Abstract
Nature has been the source of life-changing and -saving medications for centuries. Aspirin, penicillin and morphine are prime examples of Nature׳s gifts to medicine. These discoveries catalyzed the field of natural product drug discovery which has mostly focused on plants. However, insects have more than twice the number of species and entomotherapy has been in practice for as long as and often in conjunction with medicinal plants and is an important alternative to modern medicine in many parts of the world. Herein, an overview of current traditional medicinal applications of insects and characterization of isolated biologically active molecules starting from approximately 2010 is presented. Insect natural products reviewed were isolated from ants, bees, wasps, beetles, cockroaches, termites, flies, true bugs, moths and more. Biological activities of these natural products from insects include antimicrobial, antifungal, antiviral, anticancer, antioxidant, anti-inflammatory and immunomodulatory effects.
Keywords: Natural products, Insects, Entomotherapy, Traditional medicine, Drug discovery
Graphical abstract
This review presents an overview of current traditional medicinal applications of insects and characterization of isolated biologically active molecules starting from approximately 2010.
1. Introduction
The chemical and structural diversity of natural products are unparalleled by any synthetic based library and have inspired a wealth of life-changing and -saving medicines for centuries1. In 1803, the remarkable pain-relieving natural product, morphine, was isolated from the Papaver somniferum plant and in the 1870׳s served as the template for codeine2. Two decades later, salicin, the medicinally active natural product from the willow bark, Salix alba, was isolated and characterized by Henri Leroux2, 3. From this acetylsalicylic acid, an anti-inflammatory agent known commercially as aspirin was synthesized by the Bayer scientist Felix Hoffman. Aspirin has been a commercial success for the Bayer Company since its market debut in 1899. Thirty years later penicillin was isolated from the fungus Penicillium notatum by Alexander Fleming and proven to be a groundbreaking antibiotic by Howard Florey and Ernest Chain2, 4, 5. This discovery revolutionized medicine, earning Fleming, Chain and Florey the 1945 Nobel Prize in physiology or medicine for their efforts2, 5. Then in 1971, the most effective malarial treatment to date was discovered while scientists were reviewing ancient tomes on traditional Chinese medicine4. It was within these texts that artemisinin was identified as a promising drug discovery lead. The head scientist, Youyou Tu, earned the Lasker prize in 2011 and the 2015 Nobel Prize in physiology and medicine for this impressive discovery4, 5.
The aforementioned examples and the fact that 33% of drugs approved from 1981--2010 and a staggering 68% of all approved antibacterial drugs are natural products or derivatives thereof prove that nature is an important source of new target molecules6, 7. However, the focus of natural product medicine has been overly focused on terrestrial plants, various algae and fungi. An underrepresented source of inspiration, entomotherapy, has been in practice for as long as and often in conjunction with medicinal plants. Entomotherapy is the use of insects as medicine and is an important alternative to modern therapy in many parts of the world including India, Mexico, Korea, China, Spain, Brazil, Argentina, Ecuador and various African countries today8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22. Honey, a sweet bee byproduct, is valued as an antioxidant and antimicrobial agent, suitable for the battle against heart disease and skin disorders23, 24, 25, 26, 27. Propolis, a gap filling “glue” used in the construction of the beehive, has been evaluated extensively for its potential antioxidant, antimicrobial, anti-inflammatory, cardioprotective, immunomodulatory and antiangiogenic properties28, 29, 30. Venom, especially from bees, has been studied as an alternative medicine for inflammation and cancer therapy31, 32, 33, 34. Success has also been achieved in identifying biologically active isolates from insect bodies, excretions and secretions. Antimicrobial peptides, an innate component of insect immunity within the hemolymph (blood), have shown activity against fungi, parasites, viruses and most importantly antibiotic resistant bacteria35, 36, 37. Insect toxin isolates pederin and cantharidin from defense secretions of the rove and blister beetles, respectively, have peaked scientific interest as potential anticancer therapies38, 39.
Insects as natural product resources have also been featured in reviews published from 2010–201514, 38, 40, 41, 42, 43, 44. However, the scope of the aforementioned articles often includes other arthropods, covers a limited selection of insects, focuses heavily on insect byproducts and incorporates uses outside of pharmaceutical applications. This review focuses on the continued cultural use of insects as medicine worldwide, validation of their medicinal properties and identification, characterization and synthesis of insect natural products and derivatives.
Like plants and other invertebrates, insects are hosts for many types of microbial endophytes that include bacteria and fungi. These endophytic microorganisms may be involved or working together with their insect hosts in the production of a natural product reported. The potential involvement of endophytes should be taken into account when we isolate the natural products from insects45.
Insects belong to the kingdom Anamalia, phylum Arthropoda, and class Insecta. Members of this class are further divided into 29 orders though 81% belong to one of the following four orders: Coleoptera, Diptera, Hymenoptera and Lepidoptera46, 47. This review article is split into sections based on the different orders and occasionally further subdivided by families. Sections are titled with scientific nomenclature with common names in parentheses.
2. Hymenoptera (bees, wasps, ants and sawflies)
2.1. Symphyta (sawflies, wood wasps, and horntails)
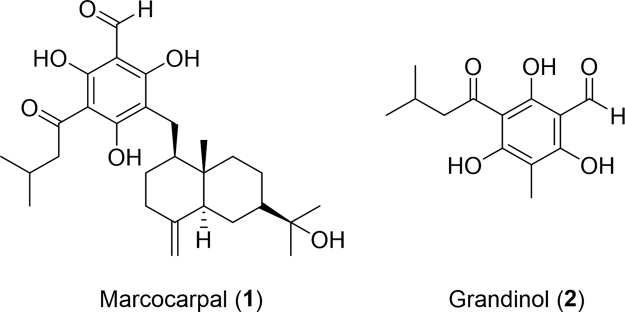
The family Symphyta is the smallest of the hymenoptera representing only 7% (8000--10,000 species) of the order48. Like ants, they feed on leaves or burrow into wood earning them the position as pests by most foresters. In 2013, researchers out of China and Australia found medicinal value in these pests. Two small molecules with strong antimicrobial activity (Fig. 1) were isolated from the methanolic extract of Australian sawfly larvae49. The novel macrocarpal (1) and known grandinol (2) were evaluated against Bacillus subtilis (B. subtilis) and exhibited inhibition zones of 8.0 and 7.0 mm, respectively.
Figure 1.
Structures of two compounds with antimicrobial activity isolated from Australian sawfly larvae.
Additionally, the hemolymph of sawfly larvae has been found to be rich in phenolic compounds, such as novel flavonol oligoglycosides, flavonoid glycosides and naphthodianthrones, all with known potential health benefits that were not evaluated in the studies50, 51.
2.2. Formicidae (ants)
Ants are one of the most familiar members of the hymenoptera order. This is a social family with an impressive life span, the record being 29 years48. In 2005, the number of valid described species was approximately 12,000. Ants and ant byproducts are widely used in traditional folk medicine across the globe. In southern India, mud from the interior portion of an anthill is applied topically for the treatment of scabies by the Paniyan tribe16. In northern India, scabies, wounds and boils are treated through the topical application of a paste made from the crushed black ants, Bothroponera rufipes11. Additionally, ground B. rufipes mixed with water is gurgled to relieve toothaches and daily consumption of 1–2 whole ants reportedly reduces blood pressure. Weaver ants (Oecophylla smaragdina) have been used in the treatment of severe cough, cold and flu in Myanmar, Africa, Australia and India52. In Thai culture, they are used for detoxification of blood, arresting hemorrhage during miscarriages, restoration of uterus and removal of any aftermath from the uterine canal after childbirth, stimulating pulse and heartbeat, and dizziness. The leaf cutting ant, genus Atta, is used for the treatment of sore throat, stomachache, chest palpitations and heart disease in Latin America12. Dinoponera are Neotropical ants used in the treatment of asthma in Latin America and earaches, rheumatism, and back pain in northeastern Brazil52, 53. In 2012, investigation into Dinoponera venom revealed antinociceptive activity supporting its use in pain treatment53. Furthermore, it showed neuroprotective effects when administered as an intraperitoneal treatment as measured by a pentylenetetrazole induced seizure model in mice54. This is an important proof of concept step in the search for new treatments for epilepsy.
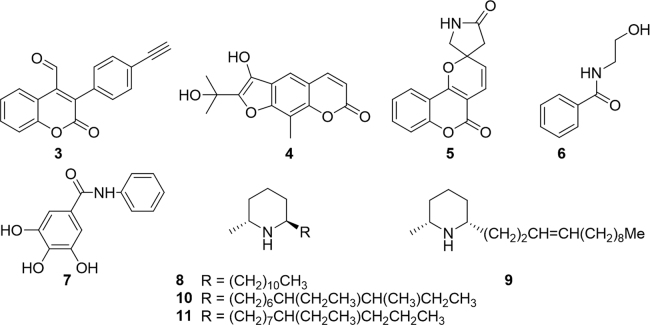
Red ants of the ChangBai mountain (Tetramorium spp.), utilized in traditional Chinese medicine, were found to contain three novel coumarin compounds and two known amide alkaloids (3–7, Fig. 2)55. Each compound was evaluated for antibacterial activity against Gram-positive B. subtilis and Gram-negative Escherichia coli. Compound 6, known alkaloid N-(2-hydroxyethyl)-benzamide, showed strong activity against B. subtilis comparable to the positive control, penicillin G. The novel coumarins, compounds 3--5, were mildly active and compound 7, known alkaloid N-phenyl-3,4,5-trihydroxybenzamide, was inactive. None of the compounds exhibited activity against Gram-negative bacteria E. coli. Solenopsin A (8), are well-known antiangiogenic active alkaloids isolated from Solenopsis invicta and Solenopsis germinate fire ants. The key mechanism of action reportedly involves selective inhibition of protein kinase B (AKT), a molecular target of growing interest in cancer18, 44, 56, 57. In 2012, three new alkaloids (9--11) were isolated from the alate queen ants of S. richteri, S.invicta and hybrid (S. richteri × S. invicta) and two years later an additional 36 alkaloids58, 59. However, the biological activities of these alkaloids have yet to be evaluated.
Figure 2.
Structures of coumarins and alkaloids isolated from ants.
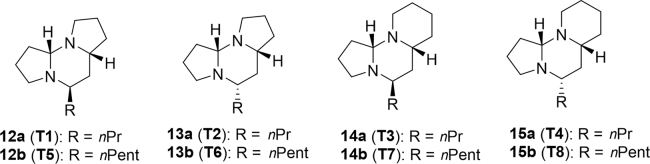
The iron ant, Tetraponera rufonigra, is used in local tribes of northeastern India to treat foot and mouth disease in cattle11. One to two ants are crushed into a powder and added to fodder which is then fed to the cattle up to 3 times a day. Studies into the venom of Tetraponera ants revealed a unique class of neurotoxic bioactive compounds termed tetraponerines. Tetraponerines are tricyclic alkaloids isolated for the first time in 1987 from venom of the New Guinean pseudomyrmecine ant60. These molecules, designated T1–T8 (12–15, Fig. 3), are split evenly into two groups based on the grouping of cyclic rings, 5-6-5 or 6-6-560. In each group, further division exists based on the stereochemistry and length of the alkyl chain (pentyl or propyl). In 2001, additional investigation into Tetraponera from India, China and Africa revealed the presence and distribution of the tetraponerines are not equivalent across the species. An Indian ant (T. allaborans) possessed T2, T4 and T8, while the Chinese ant (T. binghami) contained T5, T6, T7 and T8. The remaining tested species, T. rufonigra (India), T. penzigi (Africa), T. clypeata (Africa) and T. sp. cf. emeryi (Africa) showed no evidence of tetraponerines61. The natural application of tetraponerines as neurotoxic agents led to interest in their cytotoxic profiles. An evaluation of the anti-proliferative activity of T5–T8 against 4 different carcinoma cell lines highlighted the importance of C5 configuration on potency60. Furthermore, T7 exhibited an impressive cytotoxic profile on the breast carcinoma cell line MCF-7. A structure-activity relationship (SAR) study was conducted to analyze the effect of varying alkyl chain lengths and 6-6-6 tricyclic rings on cytotoxicity against human colorectal (HT29) cancer cells62. Results revealed the alkyl chain to have the greatest effect on cytotoxicity with a decrease in IC50 with increasing chain length, whereas the nature of the ring structure and stereochemistry had no effect.
Figure 3.
Structures of tetraponerines isolated from venom of the New Guinean pseudomyrmecine ant.
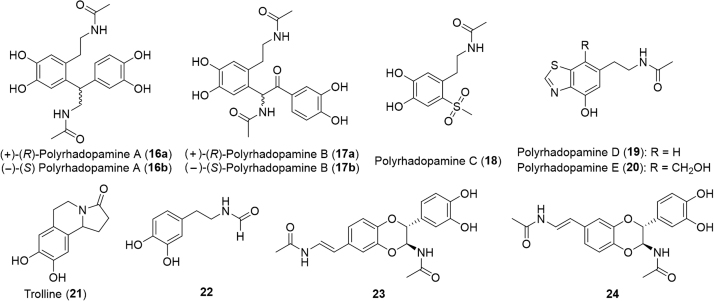
Chinese black ants, Polyrhachis dives, are traditionally used to treat numerous ailments, including rheumatoid and osteoarthritis, inflammatory diseases, and central nervous system (CNS) associated disorders63. Recently, several dopamine derivatives (Fig. 4) were isolated from an ethanol extract: (±)-polyrhadopamine A (16), (±)-polyrhadopamine B (17), polyrhadopamines C–E (18–20), trolline (21) and compounds 22–24. The compounds were evaluated for inhibition of Rho-associated protein kinase ROCK isoforms, ROCK1 and ROCK2, known targets for the potenital treatment of cardiovascular, neurological, oncological and renal diseases. Compounds (±)-polyrhadopamine A (16), (+)-polyrhadopamine B (17a) and trolline (21) inhibited both ROCK isoforms. Compounds 22–24 inhibited only the ROCK2 isoform. Potential for the treatment of CNS disorders was further evaluated utilizing a neural stem cell (NSC) proliferation assay. Only (–)-polyrhadopamine B (17b) exhibited a potent dose-dependent promotion of NSC proliferation. Additionally, the immunosuppressive and anti-inflammatory effects of the compounds were studied to verify activity against rheumatoid and osteoarthritis. One dopamine derivative, polyrhadopamine C (18), was found to have anti-proliferative effects against T-lymphocytes isolated from Kunming mice comparable to the control, cyclosporine A. Likewise, this same compound had comparable inhibitory effects on tumor necrosis factor (TNF)-α production in lipopolysaccharides (LPS)-induced RAW264.7cells as the control indomethacin63. The combined immunosuppressive and anti-inflammatory activity is strong evidence that polyrhadopamine C (18) could be effective against rheumatoid arthritis. Last year, an additional thirteen nitrogen containing compounds were isolated from P. dives ants and evaluated for their anti-inflammatory, immunosuppressive, and renoprotective activities64.
Figure 4.
Structures of dopamine derivatives isolated from Chinese black ants.
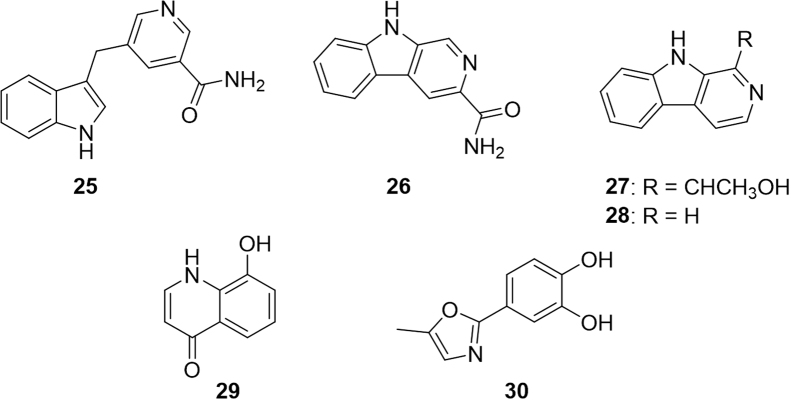
The compounds 5-(3-indolylmethyl)-nicotinsaureamide (25, Fig. 5) and β-carboline-3-carboxamide (26) suppressed the proliferation of T-cells, key cellular targets for rheumatoid arthritis therapy64, 65. Additionally, compounds 25, 27, and 3-hydroxypyridine significantly reduced collagen IV, fibronectin, and interleukin-6 (IL-6) overproduction supporting the use of the ants as a tonic for kidney problems64. Furthermore, compounds 25, 26, 29, and 30 led to moderate inhibition of TNF-α production, compound 29 inhibited cyclooxygenase-1, and compound 28 significantly inhibited janus kinase (JAK)-3 kinase all of which indicate anti-inflammatory activity.
Figure 5.
Structures of additional compounds isolated from Chinese black ants.
2.3. Vespidae (paper, potter, and hover wasps; hornets; yellow jackets)
Wasps are another familiar group of the hymenoptera known for their wondrous paper and clay nests and the act of stinging as a defense mechanism. The Vespidae have approximately 5,000 species classified into six subfamilies: Vespinae, Polistinae, Stenogastrinae, Eumeninae, Masarinae and Euparagiinae. In Latin America and Korea, the Apoica, Brachygastra and Synoeca genera of the Polistinae (paper wasps) are valued traditional therapies for asthma and cough12, 17. Additionally, Apoica and Protonectarina are thought to cure mumps, hemorrhage, and menstruation problems12. Polistes, the most diverse genus of the Polistinae, are used to treat colds and severe cough in northeastern India and parts of Latin America11, 12. Also, the buffer extract of Polistes mandarinus was cytotoxic against cervix cancer cell line, HeLa, at an equivalent level to the positive control drug mitomycin C66. As eusocial insects wasps are known to produce antimicrobial substances to provide a collective immunity against microorganisms. Malaysian female wasp venom from various genera of the Stenogastrinae (hover wasps) subfamily were evaluated for antimicrobial activity67. The venom was extracted with methanol and tested against Gram-positive bacteria, B. subtilis, Gram-negative bacteria, E. coli, and yeast, S. cerevisiae. Each extract produced inhibition zones approximately half the size of the positive controls, penicillin G and kanamycin, indicative of mild activity against the bacterial and yeast strains tested. The venom of the Brazilian social wasp, Synoeca cyanea (Polistinae), was found to inhibit bacterial growth of Gram-positive Enterococus faecalis and Gram-negative E. coli bacteria by 93% at 100 µg68. The bite or sting of the Vespa orientalis (Vespinae) is used to treat coughs, colds and stomach pains in the Galo and Nyishi tribes of northeastern India11. Crude venom of the V. orientalis wasp was found to have strong activity against B. subtilis though not as potent as the positive control tetracylcin69. The venom was also shown to have mild activity against Staphylococcus aureus, E. coli and Klesiella pneumonia. In addition to antibacterial activity venom is known to possess neurotoxic effects and in many cases is pharmacologically active against tumor cells56, 70. For example polybioside (31, Fig. 6), an isolate of Polybia paulista (Polistinae) venom, was found to be neuroactive70. Its neuroactive effect was evidenced through the expression of c-Fos protein, a known biochemical marker for stimulated neurons, in various areas of a rat brain after intracerebroventricular (ICV) application.
Figure 6.
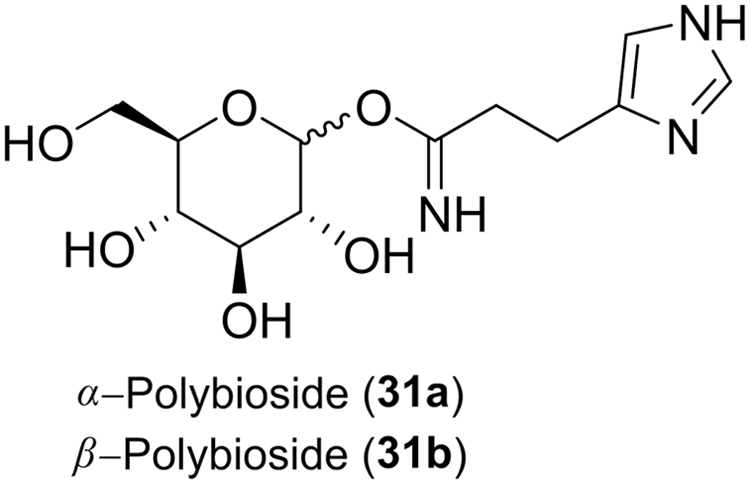
Structures of polybiosides isolated from the venom of Polybia paulista.
2.4. Apidae and Halictidae (bees)
Bees are the most popular insect of the hymenoptera albeit for the honeybees (Apis) and bumblebees (Bombus) which ironically represent a tiny fraction of the 20,000 species. Likewise, the Apis, numbering only 170 species, has been used extensively in medicine. In 2013, an Asiatic honeybee (Apis cerana) chymotrypsin inhibitor (AcCI) gene was identified to encode an 85 amino acid protein that includes a 20 amino acid signal sequence. The recombinant 65-amino acid mature peptide inhibited chymotrypsin with an IC50 of 24.71 nmol/L and showed anti-elastolytic activity via the inhibition of human elastase (IC50 = 38.50 nmol/L) and porcine elastase (IC50 = 70.21 nmol/L). This unique mode of action provides a novel approach for anti-inflammatory therapies.
The venom of A. cerana was evaluated for its anti-diabetic and anti-dandruff activities71. Researchers assessed its antidiabetic properties by measuring blood sugar, cholesterol and triglyceride content in test subjects before and 30 min after bee stinging. Prior to being stung the blood sugar levels ranged from 91–120 mg/dL, cholesterol was 189–235 mg/dL and triglycerides were 110–184 mg/dL. After bee stinging values fell to 80–94, 178–199 and 84–138 mg/dL for blood sugar, cholesterol and triglyceride levels, respectively. Anti-dandruff activity was investigated by testing the inhibitory effect of the venom on Malassezia furfur a known dandruff causing organism. Ketoconazole, a common active ingredient in anti-dandruff shampoos, was used as a positive control. Interestingly, at a concentration of 5 mg/mL the venom was more than twice as effective as ketoconazole. Last year, Indian Apis dorsata bee venom (ADBV) was found to possess anti-inflammatory, anti-nociceptive, and anti-arthritic properties. These properties were evinced through the venom׳s ability to reduce joint inflammation, paw edema and arthritic index along with the reduction of pain perception parameters such as dorsal flexion, motility, and stair climb score. Furthermore, it significantly decreased the major pro-inflammatory mediators like TNF-α and IL-6 in rats induced with arthritis. Crude bee venom extracts from A. cerana, A. dorsata and Apis florea were tested against six Gram-negative bacteria and fungal strains to investigate antimicrobial properties with ampicillin as a control72. The venoms were as effective as ampicillin against E. coli, Salmonella typhimurium and Xanthomonas subtilis and were more active than ampicillin against Klebsiella pneumonia.
Portuguese propolis from the beehive of Apis mellifera was recently assessed for total phenolic and flavonoid content, anti-inflammatory and antimicrobial activity73. The propolis samples were collected from three regions in Portugal including: Bragança, Coimbra and Beja. The Bragança propolis extract had the strongest antioxidant profile with a total phenolic and flavonoid content of 277.17 and 142.32 mg/g, respectively. Anti-inflammatory activity was assessed by inhibition of hyaluronidase, an enzyme that degrades hyaluronic acid leading to bone loss, pain and inflammation. All extracts inhibited hyaluronidase activity by 50%–75% at a concentration of 25 mg/mL and not surprisingly, the Bragança extract was the most effective. Antimicrobial activity was evaluated against S. aureus (Gram-positive), P. aeruginosa (Gram-negative), E. coli (Gram-negative) and C. albicans (yeast). All extracts showed activity against each strain with the weakest activity being against C. albicans and the strongest activity against S. aureus73. Propolis generated by the Brazilian stingless bee, Melipona orbignyi, was recently evaluated for its chemical composition and antimicrobial, antioxidant and cytotoxic activities74. The 80% ethanol extract was determined to contain aromatic acids, phenolic compounds, alcohols, terpenes and sugars in its chemical profile. Antioxidant activity was confirmed by its ability to scavenge free radicals, inhibit hemolysis and lipid peroxidation in human erythrocytes incubated with an oxidizing agent. The extract was active against S. aureus bacterium (Gram-positive) and the fungus C. albicans but not the E. coli (Gram-negative) bacterium. The cytotoxic activity of M. orbignyi ethanol extract was evaluated against the human K562 erythroleukemia cell line which showed a decrease in viability in contact with the extract which was attributed to cell necrosis74. A phytochemical screening of the ethanolic extract of Brazilian red propolis indicated the presence of phlobaphene tannins, catechins, chalcones, aurones, flavonones, flavonols, xanthones, pentacyclic triterpenoids and guttiferones75. Given the strong phenolic content, it is not surprising the extract exhibited strong antioxidant activity that was in fact far superior to the control Trolox, as measured by 2,2′-diphenyl-1-picrylhydrazyl (DPPH) sequestering. Additionally, the extract exhibited intense cytotoxicity against tumor cell lines SF-295 (human glioblastoma), OVCAR-8 (ovary) and HCT-116 (colon). Geopropolis from the Brazilian stingless bee, Scaptotrigona postica, was evaluated for chemical composition and effectiveness against antiherpes simplex virus-1 (HSV-1)76. The chemical composition was found to consist mostly of pyrrolizidine alkaloids and C-glycosyl flavones. Treatment with the extract reduced the presence of HSV-1 by 98% which was attributed to inhibition of viral replication and virus entry into cells76. Propolis from the Brazilian stingless bee, Tetragonisca fiebrigi, was evaluated for antimicrobial, antioxidant and anti-inflammatory activity77. The ethanol extract was analyzed for chemical composition utilizing gas-chromatography mass spectrometry (GC--MS). The major components of the extract, determined by GC--MS, were phenolic compounds, aromatic acids, alcohols, terpenes, and sugars. Antimicrobial activity was evaluated against various Gram-positive and Gram-negative bacteria and fungi. The extract was active against all strains tested but was most effective against Gram-positive bacteria. Antioxidant activity was also noted through scavenging free radicals, inhibiting hemolysis and lipid peroxidation in human erythrocytes incubated with an oxidizing agent. Furthermore, anti-inflammatory potential of the extract was confirmed via inhibition of the hyaluronidase enzyme and cytotoxic activity was concentration-dependent against K562 (leukemia) cells, with a predominance of death by necrosis77.
The Indian propolis from the stingless bee, Trigona sp., was evaluated for its chemical composition and antimicrobial activity78. The chemical composition of the ethanol extract contained 24 compounds belonging to the following classes: alkanes, thiophilic acids, aromatic acids, aliphatic acids, sugars, esters, and terpines. The antimicrobial activity was tested against 6 standard bacterial strains (E. coli, S. typhimurium, S. aureus, etc.) several multi drug resistant strains (K. pneumoniae and A. baumannii, etc.) and a standard yeast strain (C. glabrata). The extract proved potent against all strains tested with minimum inhibitory concentration (MIC) values ranging from 1.21–9.75 µg/mL. Although in general, MICs for Gram-positive bacteria were lower than that for Gram-negative bacteria78. Methanol extracts of Nigerian insect propolis were recently evaluated for activity against the malarial parasite Plasmodium berghei79. Mice were infected with P. berghei and evaluated for parasitaemia levels over several days. Survivorship of mice treated with the positive control, chloroquine, was not significantly higher than those treated with the extract. Thus, the extract presents a promising lead for new anti-malarial drugs.
The honey from A. mellifera is used in traditional Mexican medicine for the treatment of cough, bronchitis, asthma, rheumatism and diarrhea18. A daily spoonful of honey is believed to cure coughs, fevers and stomach pains in the Galo and Nyishi tribes of northeastern India11. Additionally, the honeycomb is rubbed on the skin to treat irritation. In northeastern India honeybee (A. cerana, A. florae, and A. mellifera) honey is thought to cure coughs, fevers, stomach pains and the comb, skin irritations. In parts of India honey from the honeybee, Apis indica, is also used to treat colds, headache, asthma and sore throat, indigestion and urinary disorders16, 80. Honey is also an important staple in Argentinian homeopathy. It is taken orally to treat dyspepsia, constipation, colds, influenza and asthma21. It is also applied topically to relieve ocular illness, insect bites, bruises, arthritis and muscular21. Likewise, in Mexican traditional medicine honey is used as a topical anti-septic agent to treat cutaneous injuries and infections and as an anti-inflammatory agent against muscle fatigue and sprains81.
Honey made from the Brazilian stingless bee, Melipona marginata, was evaluated for potential anti-inflammatory activity of honey extract on skin inflammation82. High performance liquid chromatography-electrospray ionization-tandem mass spectrometry (HPLC--UV--ESI-MS) identified 11 phenolic compounds such as kaempferol and caffeic acid. Topical treatment using M. marginata honey extract confirmed its anti-inflammatory activity by reducing edema (54%), leucocyte migration, and production of ROS (55%) in the skin of mice after 12-O-tetradecanoylphorbol-13-acetate (TPA) application82. Honey made from the Brazilian bee Melipona subnitida was evaluated for its chemical profile and antioxidant activity83. The chemical profile consisted of gallic acid, vanillic acid, 3, 4-dihydroxybenzoic acid, cinnamic acid, isorhamnetin, naringenin, quercetin, trans-trans and cis-trans abscisic acid. Three different methods were employed to verify antioxidant activity of pure honey and various extracts including inhibition of cooxidation in β-carotene/linoleic acid mixturesand the scavenging of DPPH and 2, 2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS). The pure honey and all extracts prevented the bleaching of β-carotene in the carotene/linoleic acid mixtures and scavenged free radicals (DPPH and ABTS) with varying degrees of efficiency83. Six honeys from the Republic of Mauritius were analyzed for antimicrobial and antioxidant potential84. Antimicrobial properties were evaluated against five strains and antioxidant capacity was determined using eight assays: trolox equivalent antioxidant capacity (TEAC), ferric reducing antioxidant power (FRAP), iron (II) chelating activity, hydroxyl radical (OH·) scavenging, DPPH free radical scavenging, hypochlorous acid (HOCl), nitric oxide (NO·) and ABTS diammonium salt radical (ABTS·+) scavenging. All honeys had similar antioxidant profiles and were found to have higher antibacterial activity than antifungal, supporting its reported effects in wound healing84. Four Malaysian honey samples, acacia (A. mellifera), pineapple (A. mellifera), borneo (A. cerana) and tualang (A. dorsata) were evaluated for chemical composition and antioxidant activities85. Total phenolic, flavonoid and protein content were measured. Tualang had the highest phenolic, flavonoid and protein content and strongest antioxidant activity as measured by the FRAP assay and DPPH radical scavenging activity85. The seasonal effect on antimicrobial activity of honey from the Brazilian stingless bee, Melipona compressipes manaosensis, was recently evaluated86. Honey harvested in November (rainy season) was only effective against E. coli and S. sonnei. However, honey harvested in March (dry season) was effective against S. aureus, E. coli, P. vulgaris, S. sonnei, and Klebsiella sp. Wild honey produced by four species of bees, A. cerana, A. andreniformis, A. koschevnikovi and A. nuluensis, in Malaysia were evaluated for antioxidant and acetylcholinesterase inhibitor potential87. Antioxidant properties were assessed by radical scavenging activity as determined from DDPH assay, ferric reducing/antioxidant power (FRAP) assay, and ABTS decolorization assay. The 80% methanol extract of the wild A. cerana honey performed the best as measured by DPPH assay (84%), FRAP assay (37 µmol/L Fe (II)/1 g dry sample) and ABTS decolorization assay (8 mg AEAC/1 g dry sample).
3. Coleoptera (beetles)
The coleopterans are the largest insect order with 350,000-386,000 species in 176 families occupying the land, freshwater and sea46, 88, 89. Approximately 62% of beetles belong to the following six mega families, each of which have at least 20,000 members: Staphylinidae, Carabidae, Cerambycidae Curculionidae, Chysomelidae, and Scarabaeidae88. Although diverse and abundant, with the exception of the Scarabaeidae, mention of medicinal use for these mega families in the literature within the last 5 years is scarce. For example, the Chrysomelidae have approximately 38,000 species and the Curculionidae have nearly 60,000 species, yet the only mention of medicinal use was found in Latin American folk medicine46, 88. Adult Chrysomelidae, commonly known as leaf beetles, are taken for epilepsy and their caterpillars are further utilized for earache, stroke, swelling, wounds, seborrheic dermatitis, inflammation and thrombosis treatment12. Snout beetles (Curculionidae) are leveraged for the relief of fever, headache and boils.
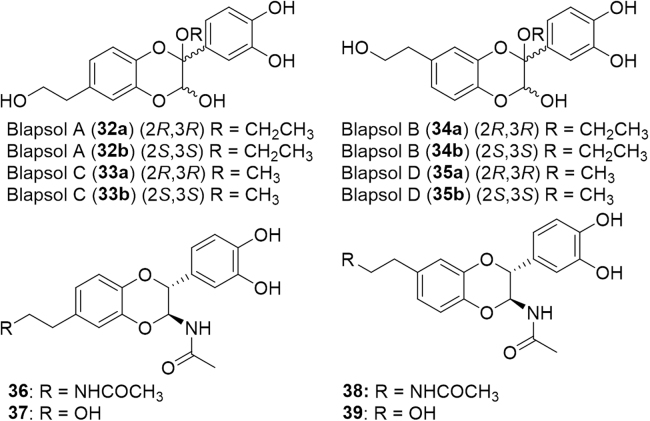
The Tenebrionidae, though not a mega family, have an extensive collection of 19,000 species. One such species is Blaps japanensis, a Chinese medicinal insect, which has been used for the treatment of fever, cough, rheumatism, cancer, and inflammatory disorders. As such, four new dimeric compounds, blapsols A–D (32--35, Fig. 7) and five known dopamine dimers (24, 36--39) isolated from Blaps japanensis were evaluated for their activity against cyclooxygenase (COX) enzymes: COX-1 and COX-290. Cyclooxygenases are responsible for catalyzing the conversion of arachidonic acid to prostaglandins, important mediators of pain, fever, and inflammation91. The COX-1 isoform is expressed constitutively in tissues and is responsible for maintaining a homeostatic concentration of prostaglandins. The second, COX-2, is induced by stimuli including mitogens, hormones, cytokines, and growth factors. Selective inhibition of COX-2 is therefore desirable as disruption of COX-1 activity leads to adverse side effects like ulcers of the upper gastrointestinal tract91.
Figure 7.
Structures of dopamine dimers isolated from Blaps japanensis, a Chinese medicinal insect.
All compounds inhibited COX-2 and only compound 32 inhibited COX-1, thus supporting the use of these insects to treat pathogenic inflammation90. The genotoxic and cytotoxic effects of darkling beetles, Ulomoides dermestoides (Tenebrionidae), used in traditional Argentinian medicine to treat asthma, Parkinson׳s disease, diabetes, arthritis, HIV and cancer, were recently evaluated92. The dichloromethane extract was cytotoxic against human lung carcinoma epithelial cells (A549) and normal mononuclear cells. Additionally, the extract induced significant DNA damage in both tumor and healthy cells. However, if targeted directly to tumor cells, there is still potential for anticancer treatment despite the deleterious side effects92.
The Scarabaeidae make up about 8% of all coleopterans and have been mentioned frequently in the literature for their medicinal value. The large chafer beetle Holotrichia parallela (Scarabaeidae), traditionally used as drug in China and East Asia for the treatment of gout, tetanus, erysipelas and superficial infections, was recently evaluated for antioxidant activity93. Both water and ethanolic extracts were subjected to various in vitro assays including linoleic acid peroxidation inhibition, metal-chelating activity and DPPH radical scavenging activity. The ethanol extract had potent metal-chelating activity and inhibition of lipid peroxidation which was attributed to the presence of Catechin. The water extract, rich in proteins, proved to be an excellent antioxidant in the scavenging of DPPH radicals and metal-chelating activity93. Holotrichia diomphalia (Scarabaeidae) larvae is a traditional crude drug in China for the treatment of chronic liver cirrhosis, contusion, edema, furuncle, and apoplexy94. A chemical evaluation of the larval extract found four known compounds including the flavonoid tricin as well as cholesterol, palmitinic acid and eicosane. Additionally, ethanol and petroleum ether extracts of H. diomphalia larvae showed antifungal activities in vitro against Pyricularia oryzae by morphologically deforming hyphal growth from conidia95. Minimum morphological deformation concentration (MMDC) values from ethanol and petroleum ether extracts were 7.8 and 125 µg/mL, respectively.
The extracts of rhinoceros beetle larvae, Allomyrina dichotoma (Scarabaeidae), were found to play a vital role in reactive oxygen species (ROS) scavenging in response to oxidative stress96. Antioxidant properties of various extracts, including methanol, water, chloroform, ethyl acetate and hexane, were measured through DPPH radical scavenging, superoxide anion radical scavenging and singlet oxygen quenching assays. The methanol extract had the most effective DPPH radical scavenging activity and was 1.7 times more efficient than the standard, ascorbic acid, at singlet oxygen quenching. Also, the extracts protected the biological systems in E. coli and lactate dehydrogenase against detrimental effects of 1O2 of type II photosensitization in vitro. Last year, A. dichotoma larvae were shown to have hepatoprotective and anticancer activities97. Oral administration of powdered larvae lessened diethylnitrosamine (DEN)-induced hepatotoxicity in mice and an ethyl acetate extract showed cytotoxicity against various cancer cells through induction of apoptosis, necrosis, and autophagy.
In 2012, the antioxidant activity of Protaetia brevitarsis (Scarabaeidae) at various growth stages, larvae, pupae and imago, was evaluated98. The larvae and imago extracts had equivalent singlet oxygen quenching ability to the control, ascorbic acid, as well as comparable DPPH and ABTS radical scavenging activity. Two years later the larvae was shown to also have liver protective and antineoplastic activity99. Oral administration of the powdered larvae reduced signs of acute and chronic liver injuries in DEN-induced hepatotoxic mice. Also, a hexane extract proved highly effective against a variety of cancer cell lines with minimal effect on healthy cells. The mechanism of action was determined to be apoptosis via the perturbation of mitochondrial membrane potential99.
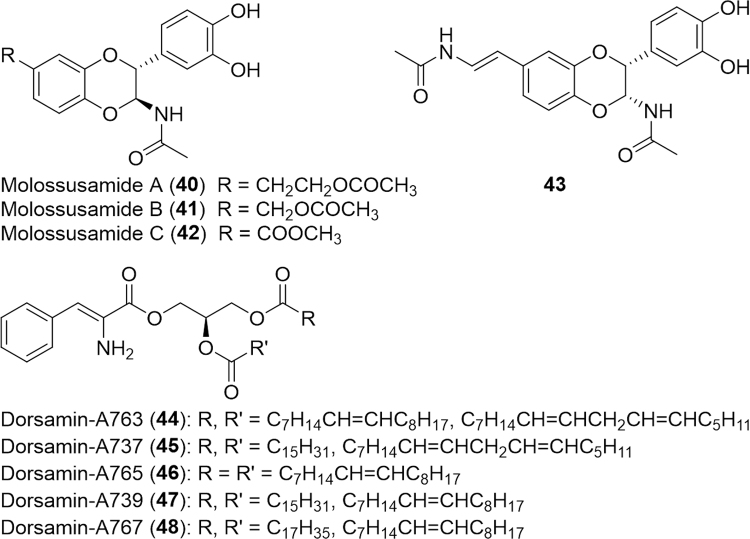
In northeastern India, the dung beetle, Catharsius sp. (Scarabaeidae), is made into a paste and taken orally for the relief of diarrhea11. Catharsius molossus is also a valued Chinese medicinal insect with multiple reported uses including arresting convulsions, removing blood stasis, relaxing the bowels, and counteracting toxins. Recently, five small molecules including three novel N-acetyldopamine dimers, termed molossusamide A--C (40--42, Fig. 8), and two known compounds (38 and 43) were isolated and evaluated for biological activities using cytotoxicity, anti-influenza, enterovirus 71 (EV71) inhibition and anti-inflammation (COX--1/2)100. None of the compounds exhibited cytotoxicity against cancer cells or anti-viral (influenza and EV71) effects. Compound 38 inhibited the COX isozymes and was several times more effective against COX-2.
Figure 8.
Structures of actyldopamine dimers isolated from Catharsius molossus and antioxidant lipids from Bruchidius dorsalis.
New antioxidant lipids, designated dorsamin-A763 (44), -A737 (45), -A765 (46), -A739 (47), and -A767 (48) were isolated via an activity-directed fractionation of the chloroform extract of Bruchidius dorsalis (Bruchidae) larvae101. Compounds 44--48 exhibited ABTS radical scavenging activity comparable with or stronger than that of the control Trolox. The lucanid beetle, Serrognathus platymelus castanicolor (Lucanidae), was evaluated for antioxidant properties at three life stages: larvae, pupae and adult102. The pupal methanol extract methanol extracts (PME) had the highest DPPH and ABTS radical scavenging activity and a 1O2 quenching ability comparable to the control ascorbic acid.
The Meloidae are a smaller group within the Coleopterans numbering only 2500 species. But thanks to their defensive secretions, the meloidae have a long history of medicinal applications. Therapeutic uses date back to 1264 in which they were reportedly used for wart removal in China and cancer treatment during the middle ages in Europe39. Presently in parts of Spain, oil from the red-striped oil beetle, Berberomeloe majalis (Meloidae), is applied externally for wound and wart treatment and as an analgesic8. Palembus dermestoides (Meloidae) is used for the treatment of sexual impotence, ophthalmological problems, rheumatism, weakness in folk Latin American medicine12. Additionally, Pseudomeloe andensis is used for the treatment of warts in folk Latin American medicine. In current traditional Korean medicine the Meloidae are used externally for boils, fungal infection and facial paralysis due to stroke. Internally they are levered for gonorrhea, lymphangitis, neoplasm, rabies, syphilis and edema17. In Mexico they are macerated with alcohol and rubbed on the head to combat baldness18.
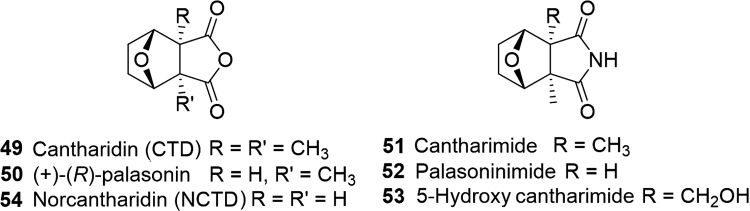
Scientific explorations into these beetles have revealed their bioactivity is attributable to terpene-related compounds. The monoterpene, cantharidin (CTD, 49, Fig. 9), was the first to be isolated and characterized (c.1914) from blister beetles colloquially known as the ‘Spanish fly’39, 46, 88. The demethylated CTD analogue, (R)-(+)-palasonin (50), was discovered in the dried bodies of the South African Hycleus oculatus, H. tinctus, and H. lunata beetles and is the only CTD analogue found in plant species as well39, 103, 104. Imide analogues of CTD and palasonin, cantharimide (51) and palasonimide (52) respectively, in which the oxygen atom in the anhydride core of the molecules is replaced by a nitrogen atom were also found in the H. lunata beetle104. Within the last decade cantharimide, 5-hydroxy cantharimide (53) and norcantharidin (NCTD, 54) were isolated from the native Korean and Chinese blister beetle, Mylabris phalerate PALLAS39.
Figure 9.
Structures of monoterpene cantharidin (CTD), norcantharidin (NCTD), and their derivatives isolated from African, Korean, and Chinese Beetles.
The earliest isolated compound, CTD, is the highest concentrated terpene in blister beetles and has been explored extensively to further understand its pharmacological and toxicological properties against cancer. Cantharidin is often reported as inducing apoptosis and cell cycle arrest through a variety of underlying pathways39, 105. The primary mechanism is the inhibition of protein phosphatase 1 (PP1) and protein phosphatase 2 A (PP2A)106. These two phosphatases are known to be involved in various cellular processes including DNA damage, cell cycle arrest, and apoptosis. Researchers from North-Eastern Hill University reported CTD treatment caused plasma membrane disintegration, the appearance of membrane vacuoles and blebbing on murine Ehrlich ascites carcinoma cells which are hallmarks of apoptosis107. Further evaluation revealed CTD acted via an intrinsic pathway involving disruption of the mitochondrial membrane, rerelease of cytochrome c and downstream activation of caspases directly involved in apoptosis108. Similar mechanisms for apoptosis were reported for in vitro tests on human skin and gastric cancer cells in addition to cell cycle arrest at the G2/M phase109, 110. Cantharidin has been reported to effect human lung cancer cells via the intrinsic pathway by increasing reactive oxygen species (ROS) and calcium concentration leading to disruption of the mitochondrial membrane or inhibition of the phosphatidylinositol 3-kinase/Akt signaling pathway111, 112. This leads to the selective attenuation of one of the gelatinases, matrix metalloproteinase (MMP)-2, which can degrade components of extracellular matrix (ECM) and inhibits migration and invasion of human lung cancer cells111. Also an extrinsic pathway whereby the death receptor is activated by an extracellular response followed by caspase 8 and caspase 3 activation leading to apoptosis has been postulated112. Recently, it was discovered CTD also suppresses human umbilical vascular endothelial cell proliferation, migration, and tube formation, abrogates vascular endothelial growth factor (VEGF)-induced activation of signal transducer and activator of transcription 3 (STAT3) and suppressed the phosphorylation of JAK1, AKT and Extracellular signal–regulated kinase (ERK)113. All of which are markers of angiogenesis which is required for the large scale growth of tumor cells114. Suppression of angiogenesis further supports the promise of cantharidin as a potential anticancer drug.
Cantharidin has also been found to induce a special type of cell suicide in erythrocytes-termed eryptosis105. The erythrocytes of healthy subjects exposed to cantharidin were found to exhibit cell membrane scrambling and cell shrinkage, both hallmarks of eryptosis. Eryptosis eliminates infected or defective erythrocytes thus counteracting parasitemia in malaria and contributes to the pathophysiology of several clinical disorders including metabolic syndrome and diabetes, malignancy, cardiac and renal insufficiency, hemolytic uremic syndrome, sepsis, mycoplasma infection, malaria, iron deficiency, sickle cell anemia, thalassemia, glucose 6-phosphate dehydrogenase deficiency, and Wilson׳s disease115. Cantharidin was recently evaluated for its potential immunomodulatory effects through regulation of dendritic cells (DC). Cantharidin modulates the differentiation and maturation of DCs with alteration of the expression of surface markers and cytokines secretion, causing deviation of standard DC differentiation toward a state of less maturation116. Thus, CTD could treat or prevent disorders involving unwanted immune responses, such as allergies, transplant rejection, or autoimmune diseases.
Though CTD has a variety of therapeutic uses, it has a number of toxic side effects, such as kidney and gastrointestinal damage and disruption of reproductive functions107, 117, 118. Its closely related naturally occurring analogue, norcantharidin (NCTD), has similar therapeutic uses but with lower toxicity risk117, 118. As a chemo-preventive agent NCTD has inhibited tumor progression in non-small cell lung cancer (NSCLC), prostate cancer and leukemia, in vitro, through cell-cycle arrest and apoptosis119, 120, 121. Just last year NCTD was also linked to the suppression of the canonical wingless type (Wnt) signaling pathway which is known to cause a variety of human cancers including NSCLC119. Additional therapeutic benefits, cited in a recent review of NTCD, include the modulation of cancer stem cell self-renewal pathways, overcoming multidrug resistance and as radiation sensitizer118.
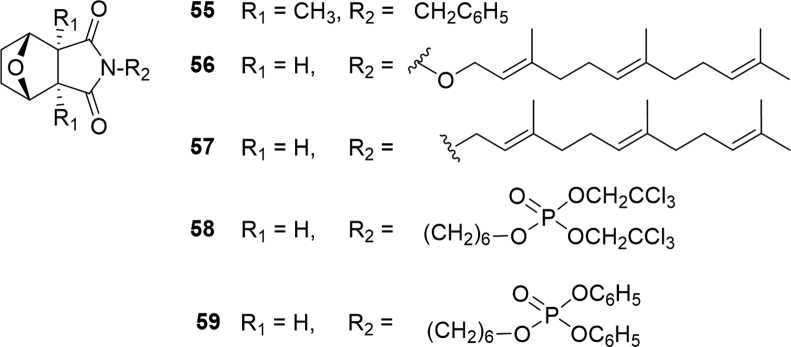
The presence of nitrogen in cantharimide and 5-substituted cantharimide improves their solubility and cytotoxicity against human hepatocellular carcinoma cell lines39. Given the therapeutic potential of CTD, NCTD and cantharimide, thousands of related analogues have been designed and synthesized with the aim of improving their anticancer potency and decreasing their toxicity. In 2014, a novel CTD analogue, N-benzylcantharidinamide (55, Fig. 10), was found to suppress the metastatic potential of Hep3B hepatocellular carcinoma cells through inhibition of MMP-9 expression122. Cancer cells produce proteolytic enzymes like MMPs to invade surrounding tissue such as extracellular connective tissues and the basement membrane. An additional twenty-three N-substituted NCTD analogues were synthesized and evaluated for cytotoxicity against five human cancer cell lines that same year123. Lipophilicity of the derivatives was altered through the N-substituents which included alkyl, alkyloxy, terpenyl or terpenyloxy groups. The lipophilicity of a molecule is an important factor in drug development as it affects solubility and permeability through membranes124. Eleven of the molecules showed significant inhibition against all five cancer cell lines tested with a general trend of increased cytotoxicity with increasing side chain length123. Thus, the lipophilicity of the N-position of norcantharimide is an important parameter affecting the biological activity of the compound. In fact, two compounds N-farnesyloxy-norcantharimide (56) and N-farnesyl-norcantharimide (57) showed a 2--5-fold increase in cytotoxicity as compared to NCTD against liver carcinoma cells. Even more impressive neither had significant adverse effects on normal murine embryonic liver cells. Not surprisingly, microscopy of the treated cells showed typical morphological features of apoptosis, such as cell shrinkage, chromatin condensation and DNA fragmentation123. The biological activity of norcantharimide analogues with terminal phosphate esters was evaluated against a panel of nine human cancer cell lines: human colon carcinoma cell lines (HT29 and SW480); human breast carcinoma cell line (MCF-7); human ovarian carcinoma cell line (A2780); human lung carcinoma cell line (H460); human skin carcinoma cell line (A431); human prostate carcinoma cell line (DU145); human neuroblastoma cell line (BE2-C); and human glioblastoma cell line (SJ-G2)125. Multiple analogues were at least equipotent with NCTD and a number displayed about a 5-fold increase in cell death with the most potent being bis-trichloroethyl (58) and diphenyl (59) analogues.
Figure 10.
Structures of synthetic cantharimide and norcantharimide derivatives.
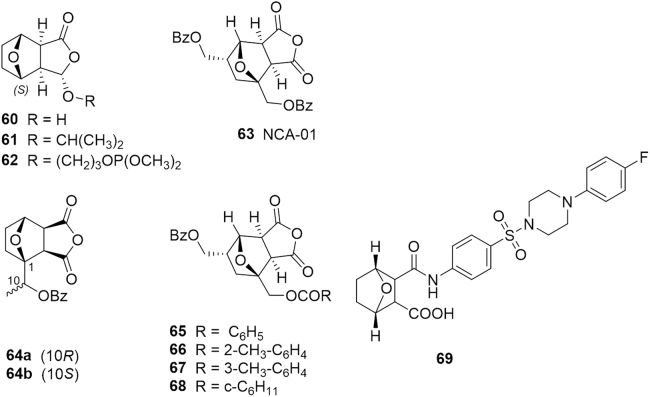
In fact, the anti-proliferative activity of these analogues generally correlated with the relative ease of phosphate ester hydrolysis. A series of synthetic NCTD analogues were evaluated against a panel of nine human cancer cell lines including colon carcinoma (HT29 and SW480), breast carcinoma (MCF-7), ovarian carcinoma (A2780), lung carcinoma (H460), skin carcinoma (A431), prostate carcinoma (DU145), neuroblastoma (BE2-C) and glioblastoma (SJ-G2). The diastereomeric analogue, Novo-6 (60, Fig. 11), displayed a high level of anticancer activity against all tumor cells but with selectivity towards colon and glioblastoma cancer cell126. Incorporation of an isopropyl tail at the hydroxyl position (61) increased the selectivity towards colon and glioblastoma but still completely void of any activity against the remaining tumor cell lines as per the parent molecule. Furthermore the introduction of a terminal phosphate moiety (62) at the same position produced a different trend in cytotoxicity with strong activity on neuroblastoma cells, suggesting an alternate mode of action. In recent years, a series of 5-benzoyloxymethylnorcantharidin derivatives with various substituents at the C1 position were synthesized in an effort to find PP2B-selective inhibitors an attribute of the well-known immunosuppressant drug cyclosporine A127. The lead compound NCA-01 (63) and its derivatives (64--68) evinced potent inhibition of PP2B, approximately 70% or more, with negligible inhibition of PP1 and PP2A.
Figure 11.
Structures of synthetic cantharidin and norcantharidin derivatives.
In a study last year, a series sulfonamide NCTD derivatives were synthesized and evaluated for their cytotoxic effects on human lung (A549), liver (HepG2), cervical (HeLa) and colon (HCT-8) carcinoma cell lines together with a genetically normal human cell line (WI-38)106. One compound in particular, 69, exhibited potent cytotoxic effects on all carcinoma cell lines, whereas it was less toxic to normal cells than its parent compound, NCTD. The mechanism of action was determined to be inhibition of PP1 and microtubule formation in tumor cells and suggests that compound 69 is a promising anticancer drug candidate.
4. Blattodea (cockroaches and termites)
Blattodea is a smaller insect order of approximately 7600 species comprised of cockroaches (4600) and termites (3000)89. The giant cockroach, Rhyparobia maderae (Blaberidae), is used as an asthma treatment in Latin American folk medicine12. Additionally, the cockroach Eurycotis manni (Blattidae) is prescribed for headache relief. The American cockroach, Periplaneta americana (Blattidae), is used for a variety of conditions including: heartburn, asthma, stomach ache, intestinal colic, earache, alcoholism, epilepsy, vomit, boil, hemorrhage, bronchitis, diarrhea, gonorrhea, panaris, cancer, stroke, burns and menstrual cramps12. In parts of India, it is cooked in a soup to treat postpartum, uterine problems and urinary obstructions80. Additionally, the whole body is consumed to treat gangrene, dyspnea, asthma and tuberculosis and applied topically for tetanus and earache relief43, 80. In China, the ethanol extract of the dried whole body of P. americana have been used as traditional Chinese medicine for the treatment of blood stasis syndrome, acne and abdominal mass for hundreds of years. Given the varied therapeutic uses for the pest, the ethanol extract of the insect was screened for bioactive compounds resulting in the discovery of four new isocoumarins periplatins A--D (70--73, Fig. 12) along with three known ones (74--76)128. The compounds were tested against various cancer cell lines and compounds 72--74 showed significant cytotoxic activities against human liver (HepG2) and breast cancer (MCF-7) cells. In a small scale clinical study, sixty septic patients were treated with P. americana extract which successfully improved the condition and prognosis in patients with sepsis129.
Figure 12.
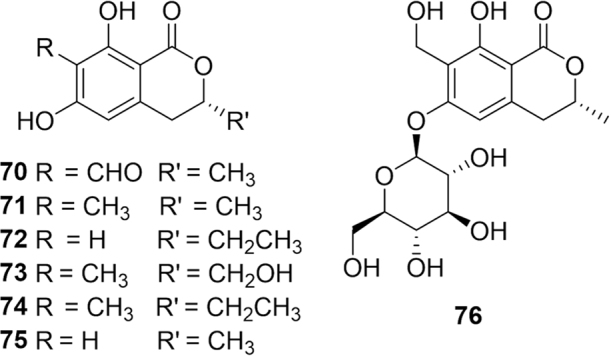
Structures of isocoumarins periplatins isolated from Periplaneta americana.
Blatta orientalis (Blattidae) is used topically for the treatment of tetanus and ear pain in Mexican traditional medicine18. Recently, the anti-asthmatic and anti-anaphylactic activities of B. orientalis mother tincture was evaluated130. The results indicated nonselective anti-cholinergic and anti-histaminic activities were responsible for its anti-asthmatic activity. Furthermore, mast cell stabilization, lowering elevating IgE antibody and reducing oeosinophil led to both passive and active anaphylactic activity.
The Chinese cockroach, Eupolyphaga sinensis (Corydiidae), is a key ingredient in a number of traditional Chinese medicinal products including Shu-Mai Tang, Dahuang Zhechong pill, Shensong Yangxin capsule, Shen-Yuan-Dan, Yi Guan Jin and Yaotongning131, 132, 133, 134, 135, 136. Shu-Mai Tang is prescribed for the treatment of ischemic heart disease and was found to have a therapeutic effect against inflammation-induced myocardial fibrosis leading to the attenuation of left ventricular remodeling and improved cardiac function131. The Dahuang Zhechong pill is clinically used for the treatment of hepatic diseases, gynecopathy, and atherosclerosis in China132. Further studies into its role in atherosclerosis treatment revealed its ability to significantly inhibit proliferation of vascular smooth muscle cells through depressing PDGF expression in the cells, retarding the cell cycle and promoting cellular apoptosis. Shenong Yangxin capsule is prescribed for the treatment of arrhythmias in China and was recently found to have potential use in diabetic cardiopathy133. The Chinese herb modified Yi Guan Jian is an effective regimen that is usually used in outpatients with chronic liver diseases such as fibrosis and cirrhosis134. Shen-Yuan-Dan is used for the treatment of ischemic heart disease, proven to relieve angina and reduces cardiac dysfunction135 Traditional Chinese medicine Yaotongning Capsule is a common remedy to treat rheumatism in China and possesses diverse biological activities including anti-inflammation136.
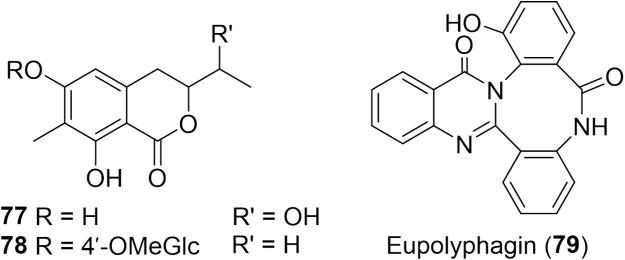
Another traditional use of Eupolyphaga sinensis is the treatment of cancer. Researchers extracted the adult insect with petroleum ether, ethyl acetate and n-butanol and evaluated the in vitro activity of the extracts in ten cancer cell lines137. All extracts had significant antiproliferative activity against all cell lines. An LC-MS evaluation of the ethyl acetate extract was analyzed further to isolate the potential bioactive compounds. Four compounds including two new isocoumarins (77--78, Fig. 13), a new alkaloid deemed eupolyphagin (79), and a known N-acetyldopamine dimer were isolated but were poorly active against the panel of tumor cells137. That same year the effect of Eupolyphaga sinensis ethanol extracts on tumor reduction was evaluated using hepatocarcinoma H22-bearing mice138. The extract exhibited significant inhibition of tumor growth, promoted Th1 type cytokine production (IFN-γ and TNF-α) and induced apoptosis of hepatocarcinoma via increasing BAX/BCL-2 ratio and activation of caspase-3. In 2014, its effect against hepatocellular carcinoma was further attributed to targeting the protein kinase C (PKC), AKT, mitogen-activated protein kinase (MAPK) signaling and related metastasis signaling which are known targets for potential cancer therapies139. That same year the 70% ethanol extract of Eupolyphaga sinensis was found to reduce tumor progression in human and murine chronic myeloid cells. The mechanism was believed to involve G2/M cell cycle arrest and inhibition of epidermal growth factor receptor (EGFR) phosphorylation in the EGFR signaling pathway of human and murine chronic myeloid tumor cells140. This extract was also found to inhibit NSCLC progression through the inhibition of angiogenesis141. The proposed underlying mechanism was interruption of the auto-phosphorylation of kinase insert domain receptor (KDR) and subsequently AKT and ERK1/2.
Figure 13.
Structures of isocoumarins and an alkaloid isolated from Eupolyphaga sinensis.
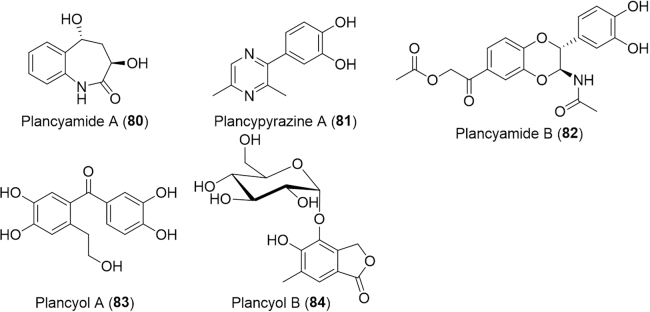
Polyphaga plancyi, a close relative of Eupolyphaga sinensis that is widely distributed in China, has also been used in Chinese traditional medicine to treat diseases such as amenorrhea and fracture and its extracts were shown to have anticancer and anti-inflammatory activities. A recent study of P. plancyi Bolivar isolated five new compounds (80--84, Fig. 14)142. Compounds 81 and 83 inhibit JAK3 kinase with IC50 values of 12.6 and 5.0 μmol/L, respectively. In addition, compound 83 inhibit DDR1 kinase with an IC50 value of 4.87 μmol/L. Both JAK3 and DDR1 are potential drug targets for cancer142.
Figure 14.
Structures of new compounds isolated from Polyphaga plancyi Bolivar.
The winged termite, Reticulitermes (Rhinotermitidae), is roasted with sugar and consumed to treat eye diseases and diabetes in certain regions within India80. In the Kerala state in southern India, the Kurichchan tribe consume a decoction of termites and Vitex negundo (a five-leaved Chinese chastetree shrub) twice daily to cure ulcers16. The tribes of Irular and Mudugar dry and powder Odontotermes formosanus (Termitidae) which is then added as needed to milk for general health improvement and relief of body pain. These termites are also fried in oil, powdered and administered orally for rheumatic diseases. The termites of Microcerotermes exignus (Termitidae) and Nasutitermes macrocephalus (Termitidae) are used for asthma, bronchitis and flu in Latin American folk medicine12. In Zambia, Hodotermes mossambicus (Hodotermitidae) is used to alleviate childhood malnutrition143. In Somalia, Macrotermes bellicosus (Termitidae) is used to suture wounds. In Nigeria, Macrotermes nigeriensis is leveraged for wounds and used to alleviate sickness in pregnant women. In Brazil, various genera of the family Termitidae are used for asthma, cough, flu, sore throat, sinusitis, tonsillitis and hoarseness. In India, Odontotermes formosanus of the Termitidae family is thought to cure ulcers, improve health, relieve body pain, rheumatism and anemia, and enhance lactation in women143.
5. Diptera (flies and mosquitoes)
Dipterans, commonly called true flies, are a large and diverse species of 120,000--150,000 colonized on all continents and aquatic environments144. Though often associated with forensic science, these insects have varied therapeutic uses as well. For example, adult horseflies (Tabanidae) are used in traditional Korean medicine to treat amenorrhea, abdominal blood stasis and indigestion17. Houseflies, Musca domestica (Muscidae), are boiled with water and lemon to relieve vomiting and diarrhea in traditional Mexican medicine18. They are also popular in Latin American medicine for boils, baldness, eyesores, external sebaceus lamps, stye, ophthalmological problems, dermatosis, cysties and erysipelas12. In China, the use of housefly larvae to cure osteomyelitis, decubital necrosis, lip boil, ecthyma and malnutrition stagnation dates back to the Ming/Qing Dynasty145.
Chitosan from housefly and blowfly, Chrysomya megacephala (Calliphoridae), larvae have been investigated for antioxidant activity in recent years146, 147. Chitosan is a partially de-acetylated polymer produced from the alkaline de-acetylation of chitin and is reportedly used as a wound healing, antimicrobial and hemostatic agent14. The chitosan extracts indeed had potent antioxidant properties comparable to ascorbic acid as measured by DPPH radical and superoxide anion scavenging activity. Antifungal activity was tested against a panel of fungi including R. stolonifer, R. solani, T. cucumeris, S. sclerotiorum, C. lunata, S. rolfsii and F. fulva147. The strongest activity was against R. stolonifer with an IC50 of 0.16 mg/mL.
Blowfly excreta and secretion extracted from Sarconesiopsis magellanica and Lucilia sericata (Calliphoridae) larvae were evaluated for antibacterial activity against a panel of six bacteria split evenly between Gram positive and Gram negative bacterium148. Both species were determined effective against the selected strains however the S. magellanica extract required a lower concentration to ensure therapeutic efficacy. Native and inoculated extracts of housefly larva were evaluated for antibacterial and anti-tumor activity in vitro against various Gram negative and Gram positive bacteria, fungi and human colon cancer cells149. The native extract included whole bodies of the larvae unmodified and the inoculated included larvae which were exposed to Shigella dysenteriae 51302. Neither extract had activity against the fungi, both were strongest against Gram positive bacteria and each exhibited dose-dependent activity against the cancer cell line.
6. Hemiptera (true bugs, aphids, cicadas and scale insects)
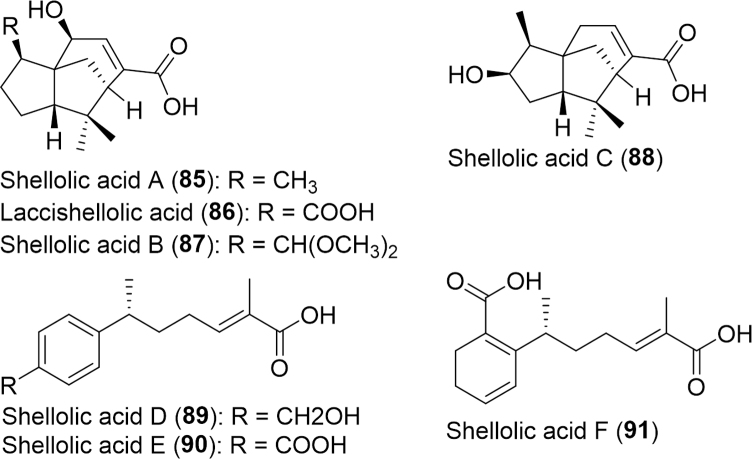
Hemiptera is the fifth largest insect order with 90,000 species described to date46, 150. Hemipterans are often cited in the literature as a healthy alternative food source and only more recently been explored for bioactive substances. The cochineal insect, Dactylopius coccus (Dactylopiidae), whole body of which is used for nasal congestion and ear pain in holistic Mexican medicine18. A major isolate of D. coccus, carminic acid, was determined to be as powerful a free radical scavenging agent as ascorbic acid and stronger than trolox151. Furthermore, it can protect against enzymatically induced β-carotene bleaching as well as quercetin151. The shell of the lac insect Kerria lacca (Kerriidae) is utilized for the treatment of diarrhea and indigestion in parts of India80. Shellac, a natural polymer resin secreted from K. lacca, is valued as a treatment for measles, macula and scabies in traditional Chinese medicine152. Recently, several novel sesquiterpene acids were isolated from this shellac including four α-cedrene types (Shellolic acid A–C, Laccishellolic acid, 85--88, Fig. 15), two R-curcumene types (Shellolic acid D–E, 89--90) and Shellolic acid F (91). The anti-tumor and antibacterial activities were evaluated revealing that none had cytotoxic activity and only shellolic acid A had antimicrobial activity against B. subtilis.
Figure 15.
Structures of sesquiterpene acids isolated from Kerria lacca.
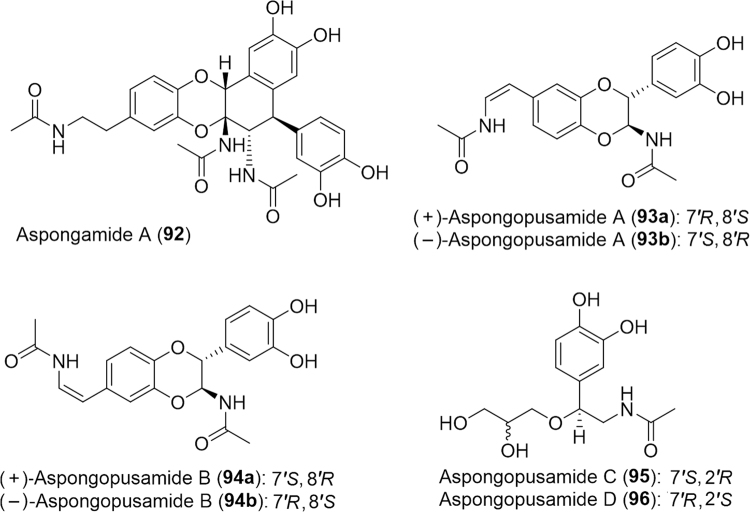
The stinkbug Encosternum delegorguei (Tessaratomidae), an edible treat in Zimbabwe and South Africa, was recently proven to contain significant amounts of protein, fat and minerals validating its use as a nutritional food source153, 154. In addition to being a healthy appetizer, the stinkbug has acclaimed traditional medicinal values, such as decreasing hypertension and curing asthma and heart diseases154. Likewise, the Chinese stinkbug, Aspongopus chinensis (Pentatomidae), is a nutrient-rich edible insect which has been employed to relieve pain, and treat nephropathy and kidney disease in China. In recent years, much research has gone into identifying bioactive small molecules from this insect. Researchers evaluated petroleum ether, ethyl acetate and n-butanol A. chinensis extracts for cytotoxicity against a panel of human cancer cell lines including colorectal (HCT-116), lung (H-125), prostate (LNCaP), melanoma (MDA), pancreatic (PANC-1), breast (MCF-7), ovarian (OVC-5), leukemia (CEM), glioma (U251N) and murine normal cells (CFU-GM) as a control155. Each exhibited significant activity against the cell lines and the ethyl acetate extract was analyzed further for active compounds. The compounds isolated from the ethyl acetate extract, however, all exhibited no or poor activity against the cancer cells. In 2014, a novel trimer of N-acetyldopamine (NADA) termed (±)-aspongamide A (92, Fig. 16) was isolated from A. chinensis along with a racemic mixture of NADA dimeric enantiomers(38)156. The compounds were tested as potential therapeutic agents to treat chronic kidney disease (CKD). Aspongamide A (92) promotes a significant decrease in expression and phosphorylation of Smad3 a key role in the TGF-B/Smad signaling pathway which regulates CKD pathogenesis. The dimeric NADA derivatives (±) 38 were observed to cause an increase in collagen I and α-smooth muscle actin (α-SMA) expression, indicating benefit for restoring skin after wounds as collagen I and α-SMA are implicated in the process of fibrosis and wound healing.
Figure 16.
Structures of N-acetyldopamine trimers and dimers isolated from Aspongopus chinensis.
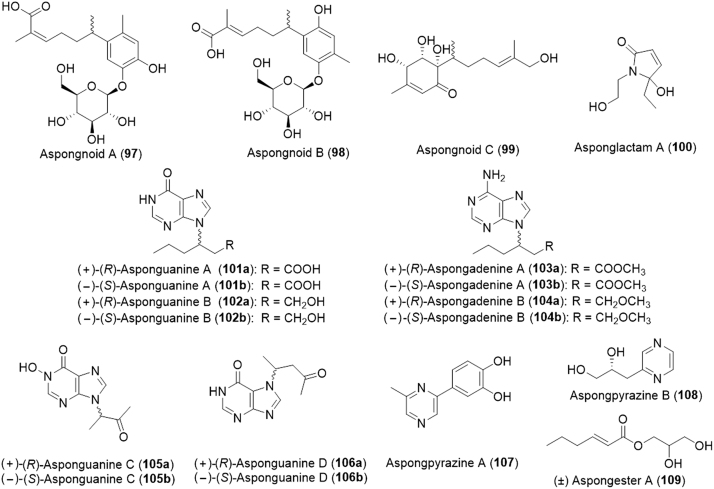
That same year an additional 29 small molecules were isolated from the insect eight of which were novel bioactive compounds including four norepinephrine derivatives (aspongopusamides A--D, 93--96), three sesquiterpenoids (aspongnoids A--C, 97--99, Fig. 17) and one lactam (asponglactam A, 100)157. A subset of the insect-derived substances was evaluated as renal protection agents in high-glucose-induced mesangial cells and COX-2 inhibition. Aspongopusamide A (103) caused a significant decrease in collagen IV, fibronectin, and IL-6 production in high-glucose-induced mesangial cells and inhibited COX-2 (IC50 = 6.50 µmol/L). Aspongopusamide B also provided protective effect on diabetic nephropathy and aspongopusamide C (95) inhibited COX-2 with an IC50 of 117 µmol/L. Last year, an additional 9 novel small molecules (101--109) were isolated from A. chinensis158. With the exception of (±) aspongadenine A (103) and (±) aspongester A (109), all had the ability to promote the proliferation of neural stem cells. The most active compound was (+)-asponguanine B (100a).
Figure 17.
Structures of sesquiterpenoids and other small molecules isolated from Aspongopus chinensis.
7. Orthoptera (grasshoppers and crickets)
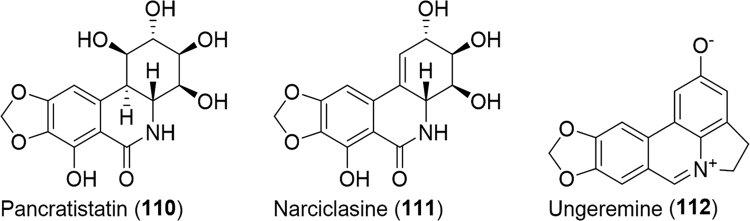
There are some 22,500 species of orthopterans described to date46. Orthopterans are sometimes singled out as pests though that determination is based on select members. One notable family includes the acridids, plague locusts that at times enter gregarious stages forming swarms numbering in the millions. In Mexico, people eat the grasshopper, Schistocerca piceifrons (Acrididae), to relieve hiccups and the cricket, Gryllus spp., for stuttering18. The mole cricket, Gryllotalpa Africana (Gryllotalpidae), is used in traditional Korean medicine for retention of urine, urolithiasis (stones in bladder), edema, lymphangitis and furuncles17. The house cricket, Acheta domesticus (Gryllidae), is used for the treatment of scabies, asthma, eczema, lithiasis, earache, oliguresis, rheumatism, urine retention, urinary incontinence and ophthalmological problems in Latin America12. The crickets, Paragryllus temulentus (Gryllidae) and Gryllus assimilis (Gryllidae), were additionally cited for rheumatism and warts, respectively. The desert locus Schistocerca gregaria (Acrididae) was found to be rich in sterols with therapeutic uses including cardiovascular protective and immune regulatory effects, anti-inflammatory and anticancer benefits159. The known anticancer agents pancratistatin (110, Fig. 18), narciclasine (111) and an additional derivative, ungeremine (112), were isolated from the Texas grasshopper, Brachystola magna (Romaleidae)160. The compounds were further tested against murine lymphocytic leukemia cells and a variety of human tumor cell lines and as expected all were active against the cell lines with narciclasine being the most potent on each cell line. The chitosan from two species of grasshoppers, Calliptamus barbarous (Acrididae) and Oedaleus decorus (Acrididae), were found to have potent antimicrobial activity against several strains of fish, clinical and food-borne pathogens163. Additionally, the chitosan was proven to have antioxidant activity as measured by DPPH radical scavenging activity and ferric reducing power. The nutritional value of the short-horned grasshopper, Chondacris rosea (Acrididae), and mole cricket, Brachytrupes orientalis (Gryllidae), were recently investigated164. These food staples of Arunachal Pradesh, India were found to possess a substantial amount of protein, minerals and energy.
Figure 18.
Structures of small molecules isolated from Brachystola magna.
8. Lepidoptera (butterflies and moths)
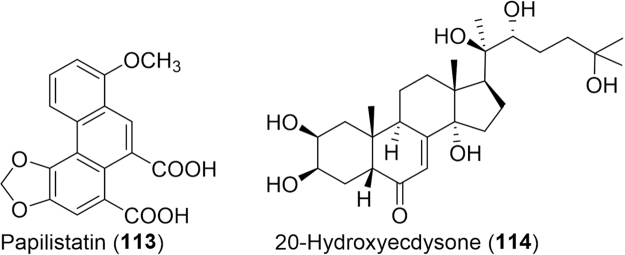
Lepidopterans, which include butterflies and moths, are one of the largest insect orders with anywhere from 155,208 to 174,312 members to date165. Moths especially have been leveraged at all life stages for medicinal purposes. In traditional Indian medicine, dog bites are treated by consummation and/or direct application of freshly laid eggs of Diacrisia obliqua (Arctiidae) to the affected area43. Dried full grown larvae of Stomphosistis thraustica (Gracillaridae) are taken with herbs to treat common fever and to increase milk flow in lactating women. In Latin American folk medicine, the moths, Oiketicus kirbyi (Psychidae), are given for the treatment of asthma, earache and haemorrhage12.
A novel small molecule, designated papilistatin (113, Fig. 19), was isolated from the wings of Byasa polyeuctes termessa, a Taiwan butterfly (Papilionidae)161. Papilistatin showed strong cytotoxicity against colon and pancreatic cancer cells and selective Gram-positive antibacterial activity. The cocoon of mulberry white caterpillar, Rondotia menciana (Bombycidae), was recently evaluated for the presence of flavonoids166. Researchers discovered flavonol galactosides quercetin 3-O-galactosyl-galactosideand kaempferol 3-O-galactosyl-galactoside, the first reported flavonoids conjugated with galactose in animal species166. The medicinal activity of these novel compounds was not evaluated however. The cocoon crude extract of Bombyx mori, a close relative of the R. menciana, was found to have significant effect on hypercholesterolemia and atherosclerosis, as evinced through lipid-lowering capability and decreasing the extent of atherosclerotic lesions in rabbits, induced with hyperlipidemia and atherosclerosis167. Three years later, a detailed chemical profile of an aqueous extract of its cocoon revealed various flavonoids including quercetin 7-O-β-D-glucoside, kaempferol 7-O-β-D-glucopyranoside, coumaric acid glucoside, 2-hydroxy-nonadecanoic acid and 9,12-dihydroxy stearic acid168. Not surprisingly, this flavonoid rich extract exhibited free radical scavenging activity and cardioprotection via reduction of apoptotic factors, oxidative stress, cardiac enzyme activity and interleukin-6 (pro-inflammatory marker).
Figure 19.
Structures of small molecules isolated from lepidopterans and their excreta.
Even B. mori excreta is known to contain bioactive compounds useful in the treatment of diabetes, cancer, high cholesterol and high blood pressure, and is also commonly prescribed in traditional Chinese medicine to treat infectious diseases, headaches and abdominal pain162, 169. For example, the excreta possess ecdysterones (114), which are known to have analgesic effects in man162. In 2014, a novel hydroxyl fatty acid termed bombyxmoric acid methyl ester was also discovered in B. mori droppings; however, it׳s medicinal properties were not evaluated169. B. mori are not the only moths valued for the medicinal qualities of their feces170. Another Chinese tradition is the consumption of insect tea which is prepared from the feces of moths, such as Aglossa dimidiata, Hydrillodes morosa and Nodaria niphona and mentioned in the “Compendium of Materia Medica” as a potential treatment for otitis media. Additional efforts in the past two years have been made to validate new therapeutic properties of insect tea. In a study on human tongue carcinoma cells, insect tea significantly induced apoptosis in cancer cells by upregulating BAX, CASP3, CASP9 and down regulating BCL2171. This was likewise the case for human liver cancer cells evaluated last year172. Anti-inflammatory activity was also evidenced through the down regulation of genes encoding nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB), inducible nitric oxide synthase (iNOS), and COX-2171. Furthermore, insect tea was found to be as effective at preventing gastric ulcers as the gastric ulcer drug, ranitidine and buccal mucosa cancer preventative effects in in vivo murine models173, 174.
9. Conclusions
Summary of small molecules isolated from insects and their biological activities is listed in Table 1. It is clear that Mother Nature has more to offer the world of medicine than just plants. Insects have a long and rich history in traditional medicine across the globe. Not to mention over one hundred small molecule isolates with bioactivity discovered within the past several years. Despite these discoveries, insect-derived products have yet to establish the recognition and market success of their plant derived counterparts. Undoubtedly, this is partly due to negative cultural attitudes towards insects, particularly in the West. However, public perception of insects should not deter scientific pioneers from advancing these largely untapped resources. Indeed, if given the proper attention, insect-derived substances hold great promise for the future of natural product drug discovery.
Table 1.
Summary of small molecules isolated from insects and their biological activities.
| Family | Genus | Common name | Molecule | Biological activity | Ref. |
|---|---|---|---|---|---|
| Hymenoptera (bees, wasps, ants and sawflies) | |||||
| Symphyta | Pergidae | Sawfly | Macrocarpal (1); grandinol (2) | Antimicrobial | 50 |
| Formicidae | Tetramorium | Red ant | Compounds 3--7 | Antimicrobial | 55 |
| Formicidae | Solenopsis | Fire ant | Solenopsin A (8) | Antiangiogenic | 18, 44, 56, 57 |
| Formicidae | Solenopsis | Fire ant | Compounds 9--11 | -- | 58, 59 |
| Formicidae | Tetraponera | Iron ant | Compounds 12--15 | Antiproliferative | 60, 62 |
| Formicidae | Polyrhachis | Black ant | Polyrhadopamines A--E (16--20); Troline (21); compounds 22--30 | Antiinflammatory; antiproliferative; renoprotective | 63--65 |
| Vespidae | Polybia | Wasp | Polybioside (31) | Neuroactive | 70 |
| Coleoptera (beetles) | |||||
| Tenebrionidae | Blaps | Stink beetle | Blapsols A--D (32--35); compounds 24, 36--39 | Antiinflammatory | 90 |
| Scarabaeidae | Catharsius | Dung beetle | Molossusamides A--C (40--42); compounds 38 and 43 | Antiinflammatory | 100 |
| Bruchidae | Bruchidius | Seed beetle | Compounds 44--48 | Antioxidant | 101 |
| Meloidae | Various | Blister beetle | Cantharidin (49) | Antiproliferative; immunomodulatory | 103, 105, 107, 109--113,116, 117 |
| Meloidae | Hycleus | Blister beetle | (R)-(+)-palasonin (50) | -- | 103, 104 |
| Meloidae | Hycleus | Blister beetle | Cantharimide (51); palasonimide (52) | -- | 104 |
| Meloidae | Mylabris | Blister beetle | 5-Hydroxy cantharimide (53); norcantharidin (NCTD, 54) | -- | 39 |
| Synthetic | -- | -- | N-benzylcantharidinamide (55) | Antiproliferative | 122 |
| Synthetic | -- | -- | N-farnesyloxy-norcantharimide (56); N-farnesyl-norcantharimide (57) | Antiproliferative | 123 |
| Synthetic | -- | -- | Compounds 58--59 | Antiproliferative | 125 |
| Synthetic | -- | -- | Compounds 60--69 | Antiproliferative | 126, 127 |
| Blattodea (cockroaches and termites) | |||||
| Blattidae | Periplaneta | American cockroach | Periplatins A--D (70--73); compounds 74--76 | Antiproliferative | 128, 129 |
| Corydiidae | Eupolyphaga | Chinese cockroach | Compounds 77--78; eupolyphagin (79) | Antiproliferative | 137 |
| Corydiidae | Polyphaga | Chinese cockroach | Plancyamides A (80) and B (82), plancypyrazine A (81); plancyols A (83) and B (84) | Antiproliferative | 142 |
| Hemiptera (true bugs, aphids, cicadas and scale insects) | |||||
| Kerriidae | Kerria | Lac insect | Shellolic acid A (85) | Antimicrobial | 152 |
| Kerriidae | Kerria | Lac insect | Shellolic acids B--C (86--87), Laccishellolic acid (88); shellolic acids D–F (89--91) | -- | 152 |
| Pentatomidae | Aspongopus | Chinese stinkbug | Aspongamide A (92) | Active against chronic kidney disease | 156 |
| Pentatomidae | Aspongopus | Chinese stinkbug | Aspongopusamides A--D (93--96) | Antiinflammatory | 157 |
| Pentatomidae | Aspongopus | Chinese stinkbug | Aspongnoids A--C (97--99); asponglactam A (100) | -- | 157 |
| Pentatomidae | Aspongopus | Chinese stinkbug | Compounds 101--109 | Promote neutral stem cell proliferation | 158 |
| Orthoptera (grasshoppers and crickets) | |||||
| Romaleidae | Brachystola | Texas grasshopper | Pancratistatin (110); narciclasine (111); ungeremine (112) | Antiproliferative | 160 |
| Lepidoptera (butterflies and moths) | |||||
| Papilionidae | Byasa | Taiwan butterfly | Papilistatin (113) | Antiproliferative | 161 |
| Bombycidae | Bombyx | Silk moth | Compound 114 | Analgesic effects | 162 |
--Not applicable.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Lauren Seabrooks, Email: les02d@gmail.com.
Longqin Hu, Email: longhu@rutgers.edu.
References
- 1.Guo Z.R. The modification of natural products for medical use. Acta Pharm Sin B. 2017;7:119–136. doi: 10.1016/j.apsb.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dias D.A., Urban S., Roessner U. A historical overview of natural products in drug discovery. Metabolites. 2012;2:303–336. doi: 10.3390/metabo2020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miner J., Hoffhines A. The discovery of aspirin׳s antithrombotic effects. Tex Heart Inst J. 2007;34:179–186. [PMC free article] [PubMed] [Google Scholar]
- 4.Cragg G.M., Newman D.J. Natural products: a continuing source of novel drug leads. Biochim Biophys Acta. 2013;1830:3670–3695. doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen B. A new golden age of natural products drug discovery. Cell. 2015;163:1297–1300. doi: 10.1016/j.cell.2015.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown D.G., Lister T., May-Dracka T.L. New natural products as new leads for antibacterial drug discovery. Bioorg Med Chem Lett. 2014;24:413–418. doi: 10.1016/j.bmcl.2013.12.059. [DOI] [PubMed] [Google Scholar]
- 7.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benítez G. Animals used for medicinal and magico-religious purposes in western Granada Province, Andalusia (Spain) J Ethnopharmacol. 2011;137:1113–1123. doi: 10.1016/j.jep.2011.07.036. [DOI] [PubMed] [Google Scholar]
- 9.Mahawar M.M., Jaroli D.P. Traditional zootherapeutic studies in India: a review. J Ethnobiol Ethnomed. 2008;4:17. doi: 10.1186/1746-4269-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaroli D.P., Mahawar M.M., Vyas N. An ethnozoological study in the adjoining areas of Mount Abu wildlife sanctuary, India. J Ethnobiol Ethnomed. 2010;6:6. doi: 10.1186/1746-4269-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chakravorty J., Ghosh S., Meyer-Rochow V.B. Practices of entomophagy and entomotherapy by members of the Nyishi and Galo tribes, two ethnic groups of the state of Arunachal Pradesh (North-East India) J Ethnobiol Ethnomed. 2011;7:5. doi: 10.1186/1746-4269-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alves R.R.N., Alves H.N. The faunal drugstore: animal-based remedies used in traditional medicines in Latin America. J Ethnobiol Ethnomed. 2011;7:9. doi: 10.1186/1746-4269-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vallejo J.R., González J.A. The use of the head louse as a remedy for jaundice in Spanish folk medicine: an overview. J Ethnobiol Ethnomed. 2013;9:52. doi: 10.1186/1746-4269-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mlcek J., Borkovcova M., Bednarova M. Biologically active substances of edible insects and their use in agriculture, veterinary and human medicine—a review. J Cent Eur Agric. 2014;15:225–237. [Google Scholar]
- 15.Chakravorty J., Ghosh S., Meyer-Rochow V.B. Comparative survey of entomophagy and entomotherapeutic practices in six tribes of eastern arunachal pradesh (India) J Ethnobiol Ethnomed. 2013;9:50. doi: 10.1186/1746-4269-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilsanand V., Varghese P., Rajitha P. Therapeutics of Insects and insect products in south indian traditional medicine. Indian J Tradit Know. 2007;6:563–568. [Google Scholar]
- 17.Pemberton P.W. Insects and other arthropods used as drugs in Korean traditional medicine. J Ethnopharmacol. 1999;65:207–216. doi: 10.1016/s0378-8741(98)00209-8. [DOI] [PubMed] [Google Scholar]
- 18.Alonso-Castro A.J. Use of medicinal fauna in Mexican traditional medicine. J Ethnopharmacol. 2014;152:53–70. doi: 10.1016/j.jep.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Kim H., Song M.J. Ethnozoological study of medicinal animals on Jeju Island, Korea. J Ethnopharmacol. 2013;146:75–82. doi: 10.1016/j.jep.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Kim H., Song M.J. Ethnomedicinal practices for treating liver disorders of local communities in the Southern Regions of Korea. Evid Based Complement Alternat Med. 2013;2013:869176. doi: 10.1155/2013/869176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kujawska M., Zamudio F., Hilgert N.I. Honey-based mixtures used in home medicine by nonindigenous population of misiones, Argentina. Evid Based Complement Alternat Med. 2012;2012:579350. doi: 10.1155/2012/579350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patricia V., Oliverio V., Triny L., Favián M. Meliponini biodiversity and medicinal uses of pot-honey from El Oro province in Ecuador. Emir J Food Agric. 2015;27:502–506. [Google Scholar]
- 23.Erejuwa O.O., Sulaiman S.A., Wahab M.S. Honey: a novel antioxidant. Molecules. 2012;17:4400–4423. doi: 10.3390/molecules17044400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maddocks S.E., Jenkins R.E. Honey: a sweet solution to the growing problem of antimicrobial resistance? Future Microbiol. 2013;8:1419–1429. doi: 10.2217/fmb.13.105. [DOI] [PubMed] [Google Scholar]
- 25.McLoone P., Warnock M., Fyfe L. Honey: a realistic antimicrobial for disorders of the skin. J Microbiol Immunol Infect. 2015;49:161–167. doi: 10.1016/j.jmii.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Mandala M.D., Mandal S. Honey: its medicinal property and antibacterial activity. Asian Pac J Trop Biomed. 2011;1:154–160. doi: 10.1016/S2221-1691(11)60016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khalil M.I., Sulaiman S.A. The potential role of honey and its polyphenols in preventing heart diseases: a review. Afr J Tradit Complement Altern Med. 2010;7:315–321. doi: 10.4314/ajtcam.v7i4.56693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daleprane J.B., Abdalla D.S. Emerging roles of propolis: antioxidant, cardioprotective, and antiangiogenic actions. Evid Based Complement Alternat Med. 2013;2013:175135. doi: 10.1155/2013/175135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freires I.A., De Alencar S.M., Rosalen P.L. A pharmacological perspective on the use of Brazilian Red Propolis and its isolated compounds against human diseases. Eur J Med Chem. 2016;110:267–279. doi: 10.1016/j.ejmech.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 30.Sforcin J.M., Bankova V. Propolis: is there a potential for the development of new drugs? J Ethnopharmacol. 2011;133:253–260. doi: 10.1016/j.jep.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 31.Bellik Y. Bee venom: its potential use in alternative medicine. Anti-Infec Agents. 2015;13:3–16. [Google Scholar]
- 32.Nabiuni M., Safaeinejad Z., Parivar K., Divsalar A., Nazari Z. Antineoplastic effects of honey bee venom. Zahedan J Res Med Sci. 2013;15:1–5. [Google Scholar]
- 33.Oršolić N. Bee venom in cancer therapy. Cancer Metast Rev. 2012;31:173–194. doi: 10.1007/s10555-011-9339-3. [DOI] [PubMed] [Google Scholar]
- 34.Lee W.R., Park K.K., Pak S.C. The protective effect of bee venom on fibrosis causing inflammatory diseases. Toxins. 2015;7:4758–4772. doi: 10.3390/toxins7114758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yi H.Y., Chowdhury M., Huang Y.D., Yu X.Q. Insect antimicrobial peptides and their applications. Appl Microbiol Biotechnol. 2014;98:5807–5822. doi: 10.1007/s00253-014-5792-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y., Xiang Q., Zhang Q., Huang Y., Su Z. Overview on the recent study of antimicrobial peptides: origins, functions, relative mechanisms and application. Peptides. 2012;37:207–215. doi: 10.1016/j.peptides.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Ntwasa M., Goto A., Kurata S. Coleopteran antimicrobial peptides: prospects for clinical applications. Int J Microbiol. 2012;2012:101989. doi: 10.1155/2012/101989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dossey A.T. Insects and their chemical weaponry: new potential for drug discovery. Nat Prod Rep. 2010;27:1737–1757. doi: 10.1039/c005319h. [DOI] [PubMed] [Google Scholar]
- 39.Galvis C.E., Méndez L.Y., Kouznetsov V.V. Cantharidin-based small molecules as potential therapeutic agents. Chem BIol Drug Des. 2013;82:477–499. doi: 10.1111/cbdd.12180. [DOI] [PubMed] [Google Scholar]
- 40.Ratcliffe N.A., Mello C.B., Garcia E.S., Butt T.M., Azambuja P. Insect natural products and processes: new treatments for human disease. Insect Biochem Mol Biol. 2011;41:747–769. doi: 10.1016/j.ibmb.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 41.Ratcliffe N., Azambuja P., Mello C.B. Recent advances in developing insect natural products as potential modern day medicines. Evid Based Complement Alternat Med. 2014;2014:904958. doi: 10.1155/2014/904958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cherniack E.P. Bugs as drugs, Part 1: Insects: the "new" alternative medicine for the 21st Century? Altern Med Rev. 2010;15:124–135. [PubMed] [Google Scholar]
- 43.Lokeshwari R.K., Shantibala T. A review on the fascinating world of insect resources: reason for thoughts. Psyche. 2010;2010:207570. [Google Scholar]
- 44.El-Tantawy N.L. Helminthes and insects: maladies or therapies. Parasitol Res. 2015;114:359–377. doi: 10.1007/s00436-014-4260-7. [DOI] [PubMed] [Google Scholar]
- 45.Newman D.J., Cragg G.M. Endophytic and epiphytic microbes as “sources” of bioactive agents. Front Chem. 2015;3:34. doi: 10.3389/fchem.2015.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grimaldi D., Engel M.S. Cambridge University Press; New York: 2005. Evolution of the insects. [Google Scholar]
- 47.Foottit R.G. Blackwell Publishing Ltd.; West Sussex: 2009. Adler PH. Insect biodiversity: science and society; pp. 1–6. [Google Scholar]
- 48.Huber J.T. Biodiversity of Hymenoptera. In: Foottit R.G., Adler P.H., editors. Insect biodiversity: science and society. Blackwell Publishing Ltd.; West Sussex: 2009. pp. 303–323. [Google Scholar]
- 49.Yin W.P., Zhao C.J., Liao C.Y., Trowell S., Rickards R.W. Preparative isolation of novel antimicrobial compounds from Pergidae sp. by reversed-phase high-performance liquid chromatography. Chem Nat Compd. 2013;49:41–45. [Google Scholar]
- 50.Vihakas M.A., Kapari L., Salminen J.P. New types of flavonol oligoglycosides accumulate in the hemolymph of birch-feeding sawfly larvae. J Chem Ecol. 2010;36:864–872. doi: 10.1007/s10886-010-9822-2. [DOI] [PubMed] [Google Scholar]
- 51.Crockett S.L., Boevé J.L. Flavonoid glycosides and naphthodianthrones in the sawfly Tenthredo zonula and its host-plants, Hypericum perforatum and H. hirsutum. J Chem Ecol. 2011;37:943–952. doi: 10.1007/s10886-011-0001-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rastogi N. Provisioning services from ants: food and pharmaceuticals. Asian Myrmecol. 2011;4:103–120. [Google Scholar]
- 53.Sousa P.L., Quinet Y., Ponte E.L., De Vale J.F., Torres A.F., Pereira M.G. Venom׳s antinociceptive property in the primitive ant Dinoponera quadriceps. J Ethnopharmacol. 2012;144:213–216. doi: 10.1016/j.jep.2012.08.033. [DOI] [PubMed] [Google Scholar]
- 54.Lopes K.S., Rios E.R., De Carvalho Lima C.N., Linhares M.I., Costa Torres A.B., Havt A. The effects of the Brazilian Ant Dinoponera quadriceps venom on chemically induced seizure models. Neurochem Int. 2013;63:141–145. doi: 10.1016/j.neuint.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 55.Song Z.W., Liu P., Yin W.P., Jiang Y.L., Ren Y.L. Isolation and identification of antibacterial neo-compounds from the red ants of ChangBai Mountain, Tetramorium sp. Bioorg Med Chem Lett. 2012;22:2175–2181. doi: 10.1016/j.bmcl.2012.01.112. [DOI] [PubMed] [Google Scholar]
- 56.Heinen T.E., De Veiga A.B. Arthropod venoms and cancer. Toxicon. 2011;57:497–511. doi: 10.1016/j.toxicon.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 57.Arbiser J.L., Kau T., Konar M., Narra K., Ramchandran R., Summers S.A. Solenopsin, the alkaloidal component of the fire ant (Solenopsis invicta), is a naturally occurring inhibitor of phosphatidylinositol-3-kinase signaling and angiogenesis. Blood. 2007;109:560–565. doi: 10.1182/blood-2006-06-029934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu Y.T., Wei H.Y., Fadamiro H.Y., Chen L. Quantitative analysis of alkaloidal constituents in imported fire ants by gas chromatography. J Agric Food Chem. 2014;62:5907–5915. doi: 10.1021/jf501423y. [DOI] [PubMed] [Google Scholar]
- 59.Chen L., Lu Y.Y., Hu Q.B., Fadamiro Y. Similarity in venom alkaloid chemistry of alate queens of imported fire ants: implication for hybridization between Solenopsis richteri and S. invicta in the southern united states. Chem Biodivers. 2012;9:702–713. doi: 10.1002/cbdv.201100109. [DOI] [PubMed] [Google Scholar]
- 60.Bosque I., Gonzalez-Gomez J.C., Loza M.I., Brea J. Natural tetraponerines: a general synthesis and antiproliferative activity. J Org Chem. 2014;79:3982–3991. doi: 10.1021/jo500446f. [DOI] [PubMed] [Google Scholar]
- 61.Garraffo H.M., Spande T.F., Jain P., Kaneko T., Jones T.H., Blum M.S. Ammonia chemical ionization tandem mass spectrometry in structural determination of alkaloids. II. Tetraponerines from pseudomyrmecine ants. Rapid Commun Mass Spectrom. 2001;15:1409–1415. doi: 10.1002/rcm.382. [DOI] [PubMed] [Google Scholar]
- 62.Rouchaud A., Braekman J.C. Synthesis of new analogues of the tetraponerines. Eur J Org Chem. 2009;2009:2666–2674. [Google Scholar]
- 63.Tang J.J., Zhang L., Jiang L.P., Di L., Yan Y.M., Tu Z.C. Dopamine derivatives from the insect Polyrhachis dives as inhibitors of ROCK1/2 and stimulators of neural stem cell proliferation. Tetrahedron. 2014;70:8852–8857. [Google Scholar]
- 64.Tang J.J., Fang P., Xia H.L., Tu Z.C., Hou B.Y., Yan Y.M. Constituents from the edible Chinese black ants (Polyrhachis dives) showing protective effect on rat mesangial cells and anti-inflammatory activity. Food Res Int. 2015;67:163–168. [Google Scholar]
- 65.Cope A.P., Schulze-Koops H., Aringer M. The central role of T cells in rheumatoid arthritis. Clin Exp Rheumatol. 2007;25:S4–S11. [PubMed] [Google Scholar]
- 66.Ahn M.Y., Ryu K.S., Lee Y.W., Kim Y.S. Cytotoxicity and L-amino acid oxidase activity of crude insect drugs. Arch Pharm Res. 2000;23:477–481. doi: 10.1007/BF02976576. [DOI] [PubMed] [Google Scholar]
- 67.Baracchi D., Mazza G., Turillazzi S. From individual to collective immunity: the role of the venom as antimicrobial agent in the stenogastrinae wasp societies. J Insect Physiol. 2012;58:188–193. doi: 10.1016/j.jinsphys.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 68.Mortari M.R., Do Couto L.L., Dos Anjos L.C., Mourão C.B., Camargos T.S., Camargos J.A. Pharmacological characterization of Synoeca cyanea venom: an aggressive social wasp widely distributed in the neotropical region. Toxicon. 2012;59:163–170. doi: 10.1016/j.toxicon.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 69.Jalaei J., Fazeli M., Rajaian H., Shekarforoush S. In vitro antibacterial effect of wasp (Vespa orientalis) venom. J Venom Anim Toxins Incl Trop Dis. 2014;20:22. doi: 10.1186/1678-9199-20-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saidemberg D.M., Da Silva-Filho L.C., Tognoli L.M., Tormena C.F., Palma M.S. Polybioside, a neuroactive compound from the venom of the social wasp Polybia paulista. J Nat Prod. 2010;73:527–531. doi: 10.1021/np900424t. [DOI] [PubMed] [Google Scholar]
- 71.Prakash S., Bhargava H.R. Apis cerana bee venom: it׳s anti-diabetic and anti-dandruff activity against Malassezia furfur. World Appl Sci J. 2014;32:343–348. [Google Scholar]
- 72.Surendra N.S., Jayaram G.N., Reddy M.S. Antimicrobial activity of crude venom extracts in honeybees (Apis cerana, Apis dorsata, Apis florea) tested against selected pathogens. Afr J Microbiol Res. 2011;5:2765–2772. [Google Scholar]
- 73.Silva J.C., Rodrigues S., Feás X., Estevinho L.M. Antimicrobial activity, phenolic profile and role in the inflammation of propolis. Food Chem Toxicol. 2012;50:1790–1795. doi: 10.1016/j.fct.2012.02.097. [DOI] [PubMed] [Google Scholar]
- 74.Campos J.F., Dos Santos U.P., Macorini L.F., De Melo A.M., Balestieri J.B., Paredes-Gamero E.J. Antimicrobial, antioxidant and cytotoxic activities of propolis from Melipona orbignyi (Hymenoptera, Apidae) Food Chem Toxicol. 2014;65:374–380. doi: 10.1016/j.fct.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 75.De Mendonça I.C., de Moraes Porto I.C., do Nascimento T.G., s de Souza N.S., dos Santos Oliveira J.M., dos Santos Arruda R.E. Brazilian red propolis: phytochemical screening, antioxidant activity and effect against cancer cells. BMC Complement Altern Med. 2015;15:357. doi: 10.1186/s12906-015-0888-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Coelho G.R., Mendonça R.Z., De Senna Vilar K., Figueiredo C.A., Badari J.C., Taniwaki N. Antiviral action of hydromethanolic extract of geopropolis from Scaptotrigona postica against antiherpes simplex virus (HSV-1) Evid Based Complement Alternat Med. 2015;2015:296086. doi: 10.1155/2015/296086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Campos J.F., Dos Santos U.P., Dos Santos Da Rocha P., Damião M.J., Balestieri J.B., Cardoso C.A. Antimicrobial, antioxidant, anti-inflammatory, and cytotoxic activities of propolis from the stingless bee Tetragonisca fiebrigi (Jataí) Evid Based Complement Alternat Med. 2015;2015:296186. doi: 10.1155/2015/296186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Choudhari M.K., Punekar S.A., Ranade R.V., Paknikar K.M. Antimicrobial activity of stingless bee (Trigona sp.) propolis used in the folk medicine of Western Maharashtra, India. J Ethnopharmacol. 2012;141:363–367. doi: 10.1016/j.jep.2012.02.047. [DOI] [PubMed] [Google Scholar]
- 79.Olayemi K.L. Therapeutic potentials of nigerian insect-propolis against the malarial parasite, Plasmodium berghei (Haemosporida: Plasmodidae) Am J Drug Disc Devel. 2014;4:241–247. [Google Scholar]
- 80.Vijayakumar S., Prabhu S., Yabesh J.E.M., Pragashraj R. A quantitative ethnozoological study of traditionally used animals in Pachamalai hills of Tamil Nadu, India. J Ethnopharmacol. 2015;171:51–63. doi: 10.1016/j.jep.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 81.Reyes-González A., Camou-Guerrero A., Reyes-Salas O., Argueta A., Casas A. Diversity, local knowledge and use of stingless bees (Apidae: Meliponini) in the municipality of Nocupétaro, Michoacan, Mexico. J Ethnobiol Ethnomed. 2014;10:47. doi: 10.1186/1746-4269-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Borsato D.M., Prudente A.S., Doll-Boscardin P.M., Borsato A.V., Luz C.F., Maia B.H. Topical anti-inflammatory activity of a monofloral honey of Mimosa scabrella provided by Melipona marginata during winter in Southern Brazil. J Med Food. 2014;17:817–825. doi: 10.1089/jmf.2013.0024. [DOI] [PubMed] [Google Scholar]
- 83.Silva T.M., dos Santos F.P., Evangelista-Rodrigues A., da Silva E.M., da Silva G.S., de Novais J.S. Phenolic compounds, melissopalynological, physicochemical analysis and antioxidant activity of jandaíra (Melipona subnitida) honey. J Food Comp Anal. 2013;29:10–18. [Google Scholar]
- 84.Dor G.O., Mahomoodally M.F. Chemical profile and in vitro bioactivity of tropical honey from mauritius. Asian Pac J Trop Dis. 2014;4:S1002–S1013. [Google Scholar]
- 85.Moniruzzaman M., Khalil M.I., Sulaiman S.A., Gan S.H. Physicochemical and antioxidant properties of malaysian honeys produced by Apis cerana, Apis dorsata and Apis mellifera. BMC Complement Altern Med. 2013;13:43. doi: 10.1186/1472-6882-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pimentel R.B., da Costa C.A., Albuquerque P.M., Junior S.D. Antimicrobial activity and rutin identification of honey produced by the stingless bee melipona compressipes manaosensis and commercial honey. BMC Complement Altern Med. 2013;13:151. doi: 10.1186/1472-6882-13-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Philip Y,Mohd Fadzelly AB. Antioxidative and acetylcholinesterase inhibitor potential of selected honey of Sabah, Malaysian Borneo. Int Food Res J 2015;22:1953–60.
- 88.Bouchard P., Grebennikov V.V., Smith A.B.T., Douglas H. Biodiversity of Coleoptera. In: Foottit R.G., Adler P.H., editors. Insect biodiversity: science and society. Blackwell Publishing Ltd.; West Sussex: 2009. pp. 265–301. [Google Scholar]
- 89.Hoffman K.H. CRC Press; Boca Raton: 2014. Insect molecular biology and ecology. [Google Scholar]
- 90.Yan Y.M., Li L.J., Qin X.C., Lu Q., Tu Z.C., Cheng Y.X. Compounds from the insect Blaps japanensis with COX-1 and COX-2 inhibitory activities. Bioorg Med Chem Lett. 2015;25:2469–2472. doi: 10.1016/j.bmcl.2015.04.085. [DOI] [PubMed] [Google Scholar]
- 91.Narsinghani T., Sharma R. Lead optimization on conventional non-steroidal anti-inflammatory drugs: an approach to reduce gastrointestinal toxicity. Chem BIol Drug Des. 2014;84:1–23. doi: 10.1111/cbdd.12292. [DOI] [PubMed] [Google Scholar]
- 92.Crespo R., Villaverde M.L., Girotti J.R., Güerci A., Juárez M.P., De Bravo M.G. Cytotoxic and genotoxic effects of defence secretion of Ulomoides dermestoides on A549 cells. J Ethnopharmacol. 2011;136:204–209. doi: 10.1016/j.jep.2011.04.056. [DOI] [PubMed] [Google Scholar]
- 93.Liu S.F., Sun J., Yu L.N., Zhang C.S., Bi J., Zhu F. Antioxidant activity and phenolic compounds of Holotrichia parallela motschulsky extracts. Food Chem. 2012;134:1885–1891. doi: 10.1016/j.foodchem.2012.03.091. [DOI] [PubMed] [Google Scholar]
- 94.Dong Q.F., Wang Z., Liu H.J., Zhang C.F., He D.X., Wu G. Flavonoid and other compounds from Holotrichia diomphalia larvae. Chem Nat Compd. 2011;47:114–115. [Google Scholar]
- 95.Dong Q.F., Wang J.L., Zhang S.F., Wang Z., Zhang C.X., Gao H. Antifungal activity of crude extracts and fat-soluble constituents of Holotrichia diomphalia larvae. Bioresource Technol. 2008;99:8521–8523. doi: 10.1016/j.biortech.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 96.Suh H.W., Kim S.R., Lee K.S., Park S., Kang S.C. Antioxidant activity of various solvent extracts from Allomyrina dichotoma (Arthropoda: Insecta) Larvae. J Photochem Photobiol B Biol. 2010;99:67–73. doi: 10.1016/j.jphotobiol.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 97.Lee J.E., Jo D.E., Lee A.J., Park H.K., Youn K., Yun E.Y. Hepatoprotective and anticancer activities of allomyrina dichotoma larvae. J Life Sci. 2015;25:307–316. [Google Scholar]
- 98.Suh H.J., Kang S.C. Antioxidant activity of aqueous methanol extracts of Protaetia brevitarsis Lewis (Coleoptera: Scarabaedia) at different growth stages. Nat Prod Res. 2012;26:510–517. doi: 10.1080/14786419.2010.530267. [DOI] [PubMed] [Google Scholar]
- 99.Lee J.E., Jo D.E., Lee A.J., Park H.K., Youn K., Yun E.Y. Hepatoprotective and antineoplastic properties of Protaetia brevitarsis larvae. Entomol Res. 2014;44:244–253. [Google Scholar]
- 100.Lu J., Sun Q., Tu Z.C., Lv Q., Shui P.X., Cheng Y.X. Identification of N-acetyldopamine dimers from the dung beetle Catharsius molossus and Their COX-1 and COX-2 inhibitory activities. Molecules. 2015;20:15589–15596. doi: 10.3390/molecules200915589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hirose Y., Ohta E., Kawai Y., Ohta S. Dorsamin-A׳s, glycerolipids carrying a dehydrophenylalanine ester moiety from the seed-eating larvae of the bruchid beetle Bruchidius dorsalis. J Nat Prod. 2013;76:554–558. doi: 10.1021/np300713c. [DOI] [PubMed] [Google Scholar]
- 102.Suh H.J., Kim S.R., Hwang J.S., Kim M.J., Kim I. Antioxidant activity of aqueous methanol extracts from the lucanid Beetle, Serrognathus platymelus castanicolor motschulsky (Coleoptera: Lucanidae) J Asia Pac Entomol. 2011;14:95–98. [Google Scholar]
- 103.Mebs D., Pogoda W., Schneider M., Kauert G. Cantharidin and demethylcantharidin (palasonin) content of blister beetles (Coleoptera: Meloidae) from southern Africa. Toxicon. 2009;53:466–468. doi: 10.1016/j.toxicon.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 104.Dettner K., Schramm S., Seidl V., Klemm K., Gäde G., Fietz O. Occurrence of terpene anhydride Palasonin and Palasoninimide in blister beetle Hycleus lunata (Coleoptera: Meloidae) Biochem Syst Ecol. 2003;31:203–205. [Google Scholar]
- 105.Alzoubi K., Egler J., Briglia M., Fazio A., Faggio C., Lang F. Induction of suicidal erythrocyte death by cantharidin. Toxins. 2015;7:2822–2834. doi: 10.3390/toxins7082822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhao J., Guan X.W., Chen S.W., Hui L. Synthesis and biological evaluation of norcantharidin derivatives as protein phosphatase-1 inhibitors. Bioorg Med Chem Lett. 2015;25:363–366. doi: 10.1016/j.bmcl.2014.11.032. [DOI] [PubMed] [Google Scholar]
- 107.Verma A.K., Prasad S.B. Bioactive component, cantharidin from Mylabris cichorii and its antitumor activity against Ehrlich ascites carcinoma. Cell Biol Toxicol. 2012;28:133–147. doi: 10.1007/s10565-011-9206-6. [DOI] [PubMed] [Google Scholar]
- 108.Verma A.K., Prasad S.B. Antitumor effect of blister beetles: anethno-medicinal practice in Karbi community and its experimental evaluation against a murine malignant tumor model. J Ethnopharmacol. 2013;148:869–879. doi: 10.1016/j.jep.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 109.Hsiao T.C., Tsai C.H., Wu P.P., Hsu S.C., Liu H.C., Yuang Y.P. Cantharidin induces G2/M phase arrest by inhibition of CDC25C and cyclin A and triggers apoptosis through reactive oxygen species and the mitochondria-dependent pathways of A375.S2 human melanoma cells. Int J Oncol. 2014;45:2393–2402. doi: 10.3892/ijo.2014.2689. [DOI] [PubMed] [Google Scholar]
- 110.Zhang C.J., Chen Z.T., Zhou X.L., Xu W., Wang G., Tang X.X. Cantharidin induces G2/M phase arrest and apoptosis in human gastric cancer SGC-7901 and BGC-823 cells. Oncol Lett. 2014;8:2721–2726. doi: 10.3892/ol.2014.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim Y.M., Ku M.J., Son Y.J., Yun J.M., Kim S.H., Lee S.Y. Anti-metastatic effect of cantharidin in A549 human lung cancer cells. Arch Pharm Res. 2013;36:479–484. doi: 10.1007/s12272-013-0044-3. [DOI] [PubMed] [Google Scholar]
- 112.Hsia T.C., Yu C.C., Hsu S.C., Tang N.Y., Lu H.F., Huang Y.P. Cantharidin induces apoptosis of H460 human lung cancer cells through mitochondria-dependent pathways. Int J Oncol. 2014;45:245–254. doi: 10.3892/ijo.2014.2428. [DOI] [PubMed] [Google Scholar]
- 113.Wang T., Liu J., Xiao X.Q. Cantharidin inhibits angiogenesis by suppressing VEGF-induced JAK1/STAT3, ERK and AKT signaling pathways. Arch Pharm Res. 2015;38:282–289. doi: 10.1007/s12272-014-0383-8. [DOI] [PubMed] [Google Scholar]
- 114.Weis S.M., Cheresh D.A. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;7:1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 115.Lang E., Lang F. Triggers, inhibitors, mechanisms, and significance of eryptosis: the suicidal erythrocyte death. BioMed Res Int. 2015;2015:513518. doi: 10.1155/2015/513518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hsieh C.H., Huang Y.C., Tsai T.H., Chen Y.J. Cantharidin modulates development of human monocyte-derived dendritic cells. Toxicol in Vitro. 2011;25:1740–1747. doi: 10.1016/j.tiv.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 117.Twu N.F., Srinivasan R., Chou C.H., Wu L.S., Chiu C.H. Cantharidin and norcantharidin inhibit caprine luteal cell steroidogenesis in vitro. Exp Toxicol Pathol. 2012;64:37–44. doi: 10.1016/j.etp.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 118.Hsieh C.H., Chao K.S.C., Liao H.F., Chen Y.J. Norcantharidin, derivative of cantharidin, for cancer stem cells. Evid Based Complement Alternat Med. 2013;2013:838651. doi: 10.1155/2013/838651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xie J.R., Zhang Y.P., Hu X.M., Lv R., Xiao D.J., Jiang L. Norcantharidin inhibits Wnt signal pathway via promoter demethylation of WIF-1 in human non-small cell lung cancer. Med Oncol. 2015;32:145. doi: 10.1007/s12032-015-0592-0. [DOI] [PubMed] [Google Scholar]
- 120.Shen B., He P.J., Shao C.L. Norcantharidin induced DU145 cell apoptosis through ROS-mediated mitochondrial dysfunction and energy depletion. PLoS One. 2013;8:e84610. doi: 10.1371/journal.pone.0084610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liao H.F., Chen Y.J., Chou C.H., Wang F.W., Kuo C.D. Norcantharidin induces cell cycle arrest and inhibits progression of human leukemic Jurkat T cells through mitogen-activated protein kinase-mediated regulation of interleukin-2 production. Toxicol In Vitro. 2011;25:206–212. doi: 10.1016/j.tiv.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 122.Lee J.Y., Chung T.W., Choi H.J., Lee C.H., Eun J.S., Han Y.T. A novel cantharidin analog N-Benzylcantharidinamide reduces the expression of MMP-9 and invasive potentials of Hep3B via inhibiting cytosolic translocation of HuR. Biochem Biophys Res Commun. 2014;447:371–377. doi: 10.1016/j.bbrc.2014.04.035. [DOI] [PubMed] [Google Scholar]
- 123.Wu J.Y., Kuo C.D., Chu C.Y., Chen M.S., Lin J.H., Chen Y.J. Synthesis of novel lipophilic N-substituted norcantharimide derivatives and evaluation of their anticancer activities. Molecules. 2014;19:6911–6928. doi: 10.3390/molecules19066911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Arnott J.A., Planey S.L. The influence of lipophilicity in drug discovery and design. Exp Op Drug Disc. 2012;7:863–875. doi: 10.1517/17460441.2012.714363. [DOI] [PubMed] [Google Scholar]
- 125.Robertson M.J., Gordon C.P., Gilbert J., McCluskey A., Sakoff J.A. Norcantharimide analogues possessing terminal phosphate esters and their anti-cancer activity. Bioorg Med Chem. 2011;19:5734–5741. doi: 10.1016/j.bmc.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 126.Tarleton M., Gilbert J., Sakoff J.A., McCluskey A. Synthesis and anticancer activity of a series of norcantharidin analogues. Eur J Med Chem. 2012;54:573–581. doi: 10.1016/j.ejmech.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 127.Shimizu T., Iizuka M., Matsukura H., Hashizume D., Sodeoka M. Synthesis of optically pure norcantharidin analogue NCA-01, a highly selective protein phosphatase 2B inhibitor, and its derivatives. Chem Asian J. 2012;7:1221–1230. doi: 10.1002/asia.201200077. [DOI] [PubMed] [Google Scholar]
- 128.Luo S.L., Huang H.J., Wang Y., Jiang R.W., Wang L., Bai L.L. Isocoumarins from American cockroach (Periplaneta americana) and their cytotoxic activities. Fitoterapia. 2014;95:115–120. doi: 10.1016/j.fitote.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 129.Zhang H.W., Wei L.Y., Zhang Z.Y., Liu S.Z., Zhao G., Zhang J. Protective effect of Periplaneta americana extract on intestinal mucosal barrier function in patients with sepsis. J Tradit Chin Med. 2013;33:70–73. doi: 10.1016/s0254-6272(13)60103-x. [DOI] [PubMed] [Google Scholar]
- 130.Nimgulkar C.C., Patil S.D., Kumar B.D. Anti-asthmatic and anti-anaphylactic activities of Blatta orientalis mother tincture. Homeopathy. 2011;100:138–143. doi: 10.1016/j.homp.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 131.Yin H.Q., Wang B., Zhang J.D., Lin H.Q., Qiao Y., Wang R. Effect of traditional Chinese medicine Shu-Mai-Tang on attenuating TNFa-induced myocardial fibrosis in myocardial ischemia rats. J Ethnopharmacol. 2008;118:133–139. doi: 10.1016/j.jep.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 132.Zhang Y.H., Liu J.T., Wen B.Y., Liu N. Mechanisms of inhibiting proliferation of vascular smooth muscle cells by serum of rats treated with Dahuang Zhechong pill. J Ethnopharmacol. 2009;124:125–129. doi: 10.1016/j.jep.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 133.Shen N.N., Li X.G., Zhou T., Bilal M.U., Du N., Hu Y.Y. Shensong Yangxin Capsule prevents diabetic myocardial fibrosis by inhibiting TGF-β1/Smad signaling. J Ethnopharmacol. 2014;157:161–170. doi: 10.1016/j.jep.2014.09.035. [DOI] [PubMed] [Google Scholar]
- 134.Lin H.J., Tseng C.P., Lin C.F., Liao M.H., Chen C.M., Kao S.T. A Chinese herbal decoction, modified Yi Guan Jian, induces apoptosis in hepatic stellate cells through an ROS-mediated mitochondrial/caspase pathway. Evid Based Complement Alternat Med. 2011;2011:459531. doi: 10.1155/2011/459531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu H.X., Shang J.J., Chu F.Y., Li A.Y., Wu B., Xie X.R. Protective effects of Shen-Yuan-Dan, a traditional Chinese medicine, against myocardial ischemia/reperfusion injury in vivo and in vitro. Evid Based Complement Alternat Med. 2013;2013:956397. doi: 10.1155/2013/956397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ni L.J., Xu X.L., Zhang L.G., Shi W.Z. Quantitative evaluation of the in vitro effect and interactions of active fractions in Yaotongning-based formulae on prostaglandin E2 production. J Ethnopharmacol. 2014;154:807–817. doi: 10.1016/j.jep.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 137.Jiang H.L., Luo X.H., Wang X.Z., Yang J.L., Yao X.J., Crews P. New isocoumarins and alkaloid from Chinese insect medicine, Eupolyphaga sinensis walker. Fitoterapia. 2012;83:1275–1280. doi: 10.1016/j.fitote.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 138.Ge G.F., Yu C.H., Yu B., Shen Z.H., Zhang D.L., Wu Q.F. Antitumor effects and chemical compositions of Eupolyphaga sinensis Walker ethanol extract. J Ethnopharmacol. 2012;141:178–182. doi: 10.1016/j.jep.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 139.Zhang Y.M., Zhan Y.Z., Zhang D.D., Dai B.L., Ma W.N., Qi J.P. Eupolyphaga sinensis Walker displays inhibition on hepatocellular carcinoma through regulating cell growth and metastasis signaling. Sci Rep. 2014;4:5518. doi: 10.1038/srep05518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dai B.L., Zhan Y.Z., Qi J.P., Zhang Y.M. Eupolyphaga sinensis Walker inhibits human chronic myeloid leukemia cell K562 growth by inducing G2–M phase cell cycle arrest and targeting EGFR signaling pathway and in S180 tumor-bearing mice. Environ Toxicol Pharmacol. 2014;37:1177–1185. doi: 10.1016/j.etap.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 141.Dai B.L., Qi J.P., Liu R., Zhang Y.M. Eupolyphaga sinensis Walker demonstrates angiogenic activity and inhibits A549 cell growth by targeting the KDR signaling pathway. Mol Med Rep. 2014;10:1590–1596. doi: 10.3892/mmr.2014.2387. [DOI] [PubMed] [Google Scholar]
- 142.Zhu H.J., Yan Y.M., Tu Z.C., Luo J.F., Liang R., Yang T.H. Compounds from Polyphaga plancyi and their inhibitory activities against JAK3 and DDR1 kinases. Fitoterapia. 2016;114:163–167. doi: 10.1016/j.fitote.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 143.De Figueirêdo R.E., Vasconcellos A., Policarpo I.S., Alves R.R. Edible and medicinal termites: a global overview. J Ethnobiol Ethnomed. 2015;11:29. doi: 10.1186/s13002-015-0016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Courtney G.W., Pape T., Skevington J.H., Sinclair B.J. Biodiversity of Diptera. In: Foottit R.G., Adler P.H., editors. Insect biodiversity: science and society. Blackwell Publishing Ltd.; West Sussex: 2009. pp. 185–222. [Google Scholar]
- 145.Lu X., Shen J., Jin X., Ma Y., Huang Y., Mei H. Bactericidal activity of Musca domestica cecropin (Mdc) on multidrug-resistant clinical isolate of Escherichia coli. Appl Microbiol Biotechnol. 2012;95:939–945. doi: 10.1007/s00253-011-3793-2. [DOI] [PubMed] [Google Scholar]
- 146.Song C., Yu H., Zhang M., Yang Y., Zhang G. Physicochemical properties and antioxidant activity of chitosan from the blowfly Chrysomya megacephala larvae. Int J Biol Macromolec. 2013;60:347–354. doi: 10.1016/j.ijbiomac.2013.05.039. [DOI] [PubMed] [Google Scholar]
- 147.Ai H., Wang F., Xia Y., Chen X., Lei C. Antioxidant, antifungal and antiviral activities of chitosan from the larvae of housefly, Musca domestica L. Food Chem. 2012;132:493–498. doi: 10.1016/j.foodchem.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 148.Díaz-Roa A., Gaona M.A., Segura N.A., Suárez D., Patarroyo M.A., Bello F.J. Sarconesiopsis magellanica (Diptera: Calliphoridae) excretions and secretions have potent antibacterial activity. Acta Trop. 2014;136:37–43. doi: 10.1016/j.actatropica.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 149.Hou L., Shi Y., Zhai P., Le G. Antibacterial activity and in vitro anti-tumor activity of the extract of the larvae of the housefly (Musca domestica) J Ethnopharmacol. 2007;111:227–231. doi: 10.1016/j.jep.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 150.Henry T.J. Biodiversity of Heteroptera. In: Foottit R.G., Adler P.H., editors. Insect biodiversity: science and society. Blackwell Publishing Ltd.; West Sussex: 2009. pp. 223–263. [Google Scholar]
- 151.González E.A., García E.M., Nazareno M.A. Free radical scavenging capacity and antioxidant activity of cochineal (Dactylopius coccus C.) extracts. Food Chem. 2010;119:358–362. [Google Scholar]
- 152.Lu J., Wang H.Y., Huang J., Li G.Y., Wang Q.H., Xu W. Sesquiterpene acids from Shellac and their bioactivities evaluation. Fitoterapia. 2014;97:64–70. doi: 10.1016/j.fitote.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 153.Teffo L.S., Toms R.B., Eloff J.N. Preliminary data on the nutritional composition of the edible stink-bug, Encosternum delegorguei Spinola, consumed in Limpopo province, South Africa. S Afr J Sci. 2007;103:434–436. [Google Scholar]
- 154.Makore T.A., Garamumhango P., Chirikure T., Chikambi S.D. Determination of Nutritional Composition of Encosternum delegorguei Caught in Nerumedzo Community of Bikita, Zimbabwe. Int. J Biol. 2015;7:13–19. [Google Scholar]
- 155.Luo X.H., Wang X.Z., Jiang H.L., Yang J.L., Crews P., Valeriote F.A. The biosynthetic products of Chinese insect medicine, Aspongopus chinensis. Fitoterapia. 2012;83:754–758. doi: 10.1016/j.fitote.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yan Y.M., Ai J., Shi Y.N., Zuo Z.L., Hou B., Luo J. (±)-Aspongamide A, an N-acetyldopamine trimer isolated from the insect Aspongopus chinensis, is an inhibitor of p-Smad3. Org Lett. 2014;16:532–535. doi: 10.1021/ol403409v. [DOI] [PubMed] [Google Scholar]
- 157.Shi Y.N., Tu Z.C., Wang X.L., Yan Y.M., Fang P., Zuo Z.L. Bioactive compounds from the insect Aspongopus chinensis. Bioorg Med Chem Lett. 2014;24:5164–5169. doi: 10.1016/j.bmcl.2014.09.083. [DOI] [PubMed] [Google Scholar]
- 158.Di L., Shi Y.N., Yan Y.M., Jiang L.P., Hou B., Wang X.L. Nonpeptide small molecules from the insect Aspongopus chinensis and their neural stem cell proliferation stimulating properties. RSC Adv. 2015;5:70985–70991. [Google Scholar]
- 159.Cheseto X., Kuate S.P., Tchouassi D.P., Ndung’u M., Teal P.E.A., Torto B. Potential of the desert locust Schistocerca gregaria (Orthoptera: Acrididae) as an unconventional source of dietary and therapeutic sterols. PLoS One. 2015:e0127171. doi: 10.1371/journal.pone.0127171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pettit G.R., Meng Y.H., Herald D.L., Knight J.C., Day J.F. Antineoplastic agents. 553. the texas grasshopper Brachystola magna. J Nat Prod. 2005;68:1256–1258. doi: 10.1021/np0402367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pettit G.R., Ye Q.H., Herald D.L., Hogan F., Pettit R.K. Antineoplastic Agents. 573. Isolation and Structure of Papilistatin from the Papilionid Butterfly Byasa polyeuctes termessa. J Nat Prod. 2010;73:164–166. doi: 10.1021/np9004689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tulp M., Bohlin L. Unconventional natural sources for future drug discovery. Drug Discov Today. 2004;9:450–458. doi: 10.1016/S1359-6446(04)03066-1. [DOI] [PubMed] [Google Scholar]
- 163.Kaya M., Baran T., Asan-Ozusaglam M., Cakmak Y.S., Tozak K.O., Mol A. Extraction and characterization of chitin and chitosan with antimicrobial and antioxidant activities from cosmopolitan orthoptera species (Insecta) Biotechnol Bioprocess Eng. 2015;20:168–179. [Google Scholar]
- 164.Chakravorty J., Ghosh S., Jung C., Meyer-Rochow V.B. Nutritional composition of Chondacris rosea and Brachytrupes orientalis: two common insects used as food by tribes of arunachal Pradesh, India. J Asia Pac Entomol. 2014;17:407–415. [Google Scholar]
- 165.Pogue M.G. Biodiversity of Lepidoptera. In: Foottit R.G., Adler P.H., editors. Insect biodiversity: science and society. Blackwell Publishing Ltd.; West Sussex: 2009. pp. 325–355. [Google Scholar]
- 166.Hirayama C., Ono H., Meng Y., Shimada T., Daimon T. Flavonoids from the cocoon of Rondotia menciana. Phytochemistry. 2013;94:108–112. doi: 10.1016/j.phytochem.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 167.Ali M.M., Arumugam S.B. Effect of crude extract of Bombyx mori coccoons in hyperlipidemia and atherosclerosis. J Ayurveda Integr Med. 2011;2:72–78. doi: 10.4103/0975-9476.82527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Khan M.S., Singh M., Khan M.A., Arya D.S., Ahmad S. Scientific validation of cardioprotective attribute by standardized extract of Bombyx mori against doxorubicin-induced cardiotoxicity in murine model. EXCLI J. 2014;13:1043–1054. [PMC free article] [PubMed] [Google Scholar]
- 169.Park J.H., Lee D.Y., Kim S.Y., Shrestha S., Bang M.H., Kim G.S., Baek N.I. New hydroxy fatty acids from Bombyx mori droppings. Chem Nat Compd. 2014;50:801–803. [Google Scholar]
- 170.Xu L., Pan H., Lei Q., Xiao W., Peng Y., Xiao P. Insect tea, a wonderful work in the Chinese tea culture. Food Res Int. 2013;53:629–635. [Google Scholar]
- 171.Qian Y., Li G.J., Wang R., Zhou Y.L., Sun P., Zhao X. In vitro anticancer effects of insect tea in TCA8113 cells. J Cancer Res Ther. 2014;10:1045–1051. doi: 10.4103/0973-1482.137979. [DOI] [PubMed] [Google Scholar]
- 172.Suo H.Y., Sun P., Wang C., Peng D.G., Zhao X. Apoptotic effects of insect tea in HepG2 human hepatoma cells. CyTA J Food. 2015;14:169–175. [Google Scholar]
- 173.Zhou Y.L., Wang R., Feng X., Zhao X. Preventive effect of insect tea against reserpine-induced gastric ulcers in mice. Exp Ther Med. 2014;8:1318–1324. doi: 10.3892/etm.2014.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhao X., Wang R., Qian Y., Li G., Zhou Y., Sun P. In vivo preventive effects of insect tea on buccal mucosa cancer in ICR mice. J Cancer Res Ther. 2014;10:651–657. doi: 10.4103/0973-1482.138081. [DOI] [PubMed] [Google Scholar]