Abstract
Peroxisome proliferator activated receptors (PPARs) α, -γ and -β/δ are ligand-activated transcription factors and members of the superfamily of nuclear hormone receptor. These receptors play key roles in maintaining glucose and lipid homeostasis by modulating gene expression. PPARs constitute a recognized druggable target and indeed several classes of drugs used in the treatment of metabolic disease symptoms, such as dyslipidemia (fibrates, e.g. fenofibrate and gemfibrozil) and diabetes (thiazolidinediones, e.g. rosiglitazone and pioglitazone) are ligands for the various PPAR isoforms. More precisely, antidiabetic thiazolidinediones act on PPARγ, while PPARα is the main molecular target of antidyslipidemic fibrates. Over the past few years, our understanding of the mechanism underlying the PPAR modulation of gene expression has greatly increased. This review presents a survey on terrestrial and marine natural products modulating the PPARα system with the objective of highlighting how the incredible chemodiversity of natural products can provide innovative leads for this “hot” target.
KEY WORDS: PPARα, Natural product, Mechanism of action, Dyslipidemia, Metabolic syndrome
Graphical abstract
This review presents a survey on terrestrial and marine natural products modulating the PPARα system with the objective of highlighting how the incredible chemodiversity of natural products can provide innovative leads for this “hot” target.
1. Introduction
Peroxisome proliferator activated receptors (PPARs) are nuclear transcription factors that, in response to the binding of small ligands, regulate the expression of genes involved in cellular development, metabolism (lipid, carbohydrate and protein) and also tumorigenesis. PPARs are activated by many environmental factors, from xenobiotics to food compounds and they have been proposed to be one of the most important connection points between genes and environmental stimuli.
PPARs were first identified in Xenopus frogs as receptors that induce the proliferation of peroxisomes1 (the organelles involved in catabolism of long fatty acids and reduction of reactive oxygen species) and they were cloned in 1990 as members of the nuclear receptor family, which includes also the classical steroid hormone receptors. At that time, they were classified as “orphan receptors” since they exhibited conserved features of the nuclear receptor family, but they were not linked to a defined family of endogenous ligands.
Among their multifaceted activities, PPARs induce or repress transcription of a large number of different genes related to the regulation of glucose, lipid, and cholesterol metabolism. Thus, natural and synthetic PPAR modulators have been identified as a promising approach to treat diabetes, dyslipidemia, obesity and hypertension2. Many of these ailments can be comprised under the big umbrella definition of metabolic syndrome, a disorder affecting more than a quarter of the world adult population related to imbalance of storage and energy utilization. In fact, metabolic syndrome includes a series of pathological risk factors of metabolic origin, such as insulin resistance, hyperinsulinemia, abdominal obesity, impaired glucose tolerance, type 2 diabetes, dyslipidemia (increased blood serum triglycerides), low high-density lipoprotein (HDL) and high low-density lipoprotein (LDL) cholesterol levels, elevated blood pressure, and a pro-inflammatory and prothrombic state, that could promote development of cardiovascular affections. Moreover, recent research indicates that metabolic syndrome—associated obesity induces chronic low-grade local tissue inflammation which is prodromic to other disease conditions, such as fatty liver, polycystic ovary syndrome, asthma, and some types of cancer2.
Three isotypes of PPARs encoded by separate genes have been identified in mammals, sharing a high level of sequence and structural homology, indicated as PPARγ, -α, and -β (also called -δ),the first being the most extensively studied. Each PPAR subtype exhibits a unique tissue expression profile and has different functions in the regulation of energy metabolism. PPARα is highly expressed in muscles, liver, heart, and kidney, and mainly regulates genes involved in the metabolism of lipids and lipoproteins; PPARβ/δ is abundantly expressed throughout the body but at low levels in the liver. It has emerged as an important regulator of lipid metabolism and energy balance primarily in adipose tissue, skeletal muscle, and the heart. The PPARγ protein exists in two isoforms: PPARγ1, abundantly expressed in adipose tissue, large intestine, and hematopoietic cells, and PPARγ2, restricted to adipose tissue under physiological conditions3.
PPARs can be activated by dietary fatty acids and their metabolites, and, upon activation, they act as lipid sensors able to markedly redirect metabolism following a gene transcription process that is identical in all three PPAR subtypes. Similarly to other nuclear receptors, the three known subtypes have N-terminal transactivation domains, central highly conserved DNA-binding domains, and C-terminal ligand-binding domains (LBD). The ligand-binding domains of the PPAR isoforms share 60%–70% sequence identity, thus enabling the three isoforms to bind naturally occurring fatty acids, which enter a pocket in the LBD activating the receptor.
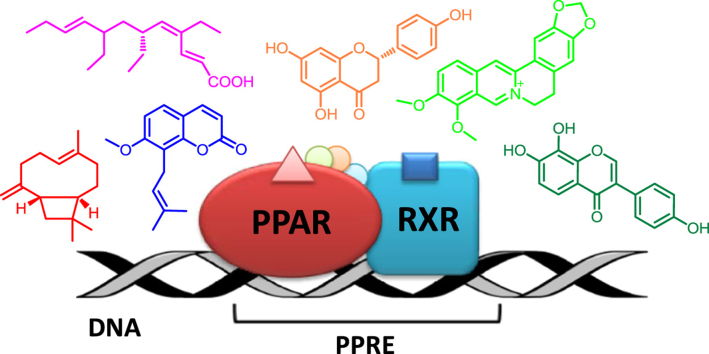
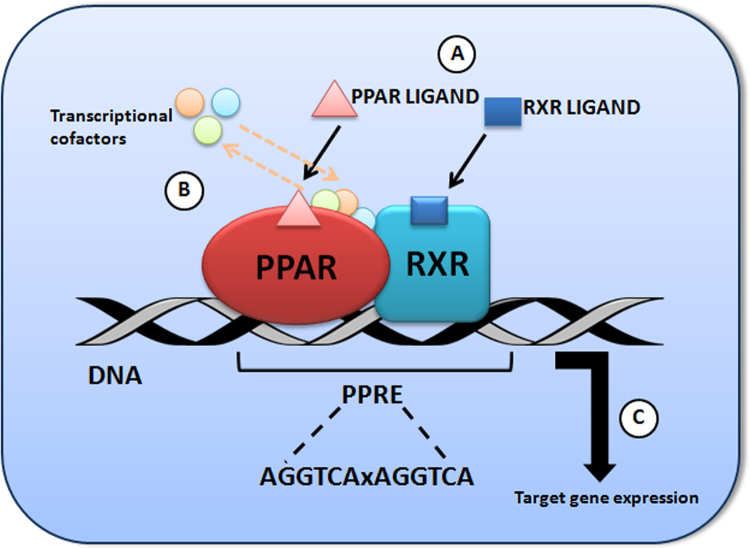
After ligand binding, the PPARs heterodimerize with their obligate partner, the retinoic acid-X receptor (RXR) and, as such, they bind to peroxisome proliferator response elements (PPREs), distinct regions of DNA in the promoter region of the respective target genes. The PPRE consensus sequence usually consists of a direct repeat of the hexameric sequence AGGTCA separated by one less well conserved spacer nucleotide (DR-1). PPARα was shown to bind to the 5′ motif of the PPRE, whereas RXR binds to the 3′ motif4 (Fig. 1).
Figure 1.
PPAR transcriptional activation in the cell nucleus. (A) Binding of PPAR/RXR ligands; (B) Changes in the associated transcriptional cofactors; (C) Activation of the transcriptional complex.
The activity of PPAR receptors is finely regulated by other intermediate compounds, collectively known as co-repressors and co-activators. In the absence of ligands, PPAR—RXR heterodimers recruit co-repressors and associated histone deacetylases and chromatin-modifying enzymes, silencing transcription by so-called active repression (ligand-independent repression). Once the ligand binds to PPAR, a conformational change in PPAR—RXR complexes causes release of repressors and their exchange with co-activators. Ligand-activated complexes recruit the basal transcriptional machinery and polymerase II, resulting in an enhanced gene expression leading to transcription of proteins. For example, carnitine palmitoyl transferase I (CPT-I), acylCoA synthase, β-ketoacyl-CoA thiolase and others, in turn regulate lipid metabolism, including uptake, synthesis, and oxidation of fatty acids, lipoprotein assembly, as well as lipid transport with the final goal of maintaining the balance of lipids and energy metabolism4.
Screening for PPAR ligands has led to identification of a plethora of natural and synthetic agonists able to activate them. For example, PPARα are activated by fibrates, lowering triglyceride levels and raising high density lipoprotein (HDL); PPARγ is activated by glitazones, drugs that can relieve insulin resistance in diabetes. PPARβ/δ is activated by an array of long-chain fatty acids and prostaglandins and, as shown recently, by retinoic acid.
2. PPARα functions and modulators
PPARα acts as a sensor of nutritional status, particularly energy balance. This key function has been better characterized in the liver, where it regulates key genes encoding proteins and enzymes involved mainly in lipid transport and β-oxidation of fatty acids5. However, the reduction in the levels of circulating or cellular lipids by PPARα activation is attributed to the stimulation of fat degradation in several others peripheral tissues expressing PPARα, including brown adipose tissue, kidney, heart, and skeletal muscle. In particular, PPARα activation stimulates the expression of lipoprotein lipase and increases its activity by stimulating apolipoproteins A—V (activator of lipoprotein lipase) and reducing apolipoprotein C-III (inhibitor of lipoprotein lipase). The effect is a reduction of triglyceride levels in chylomicrones and in very low-density lipoprotein (VLDL) particles, an increase in HDL cholesterol and a promotion of cholesterol efflux from cells to HDL, mediated by stimulation of expression of the ATP-binding cassette A1 transport protein3. Recently, it has been demonstrated that PPARα is also widely expressed in the digestive tract, where it exerts an anti-inflammatory effect. Since mice lacking PPARα develop an increased inflammation as compared to wild type (WT) mice, treatment with PPARα agonists has been proposed to inhibit inflammatory diseases development6.
Intriguingly, PPARα is also expressed in the hippocampus where it is involved in synaptic plasticity and memory through regulation of the expression of cAMP-response-element binding protein (CREB), a critical transcription factor regulating the formation of memories. Consequently, mice lacking PPARα display decreased spatial learning and memory. While targeting PPARα has been widely employed as a strategy to target dyslipidemia, the treatment of cognitive dysfunction and/or dementia has not yet been exploited as a potential indication for PPARα modulating drugs, mainly due to the pharmacokinetic problems in the crossing of the blood—brain barrier.
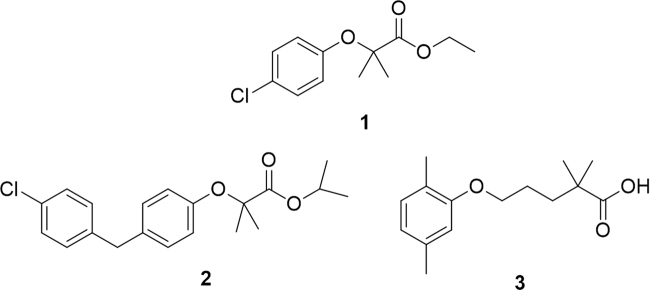
Overall, the most important and better exploited function of PPARα is the regulation of the expression of genes involved in lipid metabolism, and is thus linked to metabolic syndrome, atherosclerosis and cardiovascular diseases. The archetypal PPARα agonists are fibrates, small molecules embedding an aryloxyacetic acid moiety. The first PPARα agonist to be used in clinical therapy to treat dyslipidemia was clofibrate (1) in 1965, well before the discovery of its target about 25 years later. Successively, looking for an improvement in the pharmacological profile of this molecule, some analogues were synthesized and biologically evaluated. These compounds, commonly referred to as “second generation fibrates”, include fenofibrate (2), ciprofibrate, bezafibrate, and the dimethylphenoxypentanoic derivative gemfibrozil (3, Fig. 2)7, 8.
Figure 2.
Representative members of the fibrate family.
Paradoxically, the research to find synthetic exogenous ligands of PPARα has not been accompanied by comparable successes in the discovery of endogenous activators of this orphan receptor. In 2009, Chakravarthy et al.9 proposed that the phospholipid 1-palmitoyl-2-oleoyl-sn-glycerol-3-phosphocholine (16/18-GPC) was the main endogenous ligand of PPARα. Later, a number of other endogenous ligands have been proposed for PPARα, including saturated or unsaturated fatty acids and eicosanoids, such as palmitic acid, oleic acid, linoleic acid, arachidonic acid, oleoylethanolamide (a naturally occurring lipid related to the endocannabinoid anandamide), palmitoylethanolamide, and leukotriene B4. Recently, Roy et al.10 reported the discovery of three endogenous PPARα ligands that play a key role in modulating PPARα function in brain, 3-hydroxy-(2,2)-dimethyl butyrate, hexadecanamide, and 9-octadecenamide. It is very likely that, more than possessing a single high-affinity natural ligand, PPARα may be able to sense the total flux of fatty acids in metabolically active tissues. In addition, PPARα activity can be indirectly stimulated by phosphorylation. Not yet clearly identified amino acid residues contained in different domains of PPARα can be phosphorylated, thus promoting the transcriptional activity of PPARα even in the absence of ligands. PPARα can be phosphorylated by various kinases, such as mitogen-activated protein kinase (MAPK), protein-kinase C (PKC) and AMP-activated protein kinase11.
3. Natural products modulating PPARα
Natural products have proven historically to be a prolific and essential tool for drug discovery and the field of PPAR interacting molecules makes no exception to this general rule. A significant research effort has indeed been undertaken over the last two decades to explore the potential of a wide range of natural products originating from traditionally used medicinal plants or dietary sources. This approach has great attractiveness due to the intrinsic potential of natural sources and to the encouraging possibility of modulating PPAR activation by dietary interventions or ad hoc food supplements.
Undoubtedly, the greatest part of these research efforts has been devoted to find PPARγ modulators, in the search for natural products able to improve metabolic parameters in diabetic animal models, with reduced side effects when compared to thiazolidinedione, full agonists of this receptor. Honokiol, amorfrutins, and amorphastilbol are nice examples of molecules possessing these positive features, also because, in some cases, their mechanism of action includes the simultaneous activation of PPARα (as in the case of amorphastibol) or the PPARγ-dimer partner retinoid X receptor (as in the case of honokiol)8.
The activation of PPARα has been comparatively much less investigated, although a number of papers has been appearing in recent years. In this review, we have tried to collect the most significant and promising PPARα-modulating natural products. This collection of molecules does not aim to be complete or exhaustive, but more realistically at providing an overview on the most significant (in our opinion) researches. To this aim, we have found logical to organize the molecules according to their (likely) biogenetic origin and, consequently, their chemical scaffolds. In this way, some structural moieties crucial for the activities of a certain class of compounds could be more clearly evidenced.
While there is no shortage of reviews on PPARγ modulators12, 13, 14, including natural products, to the best of our knowledge, general reviews on natural PPARα modulators are still lacking, although some papers have reported on the activity of certain classes of natural products15.
3.1. Terpenes
3.1.1. Monoterpenes
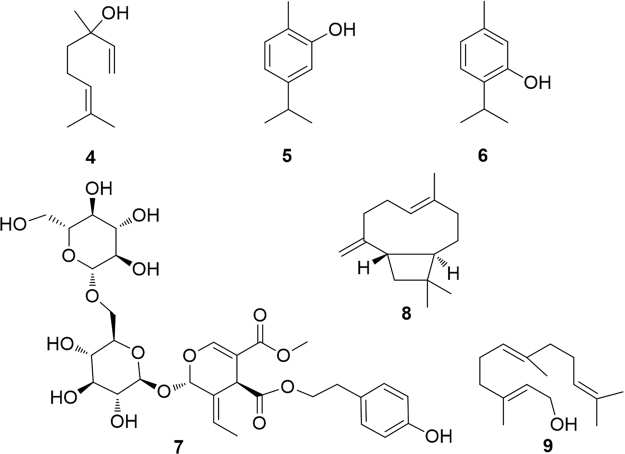
A PPARα modulating activity has been reported for the acyclic monoterpene linalool (4) and for the two isomeric aromatic monoterpenes carvacrol (5) and thymol (6)16, 17 (Fig. 3).
Figure 3.
PPARα-modulating mono- and sesquiterpenes.
Linalool is contained in most herbal essential oils and teas, where it contributes to the definition of aroma and flavors. The mixture of l- and d-linalool was found to act as a direct ligand of PPARα reducing cellular lipid accumulation, inducing fatty acid oxidation and significantly reducing the concentrations of saturated fatty acids, effects which were markedly attenuated by silencing PPARα expression. The effects of 1 mmol/L linalool appeared comparable to those of 0.1 mmol/L fenofibrate16.
Carvacrol (5) and thymol (6), monocyclic aromatic monoterpenes of thyme oil, were found to be somewhat weak agonists of PPARα and PPARγ receptors and, at the same time, to suppress the expression of COX-2. Since p-cymene was inactive on both these endpoints, Authors drew the reasonable conclusion that the —OH group is essential for these activities17.
The glycosylated secoiridoid excelside B (7) and some related metabolites extracted from Fraxinus excelsior L. were found to moderately activate PPARα, thus, at least partly, explaining the activity of the plant extract18.
3.1.2. Sesquiterpenes
trans-Caryophyllene (8), major component of the essential oils of many plants and traditionally used in cosmetics for its typical aroma, was found to be able to interact with the LBD of PPARα and, consequently, exert an effect in the regulation of cellular lipid metabolism. In particular, caryophyllene activity resulted in a significant reduction of intracellular triglyceride concentrations and increase of hepatic fatty acid uptake19. The activity of an hydrocarbon like caryophyllene, lacking any polar functional group and characterized by a small molecular weight, may appear surprising. However, the same molecule has also shown a selective and potent activity on cannabinoid CB2 receptor20, 21, whose previously known ligands invariably showed polarized bonds, thus indicating the privileged status of this natural product.
The acyclic alcohol derivative farnesol (9) which, as pyrophosphate, is the precursor of all the sesquiterpenoids and of squalene in the cholesterol biosynthetic pathway, was found to upregulate the expression of PPARα and the PPARα-regulated genes fatty acyl-CoA oxidase and carnitine palmitoyl transferase with a consequent lowering of serum triglyceride levels in rats22, 23.
3.1.3. Diterpenes
A series of diterpenes have been reported to be able to modulate PPARα although, almost invariably, these compounds were dual PPARα/γ activators. This is the case of dehydroabietic acid (10, Fig. 4), a major component of the oleoresin produced by several conifer species24. This molecule has been proposed to be useful to suppress chronic inflammation in obesity and to improve obesity-related insulin resistance. Pseudolaric acid B (11) and analogues have been isolated from the trunk bark of the Chinese tree Pseudolarix kaempferi. These diterpenes showed concentration-dependent activation of PPARα, -γ and -β isoforms. Interestingly, esterification of the free carboxy group of these compounds markedly reduced the activity, indirectly suggesting an interaction with the fatty acid binding site. However, authors suggested that pseudolaric acid B may act also by modifying the phosphorylation state of the receptor25.
Figure 4.
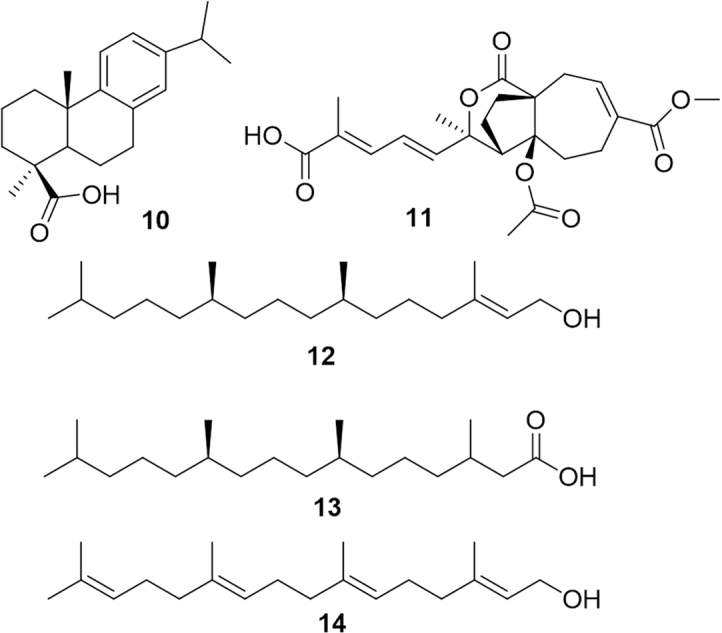
PPARα-modulating diterpenes.
Analogously to linear monoterpenes and sesquiterpenes, also in the case of diterpenes, the branched-fatty alcohol, (E)-phytol (12), ubiquitous in vegetal cells as carbon side-chain of chlorophylls, can be metabolically transformed into phytanic acid (13). Phytol itself is able to upregulate the expression of PPARα-target genes in hepatocytes, while phytanic acid has been reported to activate PPARγ, the retinoid-X-receptor (RXR) and PPARα26. Not surprisingly, also geranylgeraniol (14) proved to be able to activate both PPARα and PPARγ. Thus, branched-fatty alcohols, widespread in many dietary plants, may be collectively indicated as a class of PPAR ligands23. This class has been investigated in detail by Hostler et al.4, who concluded that unsaturated fatty acids show a wider specificity to PPAR isoforms compared to saturated fatty acids, as a consequence of the marked differences in the structural flexibility.
3.1.4. Triterpenes and steroids
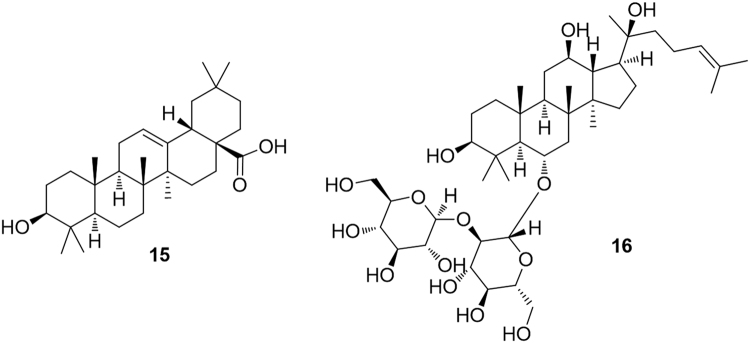
The pentacyclic triterpene oleanolic acid (15, Fig. 5) was found to stimulate PPARα activation in keratinocytes while, interestingly, the closely related ursolic acid, differing only for the methylation pattern on ring E, failed to express this activity27. The steroidal saponins ginsenosides, recognized as the main responsible for the pharmacological activities of ginseng, have been disclosed to inhibit the induction of PPARα-target genes by acting as competitive inhibitors of PPARα, with a consequent increased serum concentrations of total cholesterol, triglycerides, and HDL cholesterol28. One of the ginsenosides, namely ginsenoside Rf (16), was identified as the most potent analogue in this activity29. Thus, PPARα inhibition can be identified as an important molecular mechanism mediating ginseng induced alterations in serum lipid profiles.
Figure 5.
PPARα-modulating triterpenes.
3.1.5. Carotenoids
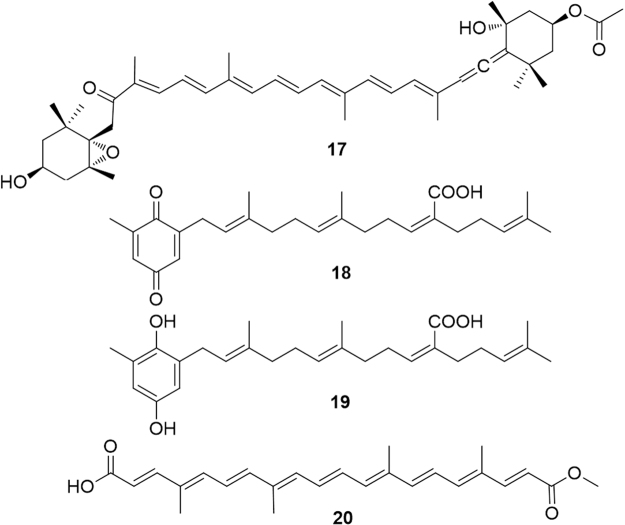
Fucoxanthin (17, Fig. 6) is a marine carotenoid characterized by an allene functionality and a conjugated ketone, widely distributed in marine algae, including edible brown algae, such as gulfweed (Sargassum fulvellum), dashima (Laminaria japonica) and hijiki (Hizikia fusiformis). This carotenoid was found to significantly down-regulate the hepatic Pparγ mRNA expression level and, in contrast, up-regulate Pparα mRNA, thus reducing triglyceride levels in the liver30. Sargaquinoic acid (18) and sargahydroquinoic acid (19) from the seaweed Sargassum yezoense were identified as novel PPARα/γ dual agonists with little effect on PPARδ activation31 (Fig. 6).
Figure 6.
PPARα-modulating carotenoids.
Bixin (20) is a carotenoid obtained from the pericarp of the seeds of Bixa orellana which was demonstrated to moderately activate PPARα, inducing the mRNA expression of PPARα-target genes involved in fatty acid oxidation in HepG2 hepatocytes. Treatment with bixin was proved to ameliorate obesity induced dysfunctions of carbohydrate metabolism (hyperglycemia and hyperinsulinemia)32.
3.2. Polyketides
Since fatty acids are physiological modulators of PPAR, it is not surprising that the C12 branched and triunsaturated fatty acid monotriajaponide A (21), obtained from a Chinese specimen of the sponge Plakortis simplex acted as a potent agonist of both PPARγ and PPARα33. Cyclic polyketides as anthraquinones and prenylated phloroglucinols also showed an interesting activity in the modulation of PPARα (Fig. 7).
Figure 7.
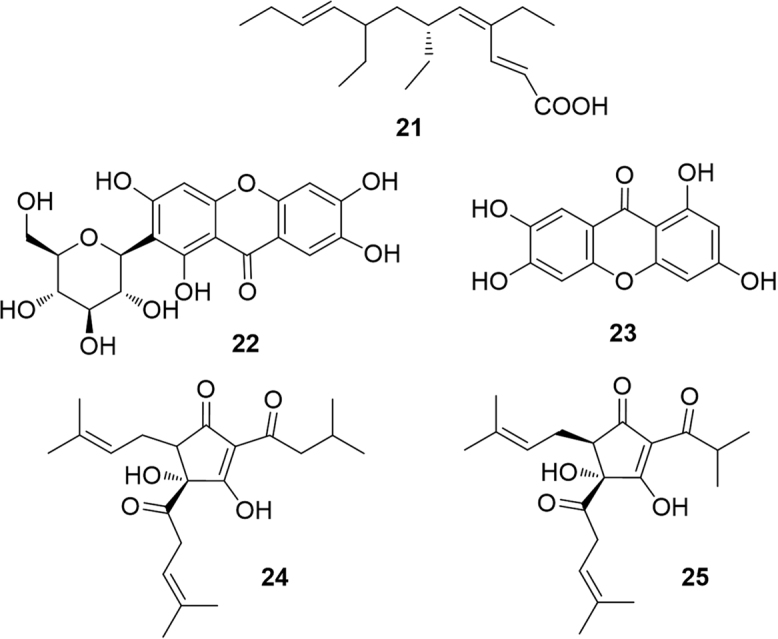
PPARα-modulating polyketides.
3.2.1. Anthraquinones
The C-glycosylated anthraquinone mangiferin (22), a secondary metabolite of Salacia oblonga root, an Ayurvedic medicine with anti-diabetic and anti-obesity properties, showed a weak effect on the transactivation of PPARγ and PPARα34. Interestingly, norathyriol (23) enhanced hepatic expression of PPARα, an effect completely suppressed by the selective PPARα antagonist MK-886. This clearly highlights the negative role played by the sugar unit of mangiferin, likely due to the increase in polarity. An enzymatic transformation of mangiferin into norathyriol has been proved both in vitro and in vivo35.
3.2.2. Prenylated polyketides
Isohumulone (24) and isocohumulone (25), are among the main bitter agents, responsible for the taste imparted by hop (Humulus lupulus L.) to beer. These compounds, formed by isomerization of humulones during the brewing process, have been found to activate PPARα and -γ with a positive effect on dyslipidemia in diabetic animals36. Another study found that treatment with isohumulones reduced plasma triglyceride and free fatty acid levels37 mediated by an up-regulation of mRNA for acyl-CoA oxidase, acyl-CoA synthetase, hydroxymethylglutaryl-CoA synthetase, lipoprotein lipase38, 39. Although it can be argued that PPARα modulation is not the single mechanism explaining the effects of isohumulones, these molecules exert an undoubted positive effect on symptoms of metabolic syndrome.
3.3. Phenylpropanoids
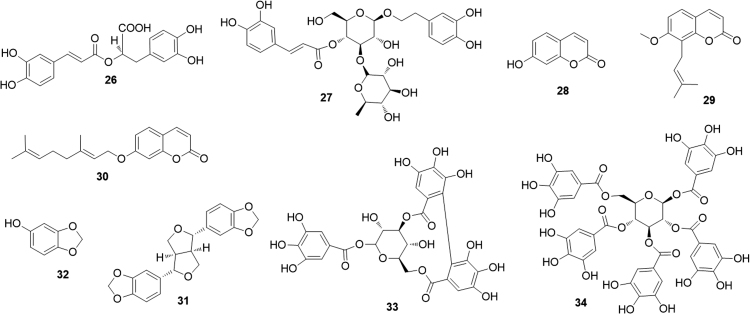
Rosmarinic acid (26), the main phenylpropanoid of oregano extract showed a moderate PPARα transactivaction activity (about 20% when compared to WY14643)40. The rhamnose bearing phenylpropanoid verbascoside (27) was demonstrated to exert its positive effects on inflammatory bowel disease, at least partly, through PPARα. Indeed, the verbascoside mediated anti-inflammatory activity is weakened in Ppar-α knock-out mice41 (Fig. 8).
Figure 8.
PPARα-modulating phenylpropanoids and tannins.
3.3.1. Coumarins
During an investigation of the mechanisms underlying the effects of the widespread coumarin umbelliferone (28) on alcoholic fatty liver, Authors found an elevated expression of the fatty acid oxidation genes (including PPARα) with a stimulated fatty acid β-oxidation activities and beneficial effects on hepatic lipid metabolism42. The prenylated coumarin osthole (29), isolated from Cnidium monnieri and Angelica pubescens, significantly activated both PPARα and PPARγ in a dose-dependent manner, thus giving a marked increase in the expression of PPAR-target genes43. Osthole was also hypothesized to activate PPARα through an AMPK-dependent pathway which induces phosphorylation, and therefore activation, of PPARα44. The O-geranoylated coumarin auraptene (30) was demonstrated to serve as a dual agonist for PPARα and PPARγ in luciferase ligand assay45. A different investigation has shown that auraptene induces up-regulation of PPAR-target genes, such as acyl-CoA oxidase (ACO), carnitinepalmitoyl transferase 1 A (CPT1A) and acyl-CoA synthetase (ACS). Authors concluded that auraptene may improve lipid abnormality through PPARα activation in the liver46.
3.3.2. Lignans
Sesamin (31), a major lignan of sesame seeds, upregulates the PPARα-associated signaling and downregulates the liver X receptor α (LXRα)-mediated pathway, a combined effect that induces an evident improvement of hepatic steatosis and related inflammation47. The biosynthetically and chemically related sesamol (32), also present in sesame seed oil, share this effect48.
3.3.3. Tannins
A recent paper from the Khan׳s group49 reported the results of an investigation of the effects of fruits of Terminalia bellerica (Combretaceae), entering in the composition of triphala, a popular Ayurvedic formulation for treating diabetes. A series of ellagitannins, e.g. corilagin (33), and of gallotannins, e.g. 1,2,3,4,6-penta-O-galloyl-β-d-glucose (34) were found to enhance PPARα and PPARγ signaling.
3.4. Polyphenols
3.4.1. Chalcones and stilbenes
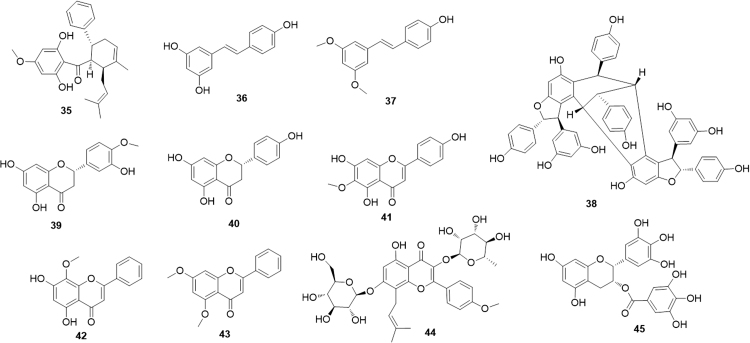
The cyclohexenyl chalcone panduratin A (35, Fig. 9), isolated from Boesenbergia pandurata rhizomes, was shown to be a natural AMPK stimulator, with consequent activation of PPARα/δ, the same mechanism as reported before for osthole (29)50. A certain structural similarity between the two compounds could provide an explanation for the common mechanism of action.
Figure 9.
PPARα-modulating chalcones, stilbenes and flavonoids.
Resveratrol (36, Fig. 9) is probably one of the most intensely investigated phytochemicals and surely the best known stilbene. A plethora of pharmacological activities have been attributed to this flavonoid, present in the skin and seeds of grapes but also in many other natural sources. A series of experiments on several cells have shown that resveratrol activates the nuclear receptors PPARα and PPARγ51. However, the activity of this stilbene seems to be higher when the oxidative state of the cell is stronger and to decrease as the effect of oxidants decrease52. A recent paper by Takizawa et al.53 evaluated in detail the chemical basis of the activation of PPARα by resveratrol. The results of experiments using the crystal structure of the PPARα LBD indicated that the 4′-hydroxyl group of resveratrol is critical for the direct activation of PPARα. In agreement with this conclusion, the activity of resveratrol is shared by its dimethylated analogue pterostilbene (37), which indeed maintains a free hydroxyl group at position 4′. Actually, the agonistic activity of 37 on PPARα is even higher than that of resveratrol, probably due to the beneficial lowering of polarity on ring A54. Vaticanol C (38), reported to be a complex resveratrol tetramer activates PPARα and PPARβ/δ, but not PPARγ, both in vitro and in vivo. The molecular size of vaticanol C is much larger than that of resveratrol and it is hard to believe that the two molecules could share the same binding pocket55.
3.4.2. Flavonoids
The flavanones hesperetin (39) and naringenin (40) (Fig. 9) and their glycosides, present in dried, immature fruit of Citrus aurantium, induced expression of PPARγ in a dose-dependent manner while only naringenin was able to activate PPARα56. The effects of this activation translated into in vivo increasing in hepatic fatty acid oxidation, decreasing in hepatic cholesterol and cholesterol ester synthesis, reduction of both VLDL derived and endogenously synthesized fatty acids57. Interestingly, naringenin also decreases cholesterol and bile acid production modulating another nuclear receptor family (LXRα)58.
Hispidulin (41), a common flavone, acts as a direct PPARα agonist and exerts hypolipidemic effect by enhancing the expression of fatty-acid β-oxidation genes. In vivo data suggested that 3-month treatment with hispidulin or fenofibrate in dyslipidaemic rat improved the lipid profile59. The related flavone wogonin (42), commonly extracted from the traditional Chinese medicine Scutellaria baicalensis, showed a somewhat similar pharmacological profile. In addition, it has been recently shown that PPARα activation by wogonin downregulates osteopontin a multifunctional protein involved in several physiological and pathological events, including cancer and cardiovascular diseases60. Another flavone, 5,7-dimethoxyflavone (43), also demonstrated to increase PPARα/γ activation, was proposed to prevent and treat skin photoaging, being able to prevent and contrast negative effects of oxidative stress and inflammation61.
Icariin (44), a glycosylated and prenylated flavonol obtained from Epimedium brevicornum Maxim (a traditional Chinese herb known as Yin Yang Huo), up-regulated PPARα and PPARγ protein levels. This effect, combined to the already known inhibition of NF-κB expression, can explain the potent neuroprotective and anti-inflammatory effects attributed to this compound62, 63.
Epigallocatechin-3-gallate (45), the major polyphenolic constituent of green tea (Camellia sinensis), increases the expression of PPARα and confers susceptibility to cancer cells via suppression of the enzyme heme oxygenase-164.
3.4.3. Isoflavonoids
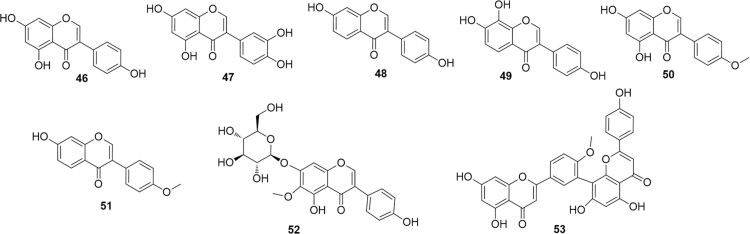
Several Authors have investigated the effects of the simple isoflavonoid genistein (46, Fig. 10) on the modulation of PPARα. This compound was found to protect against oleic acid-induced steatosis with a complex mechanism that includes an increase in PPARα expression65. Interestingly, 3′-hydroxygenistein (47) reached a higher activation efficiency than its precursor and, similarly, while the strictly related isoflavonoid daidzein (48) only slightly activated PPARα, its metabolite 6-hydroxydaidzein (49) exerted a much higher PPARα activity66. As seen before in the case of resveratrol, a comparison among isoflavonoids shows the impact of ring A functionalization on the PPARα modulating activity. However, in the case of resveratrol data available pointed to a crucial role played by the 4′-hydroxyl group for the direct activation of PPARα (see above). In the case of isoflavonoids, a methylation at that key position seems to be not only well tolerated but even to increase the activity. Thus, biochanin A (50), differing from genistein only by methylation of the 4′ OH group, was several-fold more potent than its precursor. Similarly, formononetin (51), bearing the same methylation relationship with daidzein was at least an order of magnitude more potent than its demethylated analogue67. Of course, it is not easy to unambiguously exclude that these effect are mainly related to an increase in the bioavailability of the molecules rather than on an improved interaction with the binding site. Different abilities to recruit co-activators or co-repressors, and/or cross-activation of other nuclear receptors cannot also be excluded.
Figure 10.
PPARα-modulating isoflavonoids and biflavonoids.
The glycosylated isoflavonoid tectoridin (52) was isolated from the flowers of Pueraria lobata (Willd.) Ohwi. (Puerariae Flos), used in traditional Chinese medicine as a remedy for liver injury. Since impaired fatty acid catabolism in the liver can be likely caused by the blockade of PPARα function by ethanol, it can be anticipated that administration of PPARα agonists to ethanol-fed animals could prevent fatty liver by reversing PPARα dysfunction. An investigation by Xiong et al.68 demonstrated that the effects of tectoridin are indeed mediated by a marked inhibition of the ethanol-induced decrease of PPAR expression and its target genes.
3.4.4. Biflavonoids
Bilobetin (53), a biflavonoid isolated from Ginkgo biloba was found to exert a positive effect on hyperlipidaemia, lipotoxicity and insulin resistance in rats. However, these effects could not be related to a direct PPARα agonism, while the involvement of proteon kinase A (PKA) is more likely. PKA activation in the liver by bilobetin appears to stimulate the phosphorylation (specifically of Thr129 and/or Ser163), nuclear translocation and activity of PPARα69.
3.5. Alkaloids
The class of PPARα modulators belonging to the alkaloid biogenetic pathway is comparatively small, although some promising examples have been reported. This is the case of the recent paper describing the activity of picrasidine C (54, Fig. 11), a dimeric β-carboline-type alkaloid isolated from the root of Picrasma quassioides. This compound was identified as a selective PPARα agonist (no activity on PPARβ/δ and PPARγ was observed), comparable to the positive control WY14643, with a consequent induction of the mRNA expression of several PPARα-regulated genes. In silico docking calculations confirmed that picrasidine C fitted well within the PPARα LBD forming a series of crucial interactions, including hydrogen bonds with Cys276 and Thr279 70.
Figure 11.
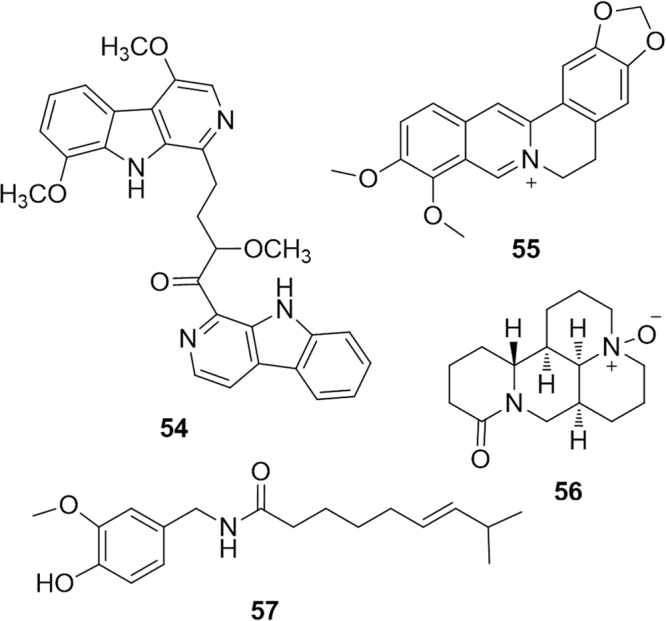
PPARα-modulating alkaloids.
The isoquinoline alkaloid berberine (55) has been shown to have a body weight reducing effect in diabetic rats, mediated by hypolipidemic effects, including restoration of normal total cholesterol, triglyceride, fatty acid and low density lipoprotein-cholesterol levels71. These effects are likely to be, at least partly, mediated by the selective activation of PPARα: berberine binds directly to the LBD of PPARα with similar affinity to fenofibrate72. Similar positive effects on body weight and dyslipidemia have been reported for oxymatrine (56) isolated from the medicinal plant Sophora flavescens73. These effects seem to be mediated by down-regulation of SREBF1 and up-regulation of PPARα mediated metabolic pathways. The pseudoalkaloid capsaicin (57), the spicy component of hot pepper, has been found to lower glucose, insulin and leptin levels, and to reduce the impairment of glucose tolerance in obese mice. Capsaicin is the archetypal agonist of transient receptor potential TRPV1 and the above effect can be modulated by the expression/activation of this endpoint. However, luciferase assays revealed that capsaicin is capable of binding PPARα and, indeed, Pparα mRNA and PPARα-target gene levels were higher in the livers of obese mice supplemented with dietary capsaicin than in those of the obese controls74.
3.6. Total extracts
Several total extracts have been reported to modulate PPARα activity and exert positive impact on dyslipidemia and metabolic syndrome symptoms. A selection of them has been collected in Table 1. The effect of a complex network of compounds, as a total extract is, on a complex and largely interrelated system as PPARα is, can be evaluated and rationalized with great difficulty. However, it is undoubted that several still unexplored natural sources of potential PPARα modulators are available. Thus, the list reported in Table 175, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112 is should encourage natural product chemists to make efforts aimed at the detailed characterization of the active principle(s) responsible for the action of these extract.
Table 1.
A selection of total extracts reported to target PPARα.
| Species name | Class of active metabolites | Ref. |
|---|---|---|
| Acacia (bark) | Polyphenols | 75 |
| Allium sativum (oil) | — | 76 |
| Anethum graveolens (seed) | — | 77 |
| Camellia sinensis (leaves) | Catechin-enriched extract | 78, 79, 80 |
| Chlorella sorokiniana | Fatty acids | 81 |
| Chrysanthemum zawadskii | — | 82 |
| Cinnamomi Cassiae (bark) | — | 83, 84 |
| Citrus limon (peel) | Polyphenols | 85, 86 |
| Clematis sp. | — | 87 |
| Crataegus pinnatifida (fruit) | — | 88 |
| Cucurbita moschata (stem parts) | — | 89 |
| Emblica officinalis | Polyphenols | 90, 91 |
| Eugenia jambolana (seeds) | Flavonoids | 92 |
| Ganoderma lucidum | — | 93 |
| Glycine max (seeds) | Isoflavones | 94, 95, 96, 97, 98 |
| Helicteres isora | Saponins | 99 |
| Hericium erinaceus | — | 100 |
| Litsea coreana | Flavonoids | 101 |
| Momordica charantia (fruit) | — | 102, 103 |
| Momordica grosvenori | Flavones | 104 |
| Pearsonothuria graeffei | Saponins | 105 |
| Pinellia ternata | — | 106 |
| Punica granatum (flower) | — | 107 |
| Rehmannia glutinosa | Oligosaccharides | 108 |
| Syzygium cumini | — | 109 |
| Vaccinium myrtillus | Anthocyanins | 110 |
| Vitis vinifera (seed) | Proanthocyanidins | 111, 112 |
— Not applicable.
4. Conclusions
The objective of this review was to collect in a single manuscript the most promising natural products having shown activity on the modulation of PPARα and, therefore, holding a potential in the treatment of metabolic syndrome. Our collection of compounds was organized on the basis of the biogenetic origin and, consequently, of the chemical structure, regardless the detailed mechanism of PPARα modulation. Our efforts were not addresses at creating a comprehensive collection of all the natural products reported to interact in some extent with PPARα, but to show the great chemodiversity of natural products able to modulate this important nuclear receptor.
Throughout this review, we have avoided reporting quantitative data since these can largely depend on type of cell line used and different cell lines might provide different results depending on the presence of cofactors (co-activators or co-repressors) and/or metabolic processes. Thus, quantitative comparisons among the different compounds would have been in many cases inappropriate. Moreover, it is now clear that in vitro assays can give only a rough idea of the quantitative effects of compounds on PPARα, and a careful investigation in vivo is in any case necessary.
In this review we have decided to focus on PPARα modulators, but we are well aware that there is growing evidence that the ligands able to bind and activate both PPARα and PPARγ can provide therapeutical advantages over PPARα selective ligands, due to synergistic increase in lipid metabolism and insulin sensitivity. Not surprisingly, PPARα/γ dual agonistic approach has been recently intensively exploited by pharmaceutical industry and compounds like muraglitazar and tesaglitazar have indeed demonstrated efficacy in glucose normalization and correction of lipid abnormalities in diabetic patients8. Unfortunately, further development of these compounds failed at clinical trials, due to heart failure and renal toxicities11, 12. Thus, again natural products or herbal medicines can be a valuable alternative strategy to find drugs for metabolic syndrome with low adverse side effects. Although, generally, activation of PPARα by natural compounds is not as strong as that by synthetic compounds, such as fibrates, the administration of a partial PPAR agonist may offer some advantages and could join the desired efficacy with a lower degree of potential adverse effects. The generic “antidiabetic” or “hypolipidemic” effects of many botanicals could likely be ascribed to activation of the PPAR signaling system. A deep investigation on these effects and the discovery and characterization of their putative PPAR-activating compounds would pave the way preparation of innovative dugs, food supplements, nutraceuticals for the management of the metabolic syndrome.
It is clear that natural products have still much to say also in the field of PPARα modulation.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Dreyer C., Krey G., Keller H., Givel F., Helftenbein G., Wahli W. Control of the peroxisomal β-oxidation pathway by a novel family of nuclear hormone receptors. Cell. 1992;68:879–887. doi: 10.1016/0092-8674(92)90031-7. [DOI] [PubMed] [Google Scholar]
- 2.Berger J.P., Akiyama T., Meinke P. PPARs: therapeutic targets for metabolic disease. Trends Pharmacol Sci. 2005;26:244–251. doi: 10.1016/j.tips.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Staels B., Fruchart J.C. Therapeutic roles of peroxisome proliferator-activated receptor agonists. Diabetes. 2005;54:2460–2470. doi: 10.2337/diabetes.54.8.2460. [DOI] [PubMed] [Google Scholar]
- 4.Hostetler H.A., Petrescu A.D., Kier A.B., Schroeder F. Peroxisome proliferator-activated receptor α interacts with high affinity and is conformationally responsive to endogenous ligands. J Biol Chem. 2005;280:18667–18682. doi: 10.1074/jbc.M412062200. [DOI] [PubMed] [Google Scholar]
- 5.Lefebvre P., Chinetti G., Fruchart J.C., Staels B. Sorting out the roles of PPARα in energy metabolism and vascular homeostasis. J Clin Investig. 2006;116:571–580. doi: 10.1172/JCI27989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Escher P., Braissant O., Basu-Modak S., Michalik L., Wahli W., Desvergne B. Rat PPARs: quantitative analysis in adult rat tissues and regulation in fasting and refeeding. Endocrinology. 2001;142:4195–4202. doi: 10.1210/endo.142.10.8458. [DOI] [PubMed] [Google Scholar]
- 7.Rubins H.B., Robins S.J. Conclusions from the VA-HIT study. Am J Cardiol. 2000;86:543–544. doi: 10.1016/s0002-9149(00)01010-9. [DOI] [PubMed] [Google Scholar]
- 8.Pirat C., Farce A., Lebègue N., Renault N., Furman C., Millet R. Targeting peroxisome proliferator—activated receptors (PPARs): development of modulators. J Med Chem. 2012;55:4027–4061. doi: 10.1021/jm101360s. [DOI] [PubMed] [Google Scholar]
- 9.Chakravarthy M.V., Lodhi I.J., Yin L., Malapaka R.R.V., Xu H.E., Turk J. Identification of a physiologically relevant endogenous ligand for PPARα in liver. Cell. 2009;138:476–488. doi: 10.1016/j.cell.2009.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roy A., Kundu M., Jana M., Mishra R.K., Yung Y., Luan C.H. Identification and characterization of PPARα ligands in the hippocampus. Nat Chem Biol. 2016;12:1075–1083. doi: 10.1038/nchembio.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burns K.A., Vanden Heuvel J.P. Modulation of PPAR activity via phosphorylation. Biochim Biophys Acta: Mol Cell Biol Lipids. 2007;1771:952–960. doi: 10.1016/j.bbalip.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L.M., Waltenberger B., Pferschy-Wenzig E.M., Blunder M., Liu X., Malainer C. Natural product agonists of peroxisome proliferator—activated receptor gamma (PPARγ): a review. Biochem Pharmacol. 2014;92:73–89. doi: 10.1016/j.bcp.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuda H., Nakamura S., Yoshikawa M. Search for new type of PPARγ agonist—like anti-diabetic compounds from medicinal plants. Biol Pharm Bull. 2014;37:884–891. doi: 10.1248/bpb.b14-00037. [DOI] [PubMed] [Google Scholar]
- 14.Feng S., Reuss L., Wang Y. Potential of natural products in the inhibition of adipogenesis through regulation of PPARγ expression and/or its transcriptional activity. Molecules. 2016;21:1278. doi: 10.3390/molecules21101278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goto T., Takahashi N., Hirai S., Kawada T. Various terpenoids derived from herbal and dietary plants function as PPAR modulators and regulate carbohydrate and lipid metabolism. PPAR Res. 2010;2010:483958. doi: 10.1155/2010/483958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jun H.J., Lee J.H., Kim J., Jia Y., Kim K.H., Hwang K.Y. Linalool is a PPARα ligand that reduces plasma TG levels and rewires the hepatic transcriptome and plasma metabolome. J Lipid Res. 2014;55:1098–1110. doi: 10.1194/jlr.M045807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hotta M., Nakata R., Katsukawa M., Hori K., Takahashi S., Inoue H. Carvacrol, a component of thyme oil, activates PPARα and γ and suppresses COX-2 expression. J Lipid Res. 2009;51:132–139. doi: 10.1194/jlr.M900255-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bai N., He K., Ibarra A., Bily A., Roller M., Chen X. Iridoids from Fraxinus excelsior with adipocyte differentiation-inhibitory and PPARα activation activity. J Nat Prod. 2010;73:2–6. doi: 10.1021/np9003118. [DOI] [PubMed] [Google Scholar]
- 19.Wu C., Jia Y., Lee J.H., Jun H.J., Lee H.S., Hwang K.Y. trans-Caryophyllene is a natural agonistic ligand for peroxisome proliferator—activated receptor-α. Bioorg Med Chem Lett. 2014;24:3168–3174. doi: 10.1016/j.bmcl.2014.04.112. [DOI] [PubMed] [Google Scholar]
- 20.Gertsch J., Leonti M., Raduner S., Racz I., Chen J.Z., Xie X.Q. Beta-caryophyllene is a dietary cannabinoid. Proc Natl Acad Sci U S A. 2008;105:9099–9104. doi: 10.1073/pnas.0803601105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chicca A., Caprioglio D., Minassi A., Petrucci V., Appendino G., Taglialatela-Scafati O. Functionalization of β-caryophyllene generates novel polypharmacology in the endocannabinoid system. ACS Chem Biol. 2014;9:1499–1507. doi: 10.1021/cb500177c. [DOI] [PubMed] [Google Scholar]
- 22.Duncan R.E., Archer M.C. Farnesol decreases serum triglycerides in rats: identification of mechanisms including up-regulation of PPARα and down-regulation of fatty acid synthase in hepatocytes. Lipids. 2008;43:619–627. doi: 10.1007/s11745-008-3192-3. [DOI] [PubMed] [Google Scholar]
- 23.Fushiki T., Goto T., Hosokawa M., Kawada T., Kimura K., Matsui N. Dual action of isoprenols from herbal medicines on both PPARγ and PPARα in 3T3-L1 adipocytes and HepG2 hepatocytes. FEBS Lett. 2002;514:315–322. doi: 10.1016/s0014-5793(02)02390-6. [DOI] [PubMed] [Google Scholar]
- 24.Kang M.S., Hirai S., Goto T., Kuroyanagi K., Lee J.Y., Uemura T. Dehydroabietic acid, a phytochemical, acts as ligand for PPARs in macrophages and adipocytes to regulate inflammation. Biochem Biophys Res Commun. 2008;369:333–338. doi: 10.1016/j.bbrc.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Jardat M.S., Noonan D.J., Wu B., Avery M.A., Feller D.R. Pseudolaric acid analogs as a new class of peroxisome proliferator-activated receptor agonists. Planta Med. 2002;68:667–671. doi: 10.1055/s-2002-33785. [DOI] [PubMed] [Google Scholar]
- 26.Goto T., Takahashi N., Kato S., Egawa K., Ebisu S., Moriyama T. Phytol directly activates peroxisome proliferator—activated receptor α (PPARα) and regulates gene expression involved in lipid metabolism in PPARα-expressing HepG2 hepatocytes. Biochem Biophys Res Commun. 2005;337:440–445. doi: 10.1016/j.bbrc.2005.09.077. [DOI] [PubMed] [Google Scholar]
- 27.Lee H.K., Nam G.W., Kim S.H., Lee S.H. Phytocomponents of triterpenoids, oleanolic acid and ursolic acid, regulated differently the processing of epidermal keratinocytes via PPAR-α pathway. Exp Dermatol. 2006;15:66–73. doi: 10.1111/j.0906-6705.2005.00386.x. [DOI] [PubMed] [Google Scholar]
- 28.Yoon M., Lee H., Jeong S., Kim J.J., Nicol C.J., Nam K.W. Peroxisome proliferator—activated receptor α is involved in the regulation of lipid metabolism by ginseng. Br J Pharmacol. 2003;138:1295–1302. doi: 10.1038/sj.bjp.0705169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee H., Gonzalez F.J., Yoon M. Ginsenoside Rf, a component of ginseng, regulates lipoprotein metabolism through peroxisome proliferator—activated receptor α. Biochem Biophys Res Commun. 2006;339:196–203. doi: 10.1016/j.bbrc.2005.10.197. [DOI] [PubMed] [Google Scholar]
- 30.Woo M.N., Jeon S.M., Kim H.J., Lee M.K., Shin S.K., Shin Y.C. Fucoxanthin supplementation improves plasma and hepatic lipid metabolism and blood glucose concentration in high-fat fed C57BL/6N mice. Chem-Biol Interact. 2010;186:316–322. doi: 10.1016/j.cbi.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Kim S.N., Choi H.Y., Lee W., Park G.M., Shin W.S., Kim Y.K. Sargaquinoic acid and sargahydroquinoic acid from Sargassum yezoense stimulate adipocyte differentiation through PPARα/γ activation in 3T3-L1 cells. FEBS Lett. 2008;582:3465–3472. doi: 10.1016/j.febslet.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 32.Goto T., Takahashi N., Kato S., Kim Y.I., Kusudo T., Taimatsu A. Bixin activates PPARα and improves obesity-induced abnormalities of carbohydrate and lipid metabolism in mice. J Agric Food Chem. 2012;60:11952–11958. doi: 10.1021/jf303639f. [DOI] [PubMed] [Google Scholar]
- 33.Chianese G., Yu H.B., Yang F., Sirignano C., Luciano P., Han B.N. PPAR modulating polyketides from a Chinese Plakortis simplex and clues on the origin of their chemodiversity. J Org Chem. 2016;81:5135–5143. doi: 10.1021/acs.joc.6b00695. [DOI] [PubMed] [Google Scholar]
- 34.Huang T.H.W., Peng G., Li G.Q., Yamahara J., Roufogalis B.D., Li Y. Salacia oblonga root improves postprandial hyperlipidemia and hepatic steatosis in Zucker diabetic fatty rats: activation of PPAR-α. Toxicol Appl Pharmacol. 2006;210:225–235. doi: 10.1016/j.taap.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Wilkinson A.S., Monteith G.R., Shaw P.N., Lin C.N., Gidley M.J., Roberts-Thomson S.J. Effects of the mango components mangiferin and quercetin and the putative mangiferin metabolite norathyriol on the transactivation of peroxisome proliferator-activated receptor isoforms. J Agric Food Chem. 2008;56:3037–3042. doi: 10.1021/jf800046n. [DOI] [PubMed] [Google Scholar]
- 36.Yajima H., Ikeshima E., Shiraki M., Kanaya T., Fujiwara D., Odai H. Isohumulones, bitter acids derived from hops, activate both peroxisome proliferator-activated receptor α and γ and reduce insulin resistance. J Biol Chem. 2004;279:33456–33462. doi: 10.1074/jbc.M403456200. [DOI] [PubMed] [Google Scholar]
- 37.Shimura M., Hasumi A., Minato T., Hosono M., Miura Y., Mizutani S. Isohumulones modulate blood lipid status through the activation of PPAR α. Biochim Biophys Acta: Mol Cell Biol Lipids. 2005;1736:51–60. doi: 10.1016/j.bbalip.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Miura Y., Hosono M., Oyamada C., Odai H., Oikawa S., Kondo K. Dietary isohumulones, the bitter components of beer, raise plasma HDL-cholesterol levels and reduce liver cholesterol and triacylglycerol contents similar to PPARα activations in C57BL/6 mice. Br J Nutr. 2005;93:559–567. doi: 10.1079/bjn20041384. [DOI] [PubMed] [Google Scholar]
- 39.Obara K., Mizutani M., Hitomi Y., Yajima H., Kondo K. Isohumulones, the bitter component of beer, improve hyperglycemia and decrease body fat in Japanese subjects with prediabetes. Clin Nutr. 2009;28:278–284. doi: 10.1016/j.clnu.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 40.Mueller M., Lukas B., Novak J., Simoncini T., Genazzani A.R., Jungbauer A. Oregano: a source for peroxisome proliferator-activated receptor γ antagonists. J Agr Food Chem. 2008;56:11621–11630. doi: 10.1021/jf802298w. [DOI] [PubMed] [Google Scholar]
- 41.Esposito E., Mazzon E., Paterniti I., Dal Toso R., Pressi G., Caminiti R. PPAR-α contributes to the anti-inflammatory activity of verbascoside in a model of inflammatory bowel disease in mice. PPAR Res. 2010;2010:917312. doi: 10.1155/2010/917312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim M.J., Sim M.O., Lee H.I., Ham J.R., Seo K.I., Lee M.K. Dietary umbelliferone attenuates alcohol-induced fatty liver via regulation of PPARα and SREBP-1c in rats. Alcohol. 2014;48:707–715. doi: 10.1016/j.alcohol.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 43.Sun F., Xie M.L., Xue J., Wang H.B. Osthol regulates hepatic PPARα-mediated lipogenic gene expression in alcoholic fatty liver murine. Phytomedicine. 2010;17:669–673. doi: 10.1016/j.phymed.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 44.Liang H.J., Suk F.M., Wang C.K., Hung L.F., Liu D.Z., Chen N.Q. Osthole, a potential antidiabetic agent, alleviates hyperglycemia in db/db mice. Chem Biol Interact. 2009;181:309–315. doi: 10.1016/j.cbi.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 45.Kuroyanagi K., Kang M.S., Goto T., Hirai S., Ohyama K., Kusudo T. Citrus auraptene acts as an agonist for PPARs and enhances adiponectin production and MCP-1 reduction in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2008;366:219–225. doi: 10.1016/j.bbrc.2007.11.119. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi N., Kang M.S., Kuroyanagi K., Goto T., Hirai S., Ohyama K. Auraptene, a citrus fruit compound, regulates gene expression as a PPARα agonist in HepG2 hepatocytes. BioFactors. 2008;33:25–32. doi: 10.1002/biof.5520330103. [DOI] [PubMed] [Google Scholar]
- 47.Zhang R., Yu Y., Hu S., Zhang J., Yang H., Han B. Sesamin ameliorates hepatic steatosis and inflammation in rats on a high-fat diet via LXRα and PPARα. Nutr Res. 2016;36:1022–1030. doi: 10.1016/j.nutres.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 48.Sharma A.K., Bharti S., Bhatia J., Nepal S., Malik S., Ray R. Sesamol alleviates diet-induced cardiometabolic syndrome in rats via up-regulating PPARγ, PPARα and e-NOS. J Nutr Biochem. 2012;23:1482–1489. doi: 10.1016/j.jnutbio.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 49.Yang M.H., Vasquez Y., Ali Z., Khan I.A., Khan S.I. Constituents from Terminalia species increase PPARα and PPARγ levels and stimulate glucose uptake without enhancing adipocyte differentiation. J Ethnopharmacol. 2013;149:490–498. doi: 10.1016/j.jep.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 50.Kim D., Lee M.S., Jo K., Lee K.E., Hwang J.K. Therapeutic potential of panduratin A, LKB1-dependent AMP-activated protein kinase stimulator, with activation of PPARα/δ for the treatment of obesity. Diabetes Obes Metab. 2011;13:584–593. doi: 10.1111/j.1463-1326.2011.01379.x. [DOI] [PubMed] [Google Scholar]
- 51.Iannelli P., Zarrilli V., Varricchio E., Tramontano D., Mancini F.P. The dietary antioxidant resveratrol affects redox changes of PPARα activity. Nutr Metab Cardiovasc Dis. 2007;17:247–256. doi: 10.1016/j.numecd.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 52.Inoue H., Jiang X.F., Katayama T., Osada S., Umesono K., Namura S. Brain protection by resveratrol and fenofibrate against stroke requires peroxisome proliferator—activated receptor α in mice. Neurosci Lett. 2003;352:203–206. doi: 10.1016/j.neulet.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 53.Takizawa Y., Nakata R., Fukuhara K., Yamashita H., Kubodera H., Inoue H. The 4′-hydroxyl group of resveratrol is functionally important for direct activation of PPARα. PLoS One. 2015;10:e0120865. doi: 10.1371/journal.pone.0120865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rimando A.M., Nagmani R., Feller D.R., Yokoyama W. Pterostilbene, a new agonist for the peroxisome proliferator-activated receptor α-isoform, lowers plasma lipoproteins and cholesterol in hypercholesterolemic hamsters. J Agric Food Chem. 2005;53:3403–3407. doi: 10.1021/jf0580364. [DOI] [PubMed] [Google Scholar]
- 55.Tsukamoto T., Nakata R., Tamura E., Kosuge Y., Kariya A., Katsukawa M. Vaticanol C, a resveratrol tetramer, activates PPARα and PPARβ/δ in vitro and in vivo. Nutr Metab. 2010;7:46. doi: 10.1186/1743-7075-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu L., Shan S., Zhang K., Ning Z.Q., Lu X.P., Cheng Y.Y. Naringenin and hesperetin, two flavonoids derived from Citrus aurantium up-regulate transcription of adiponectin. Phytother Res. 2008;22:1400–1403. doi: 10.1002/ptr.2504. [DOI] [PubMed] [Google Scholar]
- 57.Mulvihill E.E., Allister E.M., Sutherland B.G., Telford D.E., Sawyez C.G., Edwards J.Y. Naringenin prevents dyslipidemia, apolipoprotein B overproduction, and hyperinsulinemia in LDL receptor-null mice with diet-induced insulin resistance. Diabetes. 2009;58:2198–2210. doi: 10.2337/db09-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goldwasser J., Cohen P.Y., Yang E., Balaguer P., Yarmush M.L., Nahmias Y. Transcriptional regulation of human and rat hepatic lipid metabolism by the grapefruit flavonoid naringenin: role of PPARα, PPARγ and LXRα. PLoS One. 2010;5:e12399. doi: 10.1371/journal.pone.0012399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu X.C., Xu J. New role of hispidulin in lipid metabolism: PPARα activator. Lipids. 2016;51:1249–1257. doi: 10.1007/s11745-016-4200-7. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Y.M., Li M.X., Tang Z., Wang C.H. Wogonin suppresses osteopontin expression in adipocytes by activating PPARα. Acta Pharmacol Sin. 2015;36:987–997. doi: 10.1038/aps.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim J.K., Mun S., Kim M.S., Kim M.B., Sa B.K., Hwang J.K. 5, 7-dimethoxyflavone, an activator of PPARα/γ, inhibits UVB-induced MMP expression in human skin fibroblast cells. Exp Dermatol. 2012;21:211–216. doi: 10.1111/j.1600-0625.2011.01435.x. [DOI] [PubMed] [Google Scholar]
- 62.Xiong D., Deng Y., Huang B., Yin C., Liu B., Shi J. Icariin attenuates cerebral ischemia-reperfusion injury through inhibition of inflammatory response mediated by NF-κB, PPARα and PPARγ in rats. Int Immunopharmacol. 2016;30:157–162. doi: 10.1016/j.intimp.2015.11.035. [DOI] [PubMed] [Google Scholar]
- 63.Ding L., Liang X.G., Zhu D.Y., Lou Y.J. Icariin promotes expression of PGC-1α, PPARα, and NRF-1 during cardiomyocyte differentiation of murine embryonic stem cells in vitro. Acta Pharmacol Sin. 2007;28:1541–1549. doi: 10.1111/j.1745-7254.2007.00648.x. [DOI] [PubMed] [Google Scholar]
- 64.Zhang S., Yang X., Luo J., Ge X., Sun W., Zhu H. PPARα activation sensitizes cancer cells to epigallocatechin-3-gallate (EGCG) treatment via suppressing heme oxygenase-1. Nutr Cancer. 2014;66:315–324. doi: 10.1080/01635581.2014.868909. [DOI] [PubMed] [Google Scholar]
- 65.Hou S.J., Wu D., Jiang Z.Q. Effect of genistein on the changes of PPARα expression and glycolipid level in oleic acid-induced steatosis in HepG2 cells. Acta Nutr Sin. 2014;36:49–52. [Google Scholar]
- 66.Mueller M., Hobiger S., Jungbauer A. Red clover extract: a source for substances that activate peroxisome proliferator-activated receptor and ameliorate the cytokine secretion profile of lipopolysaccharide-stimulated macrophages. Menopause. 2010;17:379–387. doi: 10.1097/gme.0b013e3181c94617. [DOI] [PubMed] [Google Scholar]
- 67.Shen P., Liu M.H., Ng T.Y., Chan Y.H., Yong E.L. Differential effects of isoflavones, from Astragalus membranaceus and Pueraria thomsonii, on the activation of PPARα, PPARγ, and adipocyte differentiation in vitro. J Nutr. 2006;136:899–905. doi: 10.1093/jn/136.4.899. [DOI] [PubMed] [Google Scholar]
- 68.Xiong Y., Yang YQ., Yang J., Chai HY., Li Y., Yang J. Tectoridin, an isoflavone glycoside from the flower of Pueraria lobata, prevents acute ethanol-induced liver steatosis in mice. Toxicology. 2010;276:64–72. doi: 10.1016/j.tox.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 69.Kou X.H., Zhu M.F., Chen D., Lu Y., Song H.Z., Ye J.L. Bilobetin ameliorates insulin resistance by PKA-mediated phosphorylation of PPARα in rats fed a high-fat diet. Brit J Pharmacol. 2012;165:2692–2706. doi: 10.1111/j.1476-5381.2011.01727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao S., Kanno Y., Li W., Sasaki T., Zhang X., Wang J. Identification of picrasidine C as a subtype-selective PPARα agonist. J Nat Prod. 2016;79:3127–3133. doi: 10.1021/acs.jnatprod.6b00883. [DOI] [PubMed] [Google Scholar]
- 71.Zhou J.Y., Zhou S.W., Zhang K.B., Tang J.L., Guang L.X., Ying Y. Chronic effects of berberine on blood, liver glucolipid metabolism and liver PPARs expression in diabetic hyperlipidemic rats. Biol Pharm Bull. 2008;31:1169–1176. doi: 10.1248/bpb.31.1169. [DOI] [PubMed] [Google Scholar]
- 72.Yu H., Li C., Yang J., Zhao T., Zhou Q. Berberine is a potent agonist of peroxisome proliferator activated receptor alpha. Front Biosci. 2016;21:1052–1060. doi: 10.2741/4440. [DOI] [PubMed] [Google Scholar]
- 73.Shi L.J., Shi L., Song G.Y., Zhang H.F., Hu Z.J., Wang C. Oxymatrine attenuates hepatic steatosis in non-alcoholic fatty liver disease rats fed with high fructose diet through inhibition of sterol regulatory element binding transcription factor 1 and activation of (Srebf1) peroxisome proliferator activated receptor α (Pparα) Eur J Pharmacol. 2013;714:89–95. doi: 10.1016/j.ejphar.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 74.Kang J., Tsuyoshi G., Han I.S., Kawada T., Kim Y.M., Yu R. Dietary capsaicin reduces obesity-induced insulin resistance and hepatic steatosis in obese mice fed a high-fat diet. Obesity. 2010;18:780–787. doi: 10.1038/oby.2009.301. [DOI] [PubMed] [Google Scholar]
- 75.Ikarashi N., Toda T., Okaniwa T., Ito K., Ochiai W., Sugiyama K. Anti-obesity and anti-diabetic effects of Acacia polyphenol in obese diabetic KKAy mice fed high-fat diet. Evid Based Complement Altern Med. 2011;2011:952031. doi: 10.1093/ecam/nep241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zeng T., Zhang C.L., Song F.Y., Zhao X.L., Xie K.Q. Garlic oil alleviated ethanol-induced fat accumulation via modulation of SREBP-1, PPAR-α, and CYP2E1. Food Chem Toxicol. 2012;50:485–491. doi: 10.1016/j.fct.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 77.Takahashi N., Yao L., Kim M., Sasako H., Aoyagi M., Shono J. Dill seed extract improves abnormalities in lipid metabolism through peroxisome proliferator-activated receptor-α (PPAR-α) activation in diabetic obese mice. Mol Nutr Food Res. 2013;57:1295–1299. doi: 10.1002/mnfr.201200767. [DOI] [PubMed] [Google Scholar]
- 78.Serisier S., Leray V., Poudroux W., Magot T., Ouguerram K., Nguyen P. Effects of green tea on insulin sensitivity, lipid profile and expression of PPARα and PPARγ and their target genes in obese dogs. Br J Nutr. 2008;99:1208–1216. doi: 10.1017/S0007114507862386. [DOI] [PubMed] [Google Scholar]
- 79.Li R.W., Douglas T.D., Maiyoh G.K., Adeli K., Theriault A.G. Green tea leaf extract improves lipid and glucose homeostasis in a fructose-fed insulin-resistant hamster model. J Ethnopharmacol. 2006;104:24–31. doi: 10.1016/j.jep.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 80.Lee K. Transactivation of peroxisome proliferator-activated receptor α by green tea extracts. J Vet Sci. 2004;5:325–330. [PubMed] [Google Scholar]
- 81.Chou Y.C., Prakash E., Huang C.F., Lien T.W., Chen X., Su I.J. Bioassay-guided purification and identification of PPARα/γ agonists from Chlorella sorokiniana. Phytother Res. 2008;22:605–613. doi: 10.1002/ptr.2280. [DOI] [PubMed] [Google Scholar]
- 82.Kim B., Kim H.S. Chrysanthemum zawadskii extract activates peroxisome proliferator-activated receptor-α and has an anti-inflammatory activity: potential interest for the skin barrier function. Korean J Chem Eng. 2014;31:1831–1838. [Google Scholar]
- 83.Hee K.S., Young C.S. Antihyperglycemic and antihyperlipidemic action of Cinnamomi cassiae (Cinnamon bark) extract in C57BL/Ks db/db mice. Arch Pharm Res. 2010;33:325–333. doi: 10.1007/s12272-010-0219-0. [DOI] [PubMed] [Google Scholar]
- 84.Monden T., Hosoya T., Nakajima Y., Kishi M., Satoh T., Hashimoto K. Herbal medicine, Hachimi-jio-gan, and its component cinnamomi cortex activate the peroxisome proliferator-activated receptor α in renal cells. Endocr J. 2008;55:529–533. doi: 10.1507/endocrj.k07e-101. [DOI] [PubMed] [Google Scholar]
- 85.Fukuchi Y., Hiramitsu M., Okada M., Hayashi S., Nabeno Y., Osawa T. Lemon polyphenols suppress diet-induced obesity by up-regulation of mRNA levels of the enzymes involved in β-oxidation in mouse white adipose tissue. J Clin Biochem Nutr. 2008;43:201–209. doi: 10.3164/jcbn.2008066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li R.W., Theriault A.G., Au K., Douglas T.D., Casaschi A., Kurowska E.M. Citrus polymethoxylated flavones improve lipid and glucose homeostasis and modulate adipocytokines in fructose-induced insulin resistant hamsters. Life Sci. 2006;79:365–373. doi: 10.1016/j.lfs.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 87.Li R.W., Lin G.D., Leach D.N., Waterman P.G., Myers S.P. Inhibition of COXs and 5-LOX and activation of PPARs by Australian Clematis species (Ranunculaceae) J Ethnopharmacol. 2006;104:138–143. doi: 10.1016/j.jep.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 88.Niu C.S., Chen C.T., Chen L.J., Cheng K.C., Yeh C.H., Cheng J.T. Decrease of blood lipids induced by Shan-Zha (fruit of Crataegus pinnatifida) is mainly related to an increase of PPARα in liver of mice fed high-fat diet. Horm Metab Res. 2011;43:625–630. doi: 10.1055/s-0031-1283147. [DOI] [PubMed] [Google Scholar]
- 89.Choi H., Eo H., Park K., Jin M., Park E.J., Kim S.H. A water-soluble extract from Cucurbita moschata shows anti-obesity effects by controlling lipid metabolism in a high fat diet-induced obesity mouse model. Biochem Biophys Res Commun. 2007;359:419–425. doi: 10.1016/j.bbrc.2007.05.107. [DOI] [PubMed] [Google Scholar]
- 90.Kim H.Y., Okubo T., Juneja L.R., Yokozawa T. The protective role of amla (Emblica officinalis Gaertn.) against fructose-induced metabolic syndrome in a rat model. Br J Nutr. 2010;103:502–512. doi: 10.1017/S0007114509991978. [DOI] [PubMed] [Google Scholar]
- 91.Yokozawa T., Kim H.Y., Kim H.J., Okubo T., Chu D.C., Juneja L.R. Amla (Emblica officinalis Gaertn.) prevents dyslipidaemia and oxidative stress in the ageing process. Br J Nutr. 2007;97:1187–1195. doi: 10.1017/S0007114507691971. [DOI] [PubMed] [Google Scholar]
- 92.Sharma B., Balomajumder C., Roy P. Hypoglycemic and hypolipidemic effects of flavonoid rich extract from Eugenia jambolana seeds on streptozotocin induced diabetic rats. Food Chem Toxicol. 2008;46:2376–2383. doi: 10.1016/j.fct.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 93.Shimojo Y., Kosaka K., Shirasawa T. Effect of Ganoderma lucidum extract on adipocyte differentiation and adiponectin gene expression in the murine pre-adipocyte cell line, 3T3-L1. Phytother Res. 2011;25:202–207. doi: 10.1002/ptr.3242. [DOI] [PubMed] [Google Scholar]
- 94.Carrara V.S., Amato A.A., Neves F.A.R., Bazotte R.B., Mandarino J.M., Nakamura C.V. Effects of a methanolic fraction of soybean seeds on the transcriptional activity of peroxisome proliferator-activated receptors (PPAR) Braz J Med Biol Res. 2009;42:545–550. doi: 10.1590/s0100-879x2009000600011. [DOI] [PubMed] [Google Scholar]
- 95.Wagner J.D., Zhang L., Shadoan M.K., Kavanagh K., Chen H.Y., Tresnasari K. Effects of soy protein and isoflavones on insulin resistance and adiponectin in male monkeys. Metabolism. 2008;57:S24–S31. doi: 10.1016/j.metabol.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu L., Li X., Liu F., Deng X.W. Mechanism of soybean isoflavones on anti-atherosclerosis in metabolic syndrome rats. Chin J Arterioscler. 2008;16:928–932. [Google Scholar]
- 97.Mezei O., Li Y., Mullen E., Ross-Viola J.S., Shay N.F. Dietary isoflavone supplementation modulates lipid metabolism via PPARα-dependent and -independent mechanisms. Physiol Genom. 2006;26:8–14. doi: 10.1152/physiolgenomics.00155.2005. [DOI] [PubMed] [Google Scholar]
- 98.Mezei O., Banz W.J., Steger R.W., Peluso M.R., Winters T.A., Shay N. Soy isoflavones exert antidiabetic and hypolipidemic effects through the PPAR pathways in obese Zucker rats and murine RAW 264. 7 cells. J Nutr. 2003;133:1238–1243. doi: 10.1093/jn/133.5.1238. [DOI] [PubMed] [Google Scholar]
- 99.Bhavsar S.K., Singh S., Giri S., Jain M.R., Santani D.D. Effect of saponins from Helicteres isora on lipid and glucose metabolism regulating genes expression. J Ethnopharmacol. 2009;124:426–433. doi: 10.1016/j.jep.2009.05.041. [DOI] [PubMed] [Google Scholar]
- 100.Hiwatashi K., Kosaka Y., Suzuki N., Hata K., Mukaiyama T., Sakamoto K. Yamabushitake mushroom (Hericium erinaceus) improved lipid metabolism in mice fed a high-fat diet. Biosci Biotech Biochem. 2010;74:1447–1451. doi: 10.1271/bbb.100130. [DOI] [PubMed] [Google Scholar]
- 101.Wang J.Q., Li J., Zou Y.H., Cheng W.M., Lu C., Zhang L. Preventive effects of total flavonoids of Litsea coreana leve on hepatic steatosis in rats fed with high fat diet. J Ethnopharmacol. 2009;121:54–60. doi: 10.1016/j.jep.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 102.Shih C.C., Lin C.H., Lin W.L. Effects of Momordica charantia on insulin resistance and visceral obesity in mice on high-fat diet. Diabetes Res Clin Pract. 2008;81:134–143. doi: 10.1016/j.diabres.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 103.Chao C.Y., Huang C.J. Bitter gourd (Momordica charantia) extract activates peroxisome proliferator-activated receptors and upregulates the expression of the acyl CoA oxidase gene in H4IIEC3 hepatoma cells. J Biomed Sci. 2003;10:782–791. doi: 10.1159/000073966. [DOI] [PubMed] [Google Scholar]
- 104.Mo W.B., Gong M.M., Liu T., Yang Y.L. Effects of Momordica grosvenori flavones on myocardial energy metabolism enzymes and expression of PPARα mRNA in exercise rats. Chin J Exp Tradit Med Formulae. 2013;19:203–208. [Google Scholar]
- 105.Hu X.Q., Wang Y.M., Wang J.F., Xue Y., Li Z.J., Nagao K. Dietary saponins of sea cucumber alleviate orotic acid-induced fatty liver in rats via PPARα and SREBP-1c signaling. Lipids Health Dis. 2010;9:25. doi: 10.1186/1476-511X-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim Y.J., Shin Y.O., Ha Y.W., Lee S., Oh J.K., Kim Y.S. Anti-obesity effect of Pinellia ternata extract in Zucker rats. Biol Pharm Bull. 2006;29:1278–1281. doi: 10.1248/bpb.29.1278. [DOI] [PubMed] [Google Scholar]
- 107.Wang J., Rong X., Um I.S.I., Yamahara J., Li Y. 55-week treatment of mice with the Unani and Ayurvedic medicine pomegranate flower ameliorates ageing-associated insulin resistance and skin abnormalities. Evid Based Complement Altern Med. 2012;2012:350125. doi: 10.1155/2012/350125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang R.X., Jia Z.P., Li M.X., Wang J., Guo L.M., Zhang X.H. Molecular mechanism of Rehmannia glutinosa oligosaccharides on improvement of insulin resistance of HepG2 cell in vitro. Chin Tradit Herb Drugs. 2008;39:1184–1187. [Google Scholar]
- 109.Sharma S., Pathak S., Gupta G., Sharma S.K., Singh L., Sharma R.K. Pharmacological evaluation of aqueous extract of Syzigium cumini for its antihyperglycemic and antidyslipidemic properties in diabetic rats fed a high cholesterol diet–role of PPARγ and PPARα. Biomed Pharmacother. 2017;89:447–453. doi: 10.1016/j.biopha.2017.02.048. [DOI] [PubMed] [Google Scholar]
- 110.Takikawa M., Inoue S., Horio F., Tsuda T. Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. J Nutr. 2010;140:527–533. doi: 10.3945/jn.109.118216. [DOI] [PubMed] [Google Scholar]
- 111.Downing LE, Ferguson BS, Rodriguez K, Ricketts ML. A grape seed procyanidin extract inhibits HDAC activity leading to increased Pparα phosphorylation and target-gene expression. Mol Nutr Food Res 2017. Available from: http://dx.doi.org/10.1002/mnfr.201600347. [DOI] [PMC free article] [PubMed]
- 112.Quesada H., Pajuelo D., Fernández-Iglesias A., Díaz S., Ardevol A., Blay M. Proanthocyanidins modulate triglyceride secretion by repressing the expression of long chain acyl-CoA synthetases in CACO2 intestinal cells. Food Chem. 2011;129:1490–1494. [Google Scholar]