38 MAPK by 5.5-, 1.3- and 3.7-fold in L6 myotubes (P<0.001, P<0.05 and P<0.001). The increased phosphorylation of JNK, ERK1/2 and p38 MAPK was reversed after JQ-R (5 mg/mL) treatment, indicating that JQ-R intervention inhibits the activation of JNK and MAPK signaling.
As seen in Fig. 5B–D, exposure to palmitate for 16 h strongly stimulated phosphorylation of IκB α and NF-κB in L6 myotubes, indicating high palmitate-induced activation of inflammation-related signaling pathways. Incubation with JQ-R (5 mg/mL) significantly reduced the phosphorylation of NF-κB p65 (P<0.001). There was a corresponding decline in phosphorylation of IκB α after JQ-R treatment (P<0.001), leading to its ubiquitination and degradation and confirming NF-κB activation.
3.6. JQ-R enhances palmitate-induced, impaired insulin-stimulated glucose uptake via upregulation of phosphorylation of AKT (Ser473) in L6 myotubes
Given that insulin sensitivity was improved after JQ-R treatment in KKAy mice as described above, we wondered if JQ-R could directly act on insulin target tissues to enhance insulin signaling. To assess this, the effect of JQ-R on palmitate-induced insulin-resistant L6 myocytes was studied. As shown in Fig. 6A, palmitate incubation (shown as PA cultures) decreased glucose uptake significantly by 41.4% and 37.9% in both basal and insulin-stimulated conditions when compared with Vehicle cultures (P<0.01 and P<0.001). JQ-R at the concentration of 5 mg/mL increased glucose uptake after insulin stimulation markedly compared with PA cultures (P<0.001). On the basis of the impact of JQ-R on glucose uptake, we next tested whether JQ-R affects the insulin signaling pathway. Compared to basal conditions, phosphorylation of Ser473 in AKT (p-AKT) in the vehicle cultures was upregulated by 3.8-fold after insulin stimulation. PA incubation impaired insulin-stimulated phosphorylation of Ser473 in AKT significantly by 77% relative to the Vehicle cultures. Consistent with the results on glucose uptake, JQ-R (2.5 and 5 mg/mL) up-regulates the insulin-stimulated phosphorylation of AKT by 2.1- and 3.0-fold (P<0.05 and P<0.001), compared with the PA control cultures. In aggregate, these results indicate that JQ-R can directly enhance cellular insulin signaling in palmitate-induced insulin-resistant L6 myotubes.
Figure 6.
JQ-R enhanced insulin-stimulated glucose uptake in insulin-resistant L6 myotubes via the PI3K-AKT pathway. (A) Glucose uptake in insulin resistant L6 cells treated with JQ-R with or without insulin stimulating; (B) representative proteins expression (p-AKT and total AKT) in insulin-signaling. Data are representative images for p-AKT and total AKT, from three independent experiments. The scanned bar graph shows the fold induction over control L6 cells for each respective image. Vehicle: fully differentiated L6 cells, PA: fully differentiated L6 cells incubated with palmitic acid for 16 h. Data are presented as the mean±SD, n=3. *P<0.05, **P<0.01 and ***P<0.001 versus PA control group.
4. Discussion
In recent years, an increased focus has been placed on the use of natural products or medicinal herbal extracts. In Chinese traditional medicine various herbs are often combined into a multi-herbal formula for enhancing efficacy, as well as reducing the toxicity or adverse effects. Many herbs have been reported to be a rich source of anti-diabetic ingredients12. Multi-herbal formulas could be a more promising strategy for the prevention and treatment of insulin resistance than single herbal medicines13.
JQ-R is composed of the total alkaloids of C. chinensis, the saponins of A. membranaceus, and the polyhydric alcohols of L. japonica. These three effective fractions may be integral contributing factors in ameliorating insulin resistance in type 2 diabetic animal models. As previously reported, berberine, one of the main total alkaloid constituents of C. chinensis, was identified recently as one of the principal anti-diabetic constituents14, 15. It has also become evident that the saponins (such as astragaloside IV), as a part of the active ingredients of A. membranaceus (known as Huangqi in China), and L. japonica (also called Jinyinhua in China) were able to prevent inflammation and oxidative stress16, 17. The selected fractions of the three plant species in JQ-R were claimed to possess both anti-diabetic and anti-inflammatory activities. Therefore, we speculated that JQ-R may offer an alternative approach in treating disorders associated with insulin resistance, such as pre-diabetes and type 2 diabetes mellitus.
We previously reported that JQ-R could improve insulin resistance in HFD-induced obesity mouse model, C57BL/6J mice (HFD-C57mice), and found that JQ-R could increase insulin sensitivity by activating the AMPK signaling pathway in liver in this animal model7. We also speculated that JQ-R might improve systemic insulin sensitivity via multiple tissues and pathways in different animal and cell models.
Here we evaluated the effects of JQ-R on insulin resistance and glucose metabolism in diabetic KKAy mice, and the efficacy was compared with an oral anti-diabetic agent, metformin. We found that fasting blood glucose and insulin levels showed a notable reduction in diabetic KKAy mice after 6-week treatment (Shown in Figs. 1C and 2C), and we confirmed that decreased plasma insulin levels are due to improved insulin sensitivity in tissues. At the end of treatment, among the intervention groups, we also found that there were no significant differences in dietary intake compared to the Con group, and the body weight after JQ-R treatment (48.2 ± 3.0 g in JQ-R100 group and 47.2 ± 4.6 g in JQ-R200 group) showed no significant changes compared to the Con group (49.7 ± 1.7 g).
It is also well known that skeletal muscle insulin resistance is a common defect in type 2 diabetes. Insulin-dependent PI3K-AKT-signaling plays an important role in regulating glucose uptake and homeostasis in muscle. The impairment of PI3K-AKT-mediated insulin signaling induces insulin resistance18. Thus we wondered if JQ-R improved insulin sensitivity via activation of the PI3K-AKT-signaling pathway in skeletal muscle. To test our hypothesis, we set up a palmitate-induced insulin-resistant L6 myotubes cell model and measured insulin-stimulated glucose uptake and p-AKT levels after JQ-R treatment. The results demonstrate that palmitate induced impaired insulin-stimulated glucose uptake and p-AKT expression in L6 myotubes, while JQ-R-treated cultures exhibited enhanced insulin-stimulated glucose uptake and p-AKT expression, as seen with metformin treated cultures (Fig. 4). These data suggest that JQ-R can improve glucose uptake in palmitate-induced insulin-resistant L6 myotubes and that its mechanism may be related to the regulation of the PI3K-AKT signal transduction pathway. It is important to note that JQ-R increases glucose uptake in insulin resistant L6 myotubes previously treated with palmitate acid, which is also increased in hyperglycemic conditions and induces systemic insulin resistance in these cells.
On the other hand, it has been reported that systemic insulin resistance is characterized by chronically elevated oxidative stress and enhanced activation of pro-inflammatory signaling via activation of transcription factor and stress kinases19. Interestingly, we noted that JQ-R did modulate augmented oxidative stress in palmitate-induced insulin-resistant L6 myotubes. JQ-R increased SOD content and decreased MDA concentration and iNOS activity. The mechanism by which JQ-R exerts its protective effect on oxidative stress and inflammation-mediated pathogenesis was evaluated by estimating the transcription levels of signaling proteins. It has been demonstrated that palmitate-induced NF-κB activation occurs in response to oxidative stress20. NF-κB has been implicated as the regulator of expression of many pro-inflammatory cytokine genes including TNFα, interleukin 6 (IL6), and IL821. These inflammatory cytokines can also act, in turn, to further activate stress kinases. Our results show that palmitate exposure increased the level phosphorylation of IκB α and NF-κB in L6 myotubes, indicating direct activation of the NF-κB pathway in muscle cells. JQ-R at a concentration of 5 mg/mL diminished the IκB α and NF-κB phosphorylation to a level similar to that in the Vehicle group (Fig. 5). The same effect was seen with the metformin-treated group. Furthermore, in our insulin-resistant L6 myotubes, exposure to palmitic acid enhanced the phosphorylation of p38 MAPK, JNK and ERK1/2 (Fig. 4). Elevated activities of these stress-activated protein kinases are often found in adipose or muscle in obese and insulin-resistant rodents and humans. In our present study, the expression levels of these stress kinases have been found to be significantly down-regulated after JQ-R (5 mg/mL) treatment. Our studies could not confirm that the change of these pathways is a unique or direct effect of JQ-R, and will require further analysis to determine whether the effects and mechanisms of JQ-R on enhancing insulin signaling and reducing oxidative stress and inflammatory pathways are mediated independently in response to JQ-R treatment; using inhibitors of each pathway to evaluate the direct effect is the subject of future investigation.
5. Conclusions
In summary, JQ-R contains three biologically active fractions, including the total alkaloid fraction of C. chinensis, the saponins of A. membranaceus, and the polyhydric alcohols of L. japonica. These components effectively regulate blood glucose metabolism and ameliorate oxidative stress and related inflammatory responses. JQ-R was found to improve glucose metabolism in diabetic KKAy mice, at least in part, by stimulating glucose uptake in skeletal muscle via the insulin-dependent PI3K-AKT signaling pathway. Based on these findings, JQ-R may be useful as a candidate for the prevention and treatment of diabetes.
Acknowledgements
This work was partly supported by the CAMS Innovation Fund for Medical Sciences, China (CIFMS, No. 2016-12M-4-001) and CAMS Initiative for Innovative Medicine, China (CAMS-I2M, No. 2016-I2M-2–006).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.DeFronzo R.A., Ferrannini E., Sato Y., Felig P., Wahren J. Synergistic interaction between exercise and insulin on peripheral glucose uptake. J Clin Investig. 1981;68:1468–1474. doi: 10.1172/JCI110399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolka C.M., Harrison L.N., Lottati M., Chiu J.D., Kirkman E.L., Bergman R.N. Diet-induced obesity prevents interstitial dispersion of insulin in skeletal muscle. Diabetes. 2010;59:619–626. doi: 10.2337/db09-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abildgaard J., Henstridge D.C., Pedersen A.T., Langley K.G., Scheele C., Pedersen B.K. In vitro palmitate treatment of myotubes from postmenopausal women leads to ceramide accumulation, inflammation and affected insulin signaling. PLoS One. 2014;9:e101555. doi: 10.1371/journal.pone.0101555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pillon N.J., Arane K., Bilan P.J., Chiu T.T., Klip A. Muscle cells challenged with saturated fatty acids mount an autonomous inflammatory response that activates macrophages. Cell Commun Signal. 2012;10:30. doi: 10.1186/1478-811X-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee M.S., Choi S.E., Ha E.S., An S.Y., Kim T.H., Han S.J. Fibroblast growth factor-21 protects human skeletal muscle myotubes from palmitate-induced insulin resistance by inhibiting stress kinase and NF-κB. Metabolism. 2012;61:1142–1151. doi: 10.1016/j.metabol.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Cao H., Ren M., Guo L., Shang H., Zhang J., Song Y. JinQi-Jiangtang tablet, a Chinese patent medicine, for pre-diabetes: a randomized controlled trial. Trials. 2010;10:27. doi: 10.1186/1745-6215-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao L.H., Liu Q., Liu S.N., Chen Z.Y., Li C.N., Lei L. A refined-JinQi-JiangTang tablet ameliorates prediabetes by reducing insulin resistance and improving beta cell function in mice. J Ethnopharmacol. 2014;151:675–685. doi: 10.1016/j.jep.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 8.Shen Z, Xie M, Feng X, Wang A, Sun S, Chen Y, et al, inventors. Traditional Chinese medicine effective part compound recipe composition, preparation technique and anti-diabetes use thereof. PR China patent CN 200710122035. 2007 Mar 25.
- 9.Liu S.N., Liu Q., Li L.Y., Huan Y., Sun S.J., Shen Z.F. Long-term fenofibrate treatment impaired glucose-stimulated insulin secretion and up-regulated pancreatic NF-κB and iNOS expression in monosodium glutamate-induced obese rats: is that a latent disadvantage?. J Transl Med. 2011;9:176. doi: 10.1186/1479-5876-9-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zou C., Wang Y., Shen Z. 2-NBDG as a fluorescent indicator for direct glucose uptake measurement. J Biochem Biophys Methods. 2005;64:207–215. doi: 10.1016/j.jbbm.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Li P., Oh D.Y., Bandyopadhyay G., Lagakos W.S., Talukdar S., Osborn O. LTB4 promotes insulin resistance in obese mice by acting on macrophages, hepatocytes and myocytes. Nat Med. 2015;21:239–247. doi: 10.1038/nm.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng J., Sitou K., Zhang Y., Yan R., Hu Y. Analyzing the Chinese landscape in anti-diabetic drug research: leading knowledge production institutions and thematic communities. Chin Med. 2016;11:13–22. doi: 10.1186/s13020-016-0084-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W.L., Zheng H.C., Bukuru J., De Kimpe N. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J Ethnopharmacol. 2004;92:1–21. doi: 10.1016/j.jep.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 14.Cui G., Qin X., Zhang Y., Gong Z., Ge B., Zang Y.Q. Berberine differentially modulates the activities of ERK, p38 MAPK, and JNK to suppress Th17 and Th1 T cell differentiation in type 1 diabetic mice. J Biol Chem. 2009;284:28420–28429. doi: 10.1074/jbc.M109.012674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yin J., Hu R., Chen M., Tang J., Li F., Yang Y. Effects of berberine on glucose metabolism in vitro. Metabolism. 2002;51:1439–1443. doi: 10.1053/meta.2002.34715. [DOI] [PubMed] [Google Scholar]
- 16.Yu W.N., Sun L.F., Yang H. Inhibitory effects of astragaloside IV on bleomycin-induced pulmonary fibrosis in rats via attenuation of oxidative stress and inflammation. Inflammation. 2016;39:1835–1841. doi: 10.1007/s10753-016-0420-5. [DOI] [PubMed] [Google Scholar]
- 17.Ko H.J., Oh S.K., Jin J.H., Son K.H., Kim H.P. Inhibition of experimental systemic inflammation (septic inflammation) and chronic bronchitis by new phytoformula BL containing Broussonetia papyrifera and Lonicera japonica. Biomol Ther. 2013;21:66–71. doi: 10.4062/biomolther.2012.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma B.R., Kim H.J., Rhyu D.Y. Caulerpa lentillifera extract ameliorates insulin resistance and regulates glucose metabolism in C57BL/KsJ-db/db mice via PI3K/AKT signaling pathway in myocytes. J Transl Med. 2015;13:62. doi: 10.1186/s12967-015-0412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans J.L., Goldfine I.D., Maddux B.A., Grodsky G.M. Are oxidative stress-activated signaling pathways mediators of insulin resistance and β-cell dysfunction? Diabetes. 2003;52:1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- 20.Baker R.G., Hayden M.S., Ghosh S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Craig R., Larkin A., Mingo A.M., Thuerauf D.J., Andrews C., McDonough P.M. p38 MAPK and NF-κB collaborate to induce interleukin-6 gene expression and release. Evidence for a cytoprotective autocrine signaling pathway in a cardiac myocyte model system. J Biol Chem. 2000;275:23814–23824. doi: 10.1074/jbc.M909695199. [DOI] [PubMed] [Google Scholar]