Abstract
Syringaresinol-4-O-β-d-glucoside (SSG), a furofuran-type lignan, was found to modulate lipid and glucose metabolism through an activity screen of lipid accumulation and glucose consumption, and was therefore considered as a promising candidate for the prevention and treatment of metabolic disorder, especially in lipid and glucose metabolic homeostasis. In this study, the effects of SSG on lipogenesis and glucose consumption in HepG2 cells and C2C12 myotubes were further investigated. Treatment with SSG significantly inhibited lipid accumulation by oil red O staining and reduced the intracellular contents of total lipid, cholesterol and triglyceride in HepG2 cells. No effect was observed on cell viability in the MTT assay at concentrations of 0.1–10 μmol/L. SSG also increased glucose consumption by HepG2 cells and glucose uptake by C2C12 myotubes. Furthermore, real-time quantitative PCR revealed that the beneficial effects were associated with the down-regulation of sterol regulatory element-binding proteins-1c, -2 (SREBP-1c, -2), fatty acid synthase (FAS), acetyl CoA carboxylase (ACC) and hydroxyl methylglutaryl CoA reductase (HMGR), and up-regulation of peroxisome proliferator-activated receptors alpha and gamma (PPARα and PPARγ). SSG also significantly elevated transcription activity of PPARγ tested by luciferase assay. These results suggest that SSG is an effective regulator of lipogenesis and glucose consumption and might be a candidate for further research in the prevention and treatment of lipid and glucose metabolic diseases.
KEY WORDS: Syringaresinol-4-O-β-d-glucoside, Lipid accumulation, Glucose consumption, Insulin resistance, HepG2, C2C12, Oil red O
Graphic abstract
Syringaresinol-4-O-β-D-glucoside (SSG) is a promising candidate for the prevention and treatment of metabolic disorder. This study demonstrated that SSG has regulative effect on lipogenesis and glucose consumption in vitro. Mechanism studies revealed that the beneficial effects were associated with regulating the expression and transcription of lipid and glucose related genes.
1. Introduction
Type 2 diabetes mellitus (T2DM) and obesity are associated with lipid accumulation in the liver, which is commonly diagnosed as non-alcoholic fatty liver diseases (NAFLD) and easily induces metabolic syndrome1, 2, 3. Obesity and related metabolic diseases, including T2DM, are the most prevailing nutrition-related issues in all parts of the world4, which imposes a heavy burden on national healthcare systems, particularly in developing countries5, 6. Many studies have shown that over-accumulation of lipids in the liver and reduced glucose metabolism in muscle are closely related to insulin resistance7, 8.
Many natural products or their derivatives, such as galegine (the lead compound metformin and phenformin) and berberine, have been demonstrated to be effective regulators of the metabolic syndrome9. Therefore, it is a feasible and effective strategy to explore lead compounds from natural products with the potential to modulate lipid and glucose regulation. Our previous study demonstrated that the extracts of Pandanus tectorius fruit (PTF) could enhance insulin sensitivity and accommodate lipid and glucose metabolism in vitro and in vivo10, 11, 12. Caffeoylquinic acid, as the main component in PTF extracts, plays an important role in the anti-hyperlipidemic11 and anti-diabetic effect10. However, detailed chemical analysis of PTF extracts revealed the presence of a series of important low-abundance compounds. By screening compounds on lipid and glucose metabolism, we found that syringaresinol-4-O-β-d-glucoside (SSG) had potent activity which could possibly account for some of the effects the PTF extract. SSG is a natural furofuran-type lignan with a glucose linked to the benzene ring. Its aglycone, syringaresinol, possesses diverse pharmacological activities including anti-hepatic fibrosis7, anticancer8, antithrombotic13, as well as ant-inflammatory14, cardiomyocytic protection15, and vascular relaxation16. Various mechanisms are involved in syringaresinol-mediated health benefits such as protection cells from damage15, stimulation cell cycle arrest8 and enhancing nitric oxide (NO) production. Furthermore, syringaresinol could also activate adenosine 5ʹ-monophosphate-activated protein kinase (AMPK, a vital energy sensor), and attenuate inflammation13. Both of these activities are promising for the possible prevention and treatment of metabolic disorder, especially as related to lipid and glucose homeostasis. However, the effect of SSG on lipid accumulation and glucose metabolism has not been previously confirmed.
In the present work, we performed an in vitro study investigating the effects of SSG on lipid accumulation, glucose consumption, glucose uptake and insulin resistance, hoping to provide evidence for the utility of SSG as a candidate for the prevention and treatment of obesity and T2DM.
2. Materials and methods
2.1. Materials
HepG2 and 293 T cells, along with C2C12 myotubes, were obtained from the Peking Union Medical College (Beijing, China). SSG (Fig. 1) was isolated in our lab and the purity is 98% determined by high performance liquid chromatography (HPLC) as previously reported10. High glucose Dulbecco׳s modified Eagle׳s medium (DMEM), oleic acid (OA), 3,5-dinitrosalicylic acid, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and 2-deoxy-2-[(7-nitro-2,1,3-benzoxadiazol-4-yl)amino]-d-glucose (2-NBDG), were procured from Sigma–Aldrich, Inc. (St Louis, MO, USA).
Figure 1.
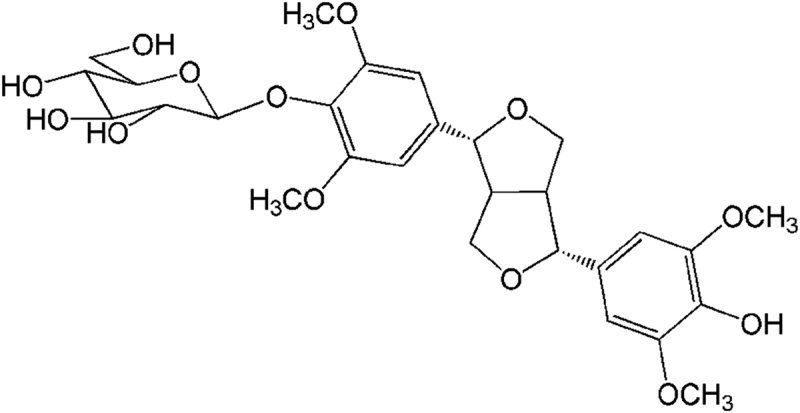
The structure of SSG.
2.2. MTT assay
HepG2 cells were maintained in high glucose DMEM medium supplemented with 10% fetal bovine serum (Gibco-BRL, Grand Island, NY, USA), streptomycin and penicillin (both 100 μg/mL, Gibco-BRL, Grand Island, NY, USA) at 37 °C in 5% CO2. After reaching 90% confluence, the medium was removed and the cells were incubated in fresh medium containing different concentrations of SSG (0, 0.1, 0.5, 1, 5 and 10 μmol/L) for 20 h. Subsequently, sterile-filtered MTT was added with the final concentration of 0.5 mg/mL for 4 h, then the medium was replaced by 100 μL/well isopropanol to dissolve the insoluble formazan crystals. Finally, the absorbance was measured at 570 nm and cell viability was expressed as a percentage of viable cells in SSG treated wells relative to untreated wells.
2.3. Lipid accumulation assay
After being grown to 90% confluence, HepG2 cells were treated with simvastatin (10 μmol/L) or different concentrations of SSG (0.1, 1 and 10 μmol/L) in high glucose DMEM containing 100 μmol/L OA for 24 h. Oil red O staining was carried out as previous reported11. The quantification of total lipids, total cholesterol and triglyceride were tested by specified kits (Nanjing Jiancheng bioengineering institute, Nanjing, China) based on the manufacturer׳s instructions.
2.4. Glucose consumption assay
Glucose consumption experiments were carried out through testing the glucose concentration in medium as previously described17. After HepG2 cells reached confluence, the culture medium was removed and replaced with serum-free high glucose DMED medium containing rosiglitazone (10 μmol/L) or different concentrations of SSG (0.1, 1 or 10 μmol/L) in the presence or absence of insulin (100 nmol/L) for 24 h. Rosiglitazone, a marketed diabetes treatment drug and PPARs selective stimulator, was selected as the positive control. Then the remaining glucose concentration in medium was measured by Glucose Assay kit (Nanjing Jiancheng bioengineering institute, Nanjing, China) accordance with the operation manual.
2.5. Glucose uptake assay
C2C12 myotubes were maintained in high glucose DMEM medium supplemented with 10% fetal bovine serum (Gibco-BRL, Grand Island, NY, USA), streptomycin and penicillin (both 100 μg/mL, Gibco-BRL, Grand Island, NY, USA) at 37 °C in 5% CO2. Glucose uptake assay was carried out as previously reported18. Briefly, when cells reached confluence, the medium was replaced with high glucose DMEM containing 2% horse serum to induce cells differentiation. After 5 days, the fully differentiated cells were treated with SSG (0.1, 1 or 10 μmol/L) or rosiglitazone (10 μmol/L), as positive control, in serum-free high glucose DMEM containing the fluorescent glucose analog 2-NBDG (10 μmol/L) with the presence or absence of insulin (100 nmol/L). After 12 h later, medium was replaced with phosphate-buffered saline (PBS) and the fluorescence intensities was measured at Ex/Em = 475 nm/550 nm using a Microplate Reader (TECAN Group Ltd., Shanghai, China).
2.6. Insulin sensitivity assay
Insulin sensitivity assay was carried out by testing the glucose concentration remaining in the medium as previously reported19 with a little modified. Briefly, differentiated C2C12 cells were treated with insulin (1 μmol/L) in serum-free high glucose DMEM for 24 h, then medium was replaced with serum-free high glucose DMED containing insulin (1 μmol/L) and different concentrations of SSG (0.1, 1 or 10 μmol/L) or rosiglitazone (10 μmol/L). Glucose concentration and glucose uptake assay were performed respectively. The medium glucose concentration was measured by glucose kit after 24 h incubation according to the operation manual. And glucose uptake ability was measured by testing the amount of 2-NBDG take up by the cells after incubation for 30 min as Section 2.5.
2.7. Quantitative real-time PCR
The regulative effect of SSG on lipid and glucose metabolism-related genes was determined by real-time quantitative PCR. Total RNA extraction, cDNA synthesis and quantitative PCR assays were performed as reported previously20. The date reproducibility was checked by measuring at least three different independent biological replicates. The primers are listed in Table 1.
Table 1.
Primers used in real-time quantitative PCR analysis.
| Name | Forward (5ʹ–3ʹ) | Reverse (3ʹ–5ʹ) |
|---|---|---|
| SREBP-1a | TGCTGACCGACATCGAAGAC | CCAGCATAGGGTGGGTCAA |
| SREBP-1c | CCATGGATGCACTTTCGAA | CCAGCATAGGGTGGGTCAA |
| SREBP-2 | CTGCAACAACAGACGGTAATGA | CCATTGGCCGTTTGTGTCAG |
| FAS | CGGTACGCGACGGCTGCCTG | GCTGCTCCACGAACTCAAACACCG |
| ACC | TGATGTCAATCTCCCCGCAGC | TTGCTTCTTCTCTGTTTTCTCCCC |
| HMGR | GGACCCCTTTGCTTAGATGAAA | CCACCAAGACCTATTGCTCTG |
| PPARα | AGGCTTTGCAAACTTGGACT | ACGGCTTCCTCAGGTTCTTA |
| PPARγ | GCAGCTACTGCATGTGATCAAGA | GTCAGCGGGTGGGACTTTC |
| β-Actin | CCTGGCACCCAGCACAAT | GCCGATCCACACACGGAGTACT |
2.8. Luciferase assay
The transcription-promoting effects of SSG on PPARγ was performed in 293T cells by Luciferase assay as previously reported21. In brief, cells were transiently transfected by lipo2000 (Invitrogen, Shanghai, China) with PPARγ expression vector and DR-1 luciferase reporter vector according to the manufacturer׳s instrument. After transfection for 6 h, the cells were treated with SSG (0.1, 1 or 10 μmol/L) or rosiglitazone (10 μmol/L) in serum-free high glucose DMEM for another 24 h. Finally, the fluorescence intensities were measured using luciferase assay kit (Promega, Beijing, China) according to manufacturer׳s instruction.
2.9. Statistics analysis
Data are expressed as mean±SD. A one-way analysis of variance (ANOVA) was done to assess the difference between groups using the SPSS 17.0 for Windows (SPSS, Chicago, IL, USA). Significant differences were accepted at P < 0.05.
3. Results
3.1. SSG inhibits lipid accumulation in HepG2 cells
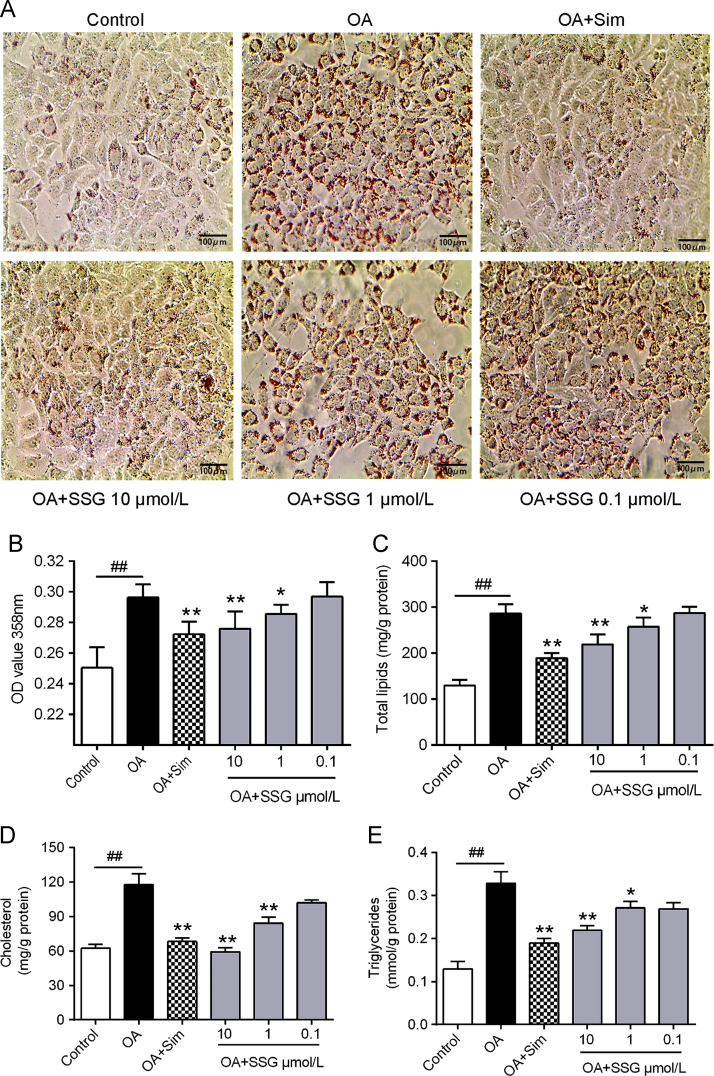
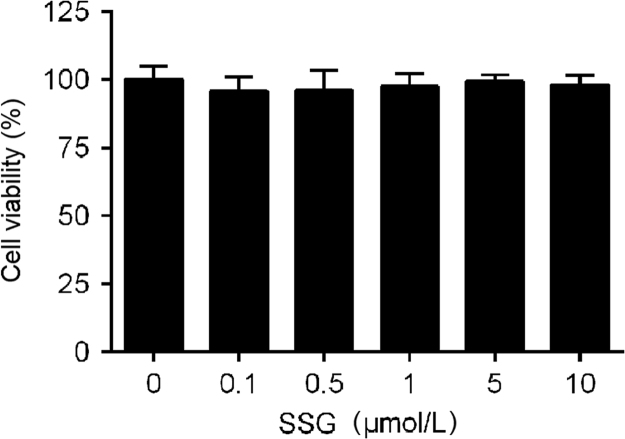
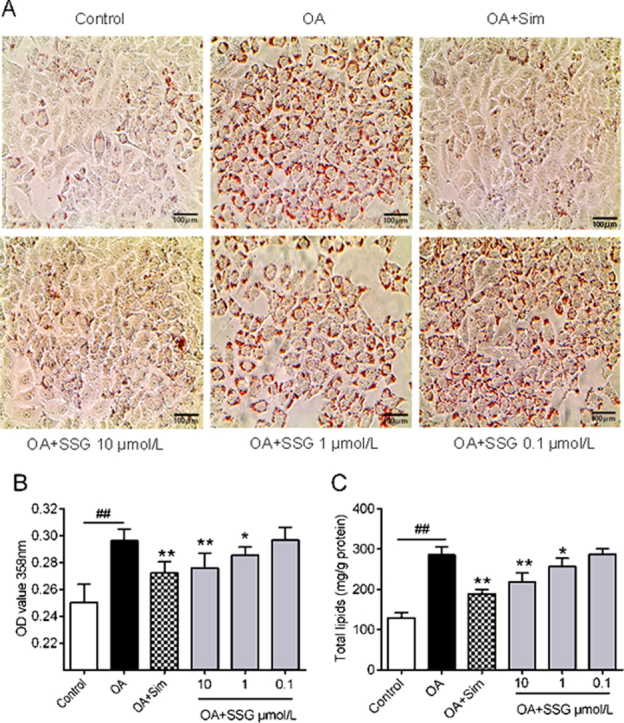
The functions of SSG on lipid metabolism were explored by studying neutral lipid accumulation elicited by OA in HepG2 cells. The results showed (Fig. 2) that OA (model group) could significantly elicit lipid accumulation in HepG2 cells. Incubation with SSG dose-dependently decreased lipid accumulation (Fig. 2A and B). At the same time, intracellular concentration of total lipids (Fig. 2C), total cholesterol (Fig. 2D) and triglyceride (Fig. 2E) were also decreased. Additionally, no cytotoxic influence on HepG2 cells was observed, assessed by MTT assay at the concentrations of 0.1–10 μmol/L (Fig. 3).
Figure 2.
Effects of SSG on lipid accumulation. (A) Typical picture of oil red O staining. Scale bar: 100 μm; (B) The OD 358 nm after oil red O staining; (C) Intracellular levels of total lipids, (D) total cholesterol and (E) triglyceride. Values represent mean±SD. Results are representative of three different experiments with n = 3. ##P < 0.01 versus control group; *P < 0.05, **P < 0.01 versus OA group. SSG, syringaresinol-4-O-β-d-glucoside; Sim, simvastatin.
Figure 3.
Effect of SSG on cell viability determined by a MTT assay. Values represent mean ± SD. Results are representative of three different experiments with n = 8. SSG, syringaresinol-4-O-β-d-glucoside.
3.2. SSG stimulates glucose consumption by HepG2 cells
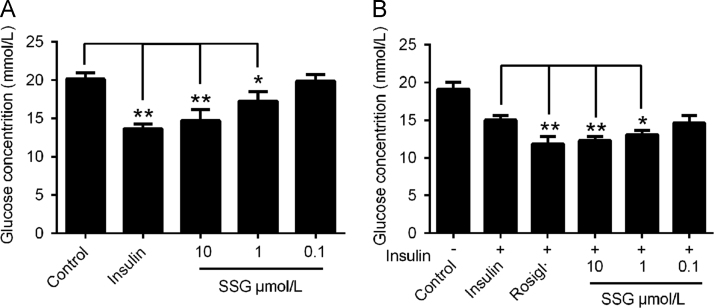
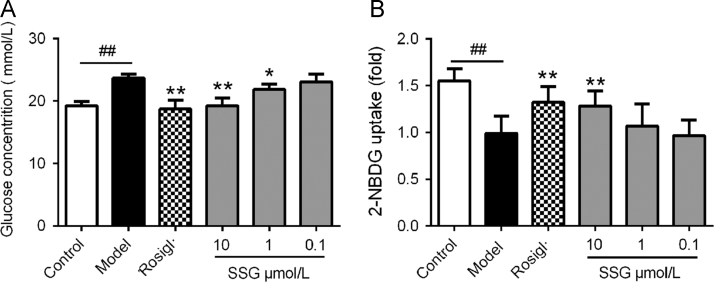
To assess the influence of SSG on glucose metabolism, we evaluated the activity of SSG on glucose consumption by HepG2 cells. The results (Fig. 4) showed that supplementation with insulin (100 nmol/L) largely decreased the concentration of glucose. Treatment with SSG significantly and dose-dependently increased the consumption of glucose. However, the effect of SSG was weaker than insulin (Fig. 4A). Interestingly, when we additionally supplied insulin (100 nmol/L) with SSG simultaneously, the results showed that the effect of glucose consumption became stronger than insulin (100 nmol/L) or SSG (10 μmol/L) alone (Fig. 4B). These results suggest that SSG can significantly stimulate the consumption of glucose and act synergistically with insulin.
Figure 4.
Effect of SSG on glucose consumption by HepG2 cells in absence (A) or presence (B) of insulin (100 nmol/L). Values are represented as mean ± SD. Results are representative of three independent experiments with n = 3. *P < 0.05, **P < 0.01. SSG, syringaresinol-4-O-β-d-glucoside; Rosigl, rosiglitazone.
3.3. SSG increases glucose uptake by C2C12 myotubes
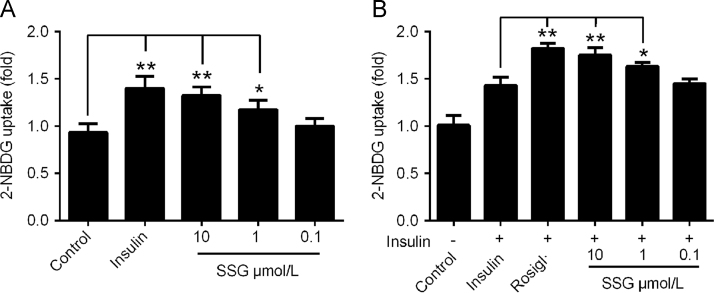
Since SSG stimulated the consumption of glucose in HepG2 cells, we also assessed the influence of SSG on glucose uptake by 2-NBDG uptake assay. As shown in Fig. 5, incubation with SSG for 12 h dose-dependently enhanced 2-NBDG uptake by C2C12 myotubes. The efficacy of SSG (10 μmol/L) was similar to that insulin (100 nmol/L), suggesting a powerful function in enhancing glucose uptake. Meanwhile, SSG could also cooperate with insulin to increase glucose uptake, exerting synergistic action with insulin (Fig. 5B).
Figure 5.
Effect of SSG on glucose uptake by C2C12 myotubes in absence (A) or presence (B) of insulin (100 nmol/L). Values are represented as mean ± SD. Results are representative of three independent experiments with n = 3. *P < 0.05, **P < 0.01. SSG, syringaresinol-4-O-β-d-glucoside; Rosigl, rosiglitazone.
3.4. SSG enhances insulin sensitivity
Inspired by the cooperative effect of SSG with insulin, we also assessed the effect of SSG on insulin resistance in differentiated C2C12 myotubes. As shown in Fig. 6, supplementing with a high concentration (1 μmol/L) (model group) of insulin could enormously inhibit insulin sensitivity, resulting in decreases in glucose consumption and glucose uptake. Treatment with SSG significantly and dose-dependently enhanced insulin sensitivity with glucose consumption and glucose uptake. Furthermore, the efficacy of SSG at a concentration of 10 μmol/L was comparable to the effects of the popular insulin sensitizing agent rosiglitazone (10 μmol/L).
Figure 6.
Effect of SSG on insulin-elicited glucose consumption (A) and glucose uptake (B) in C2C12 myotubes. Values are represented as mean ± SD. Results are representative of three independent experiments with n=3. *P < 0.05, **P < 0.01. SSG, syringaresinol-4-O-β-d-glucoside; Rosigl, rosiglitazone.
3.5. SSG regulates the transcription of lipogenesis and glucose metabolism-related genes
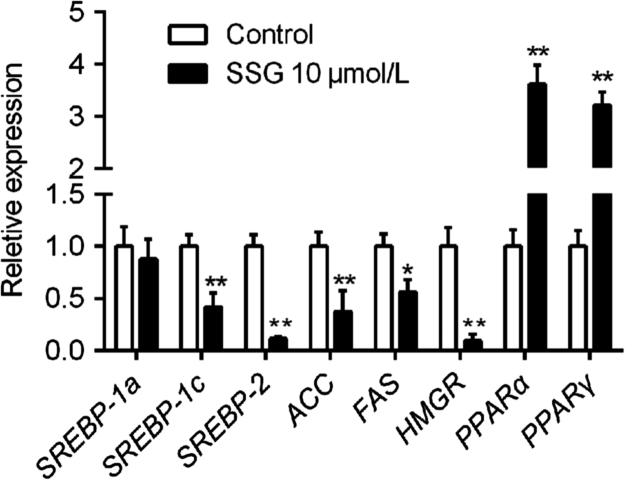
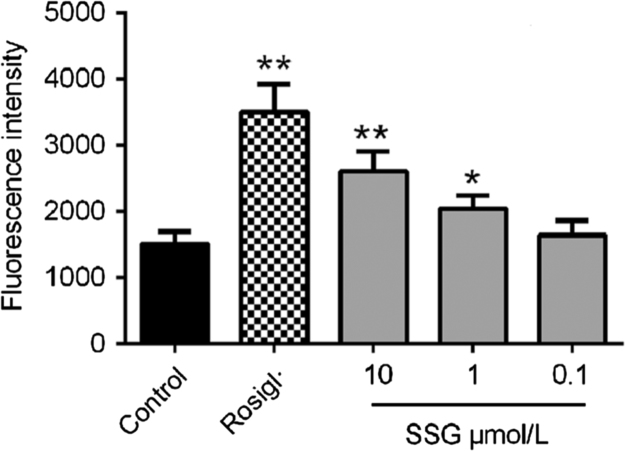
Lipid and glucose synthetic processes are both controlled by SREBPs and their downstream targets, such as ACC, FAS and HMGR22, 23. Simultaneously, many pathways related to lipid transport and glucose metabolism close relationships to PPARs24. Real-time quantitative PCR results showed that treatment with SSG (10 μmol/L) significantly decreased the transcription of SREBP-1c and SREBP-2, as well as the expression of ACC, FAS and HMGR. At the same time, SSG also increased the expression of PPARα and PPARγ (Fig. 7). Additionally, luciferase assay results revealed that (Fig. 8) SSG significantly enhanced the transcription activity of PPARγ in a dose-dependent manner, even though its stimulatory activity was weaker than that of rosiglitazone at a concentration of 10 μmol/L.
Figure 7.
Effect of SSG on mRNA levels of SREBP-1a, SERBP-1c, SREBP-2, ACC, FAS, HMGR, PPARα and PPARγ. Values are represented as mean ± SD. Results are representative of three independent experiments with n = 3. *P < 0.05, **P < 0.01 versus control group.
Figure 8.
Effect of SSG on PPARγ transcription activity by 293 T cells. Values are represented as mean ± SD. Results are representative of three independent experiments with n = 3. *P < 0.05, **P < 0.01. SSG, syringaresinol-4-O-β-d-glucoside; Rosigl, rosiglitazone.
4. Discussion
Among SSG pharmacological activities, the regulatory effects on the metabolic syndrome, including obesity and T2DM, have not been previously described. However, it has been reported that syringaresinol could induce vasorelaxation by enhancing NO production through the mechanism of activating AMPK16. Generally, AMPK is also an important interruptor of cholesterol and glucose metabolism, playing critical actions on lipid and glucose synthesis, storage, and oxidation25, 26. Additionally, AMPK activation turns on ATP-generating processes such as glucose oxidation while switching off energy-consuming mechanisms like triglyceride and lipid synthesis25, 27. Meanwhile, many natural functional molecules, such as cordycepin28, sophoricoside20, caffeoylquinic acids12 and irisin29, also have the regulatory actions on lipid or glucose metabolism through activating AMPK wholly or partially. Therefore, in this paper, we investigated the efficacy of SSG on lipid-glucose metabolism and insulin sensitivity.
Lipid accumulation in the liver is a key cause of insulin resistance30, 31, and is also closely related to obesity and metabolic disease, including T2DM. Therefore, we first explored the lipid regulatory effect of SSG in HepG2 cells. The results revealed that SSG significantly and dose-dependently suppressed OA-elicited lipid accumulation (Fig. 2A–C) with the comparable efficiency with popular hypo-cholesterol agent simvastatin in the concentration of 10 μmol/L (Fig. 2A and B). To further illuminate the inhibitory effects on lipid accumulation, we measured the intracellular concentrations of cholesterol and triglyceride, which account for the most intracellular lipids. Results showed that SSG could significantly decrease the concentration of cholesterol and triglyceride in HepG2 cells (Fig. 2D and E), among which the cholesterol inhibitory efficacy is slightly stronger than that of simvastatin. The triglyceride inhibitory effect is weaker than that of simvastatin. Even though the inhibitory function of SSG on cholesterol is stronger than on triglyceride, suppressing total lipids, particularly cholesterol and triglyceride, makes vital contributions to the inhibition of lipid accumulation.
Besides the regulatory effect on lipid metabolism, we also investigated the effect of SSG on glucose metabolism in HepG2 cells and C2C12 myotubes. Treatment with SSG significantly increased the activity of glucose consumption in HepG2 cells (Fig. 4A) and glucose uptake in C2C12 myotubes (Fig. 5A) in a dose-dependent manner. However, the effects were weaker than that of insulin (100 nmol/L). Interestingly, when co-administrated with insulin (100 nmol/L), SSG showed stronger activity on glucose consumption (Fig. 4B) and glucose uptake ability (Fig. 5B) than treatment with either SSG or insulin singly. Inspired by the obvious synergistic effect with insulin, we speculated that SSG might have the function on altering insulin resistance. So we carried out an insulin sensitivity assay. As the results shown in Fig. 6, SSG significantly and dose-dependently stimulated insulin sensitivity induce by high concentration of insulin (1 μmol/L), resulting in glucose consumption increasing (Fig. 6A). It is well known that insulin initiates its signal in a relatively short time. The result (Fig. 6B) also showed that treatment with SSG (10 μmol/L) could significantly increase glucose uptake in 30 min after desensitized by insulin (1 μmol/L) for 24 h (Fig. 6B). All these findings demonstrate that SSG might be an insulin sensitizing agent in which activation of AMPK and AKT might be mechanistically relevant15. However, this interesting in vitro function need to be further researched to distinguish the regulators directly contributing to the observation made here.
Over synthesis and impaired metabolism of cholesterol and triglyceride are the key causes of lipid accumulation, in which a number of proteins are involved, including PPARs and SREBPs, as well as downstream gene targets HMGR, FAS and ACC. Real-time quantitative PCR showed that treatment with SSG (10 μmol/L) significantly decreased the expression of SREBP-1c and SREBP-2 transcription factors, while SREBP-1a did not decreased significantly. In addition, the level of FAS, ACC and HMGR were also decreased after supplement with SSG. Furthermore, supplement with SSG could also enormously increase the transcription of PPARα and PPARγ with 11.6 and 13.2 times higher than control group respectively even though PPARγ is mainly expressed in adipose tissue. However, the luciferase assay also confirmed the enhancing ability of SSG on PPARγ expression in a dose-dependent manner. Above all, these results suggested that SSG inhibits both cholesterol and triglyceride synthesis and metabolism, and, therefore, suggests a beneficial effect on lipid accumulation in hepatic cells.
5. Conclusions
In conclusion, this work first revealed that SSG isolated from PTF is effective in alleviating lipid accumulation and regulating glucose metabolism in HepG2 cells and C2C12 myotubes. PPARs and SREBPs, as well as downstream genes like HMGR, FAS and ACC, are at least in part, if not wholly, relevant participants in the benefit of lipid and glucose homeostasis. The results of this study suggest that SSG might find utility in the treatment of obesity and T2DM.
Acknowledgements
The financial assistance is provided by the National Natural Science Foundation of China (Nos. 81573436, 81560696 and 81202994) and Peking Union Medical College Youth Fund (3332016142).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Xiaopo Zhang, Email: z_xp1412@163.com.
Peng Guo, Email: pguo@implad.ac.cn.
References
- 1.Wang Y., Li Y.Y., Nie Y.Q., Zhou Y.J., Cao C.Y., Xu L. Association between metabolic syndrome and the development of non-alcoholic fatty liver disease. Exp Ther Med. 2013;6:77–84. doi: 10.3892/etm.2013.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DiStefano J.K., Kingsley C., Wood G.C., Chu X., Argyropoulos G., Still C.D. Genome-wide analysis of hepatic lipid content in extreme obesity. Acta Diabetol. 2015;52:373–382. doi: 10.1007/s00592-014-0654-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perry R.J., Samuel V.T., Petersen K.F., Shulman G.I. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510:84–91. doi: 10.1038/nature13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali F., Ismail A., Kersten S. Molecular mechanisms underlying the potential antiobesity-related diseases effect of cocoa polyphenols. Mol Nutr Food Res. 2014;58:33–48. doi: 10.1002/mnfr.201300277. [DOI] [PubMed] [Google Scholar]
- 5.Smith-Spangler C.M., Bhattacharya J., Goldhaber-Fiebert J.D. Diabetes, its treatment, and catastrophic medical spending in 35 developing countries. Diabetes Care. 2012;35:319–326. doi: 10.2337/dc11-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCall K.D., Schmerr M.J., Thuma J.R., James C.B., Courreges M.C., Benencia F. Phenylmethimazole suppresses dsRNA-induced cytotoxicity and inflammatory cytokines in murine pancreatic β cells and blocks viral acceleration of type 1 diabetes in NOD mice. Molecules. 2013;18:3841–3858. doi: 10.3390/molecules18043841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeong E.J., Kim N.H., Heo J.D., Lee K.Y., Rho J.R., Kim Y.C. Antifibrotic compounds from Liriodendron tulipifera attenuating HSC-T6 proliferation and TNF-α production in RAW264.7 cells. Biol Pharm Bull. 2015;38:228–234. doi: 10.1248/bpb.b14-00583. [DOI] [PubMed] [Google Scholar]
- 8.Park B.Y., Oh S.R., Ahn K.S., Kwon O.K., Lee H.K. (–)-Syringaresinol inhibits proliferation of human promyelocytic HL-60 leukemia cells via G1 arrest and apoptosis. Int Immunopharmacol. 2008;8:967–973. doi: 10.1016/j.intimp.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Hardie G.D. Regulation of AMP-activated protein kinase by natural and synthetic activators. Acta Pharm Sin B. 2016;6:1–19. doi: 10.1016/j.apsb.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu C., Zhang X., Zhang X., Luan H., Sun G., Sun X. The caffeoylquinic acid-rich Pandanus tectorius fruit extract increases insulin sensitivity and regulates hepatic glucose and lipid metabolism in diabetic db/db mice. J Nutr Biochem. 2014;25:412–419. doi: 10.1016/j.jnutbio.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X., Wu C., Wu H., Sheng L., Su Y., Zhang X. Anti-hyperlipidemic effects and potential mechanisms of action of the caffeoylquinic acid-rich Pandanus tectorius fruit extract in hamsters fed a high fat-diet. PLoS One. 2013;8:e61922. doi: 10.1371/journal.pone.0061922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu C., Luan H., Wang S., Zhang X., Liu H., Guo P. Pandanus tectorius derived caffeoylquinic acids inhibit lipid accumulation in HepG2 hepatoma cells through regulation of gene expression involved in lipid metabolism. Acta Pharm Sin. 2015;50:278–283. [PubMed] [Google Scholar]
- 13.Shi Y.N., Shi Y.M., Yang L., Li X.C., Zhao J.H., Qu Y. Lignans and aromatic glycosides from Piper wallichii and their antithrombotic activities. J Ethnopharmacol. 2015;162:87–96. doi: 10.1016/j.jep.2014.12.038. [DOI] [PubMed] [Google Scholar]
- 14.Ahmad I., Waheed A., Tahir N.B., Rais A.K. Anti-inflammatory constituents from Perovskia atriplicifolia. Pharm Biol. 2015;53:1628–1631. doi: 10.3109/13880209.2014.997250. [DOI] [PubMed] [Google Scholar]
- 15.Cho S., Cho M., Kim J., Kaeberlein M., Lee S.J., Suh Y. Syringaresinol protects against hypoxia/reoxygenation-induced cardiomyocytes injury and death by destabilization of HIF-1α in a FOXO3-dependent mechanism. Oncotarget. 2014;6:43–55. doi: 10.18632/oncotarget.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung B.H., Kim S., Kim J.D., Lee J.J., Baek Y.Y., Jeoung D. Syringaresinol causes vasorelaxation by elevating nitric oxide production through the phosphorylation and dimerization of endothelial nitric oxide synthase. Exp Mol Med. 2012;44:191–201. doi: 10.3858/emm.2012.44.3.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X., Wang S., Li Y., Zhao D., An N., Wu J. Tadehaginoside modulates lipogenesis and glucose consumption in HepG2 cells. Nat Prod Res. 2015;29:2287–2290. doi: 10.1080/14786419.2014.1001387. [DOI] [PubMed] [Google Scholar]
- 18.Alonso-Castro A.J., Zapata-Bustos R., Dominguez F., Garcia-Carranca A., Salazar-Olivo L.A. Magnolia dealbata Zucc and its active principles honokiol and magnolol stimulate glucose uptake in murine and human adipocytes using the insulin-signaling pathway. Phytomedicine. 2011;18:926–933. doi: 10.1016/j.phymed.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 19.Guillen A., Granados S., Rivas K.E., Estrada O., Echeverri L.F., Balcazar N. Antihyperglycemic activity of Eucalyptus tereticornis in insulin-resistant cells and a nutritional model of diabetic mice. Adv Pharmacol Sci. 2015;2015:418673. doi: 10.1155/2015/418673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu C., Luan H., Wang S., Zhang X., Wang R., Jin L. Modulation of lipogenesis and glucose consumption in HepG2 cells and C2C12 myotubes by sophoricoside. Molecules. 2013;18:15624–15635. doi: 10.3390/molecules181215624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang S., Zhang X., Liu M., Luan H., Ji Y., Guo P. Chrysin inhibits foam cell formation through promoting cholesterol efflux from RAW264.7 macrophages. Pharm Biol. 2015;53:1481–1487. doi: 10.3109/13880209.2014.986688. [DOI] [PubMed] [Google Scholar]
- 22.Huang Y.L., Zhao F., Luo C.C., Zhang X., Si Y., Sun Z. SOCS3-mediated blockade reveals major contribution of JAK2/STAT5 signaling pathway to lactation and proliferation of dairy cow mammary epithelial cells in vitro. Molecules. 2013;18:12987–13002. doi: 10.3390/molecules181012987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao X., Song B.L. SREBP: a novel therapeutic target. Acta Biochim Biophys Sin. 2013;45:2–10. doi: 10.1093/abbs/gms112. [DOI] [PubMed] [Google Scholar]
- 24.Kersten S., Desvergne B., Wahli W. Roles of PPARs in health and disease. Nature. 2000;405:421–424. doi: 10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- 25.Lanaspa M.A., Cicerchi C., Garcia G., Li N., Roncal-Jimenez C.A., Rivard C.J. Counteracting roles of AMP deaminase and AMP kinase in the development of fatty liver. PLoS One. 2012;7:e48801. doi: 10.1371/journal.pone.0048801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shih C.C., Ciou J.L., Lin C.H., Wu J.B., Ho H.Y. Cell suspension culture of Eriobotrya japonica regulates the diabetic and hyperlipidemic signs of high-fat-fed mice. Molecules. 2013;18:2726–2753. doi: 10.3390/molecules18032726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muoio D.M., Seefeld K., Witters L.A., Coleman R.A. AMP-activated kinase reciprocally regulates triacylglycerol synthesis and fatty acid oxidation in liver and muscle: evidence that sn-glycerol-3-phosphate acyltransferase is a novel target. Biochem J. 1999;338:783–791. [PMC free article] [PubMed] [Google Scholar]
- 28.Wu C., Guo Y., Su Y., Zhang X., Luan H., Zhang X. Cordycepin activates AMP-activated protein kinase (AMPK) via interaction with the γ1 subunit. J Cell Mol Med. 2014;18:293–304. doi: 10.1111/jcmm.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xin C., Liu J., Zhang J., Zhu D., Wang H., Xiong L. Irisin improves fatty acid oxidation and glucose utilization in type 2 diabetes by regulating the AMPK signaling pathway. Int J Obes. 2016;40:443–451. doi: 10.1038/ijo.2015.199. [DOI] [PubMed] [Google Scholar]
- 30.Kumashiro N., Erion D.M., Zhang D., Kahn M., Beddow S.A., Chu X. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc Natl Acad Sci U S A. 2011;108:16381–16385. doi: 10.1073/pnas.1113359108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adamo E., Cali A.M., Weiss R., Santoro N., Pierpont B., Northrup V. Central role of fatty liver in the pathogenesis of insulin resistance in obese adolescents. Diabetes Care. 2010;33:1817–1822. doi: 10.2337/dc10-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]